Aniracetam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ampamet, Memodrin, Pergamid |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 1-2.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.108.230 |
| Chemical and physical data | |
| Formula | C12H13NO3 |
| Molar mass | 219.237 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Aniracetam (Draganon, Sarpul, Ampamet, Memodrin) is an ampakine and nootropic of the racetam chemical class purported to be considerably more potent than piracetam. It is lipid-soluble and has possible cognition-enhancing effects. It has been tested in animals extensively, Alzheimer's patients, and temporarily-impaired healthy subjects. It has shown potential as an anxiolytic in three clinical animal models. It is sold in Europe as a prescription drug.
Pharmacology
After a confirmed test of the anxiolytic efficacy in a mouse model, haloperidol, mecamylamine, and ketanserin were applied to determine the pathways aniracetam depends on to exert its anti-anxiety effects. Haloperidol completely reversed the anxiolytic effects, and mecamylamine and ketanserin nearly completely reversed the effects. This shows that aniracetam's anxiolytic mechanism is facilitated by D2/D3, nACh, and 5-HT2A receptors.[1]
Aniracetam has also been shown to selectively modulate the AMPA receptor[2] and was used as the parent compound to derive a class of drugs known as the ampakines that are being investigated as nootropics and neuroprotective drugs for the treatment of Alzheimer's disease and other neurodegenerative conditions.1
Despite the fat solubility of aniracetam, its half-life is much shorter than that of common racetam analogs such as piracetam. [citation needed]
Commonly used doses are 750-3,000 mg daily usually taken in 2-3 doses.
Side effects can include nausea and headache.[citation needed]
Synthesis
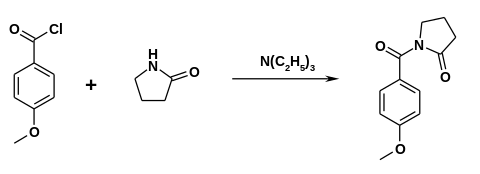
The drug was first made in the 1970s by Hoffmann-La Roche.[3][4] Synthesis can be caused by 2-Pyrrolidone reacting with Anisoyl chloride in the presence of Triethylamine.[5]
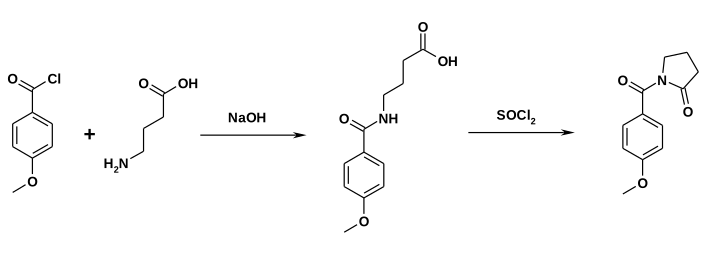
Alternatively, gamma-Aminobutyric acid can react with anisoyl chloride. Ring closure can be accomplished with the presence of Thionyl chloride.[5]
See also
References
- ^ Nakamura K (2001). "Anxiolytic effects of aniracetam in three different mouse models of anxiety and the underlying mechanism". Eur J Pharmacol. (Kanagawa, Japan). 420 (1): 33–43. doi:10.1016/S0014-2999(01)01005-6. PMID 11412837.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Ito et al. J. Physiol. 1990; 424: 533-543.
- ^ Patent EP 5 143 Hoffmann-La Roche 1978.
- ^ Patent EP 44 088 Hoffmann-La Roche 1978.
- ^ a b A. Kleemann, J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications, 4. Auflage, Thieme 2001, ISBN3-13-115134-X.
This article needs additional citations for verification. (February 2010) |
External links