Bleomycin
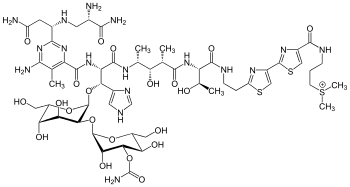 Bleomycin A2 | |
| Clinical data | |
|---|---|
| Trade names | Blenoxane |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682125 |
| License data | |
| Pregnancy category | |
| Routes of administration | intravenous, intramuscular, subcutaneous, intrapleural[2] |
| Drug class | Glycopeptide antibiotic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% and 70% following intramuscular and subcutaneous administrations, respectively, and 45% following both intraperitoneal and intrapleural administrations[2] |
| Elimination half-life | two hours[2] |
| Excretion | renal (60–70%)[2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C55H84N17O21S3 |
| Molar mass | 1415.551 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bleomycin is a medication used to treat cancer.[5] This includes Hodgkin's lymphoma, non-Hodgkin's lymphoma, testicular cancer, ovarian cancer, and cervical cancer among others.[5] Typically used with other cancer medications,[5] it can be given intravenously, by injection into a muscle or under the skin.[5] It may also be administered inside the chest to help prevent the recurrence of a pleural effusion due to cancer; however talc is better for this.[5][6]
Common side effects include fever, weight loss, vomiting, and rash.[5] A severe type of anaphylaxis may occur.[5] It may also cause inflammation of the lungs that can result in lung scarring.[5] Chest X-rays every couple of weeks are recommended to check for this.[5] Bleomycin may cause harm to the baby if used during pregnancy.[5] It is believed to primarily work by preventing the synthesis of DNA.[5]
Bleomycin was discovered in 1962.[7][8] It is on the World Health Organization's List of Essential Medicines.[9] It is available as a generic medication.[5] It is made by the bacterium Streptomyces verticillus.[5]
Medical uses
Cancer
Bleomycin is mostly used to treat cancer.[5] This includes testicular cancer, ovarian cancer, and Hodgkin's disease, and less commonly non-Hodgkin's disease.[5] It can be given intravenously, by intramuscular injection, or under the skin.[5]
Other uses
It may also be put inside the chest to help prevent the recurrence of a pleural effusion due to cancer.[5] However, for scarring down the pleura, talc appears to be the better option although indwelling pleural catheters are at least as effective in reducing the symptoms of an effusion(such as dyspnea).[10][11]
While potentially effective against bacterial infections, its toxicity prevents its use for this purpose.[5] It has been studied in the treatment of warts but is of unclear benefit.[12]
Side effects
The most common side effects are flu-like symptoms and include fever, rash, dermatographism, hyperpigmentation, alopecia (hair loss), chills, and Raynaud's phenomenon (discoloration of fingers and toes). The most serious complication of bleomycin, occurring upon increasing dosage, is pulmonary fibrosis and impaired lung function. It has been suggested that bleomycin induces sensitivity to oxygen toxicity[13] and recent studies support the role of the proinflammatory cytokines IL-18 and IL-1beta in the mechanism of bleomycin-induced lung injury.[14] Any previous treatment with bleomycin should therefore always be disclosed to the anaesthetist prior to undergoing a procedure requiring general anaesthesia. Due to the oxygen sensitive nature of bleomycin, and the theorised increased likelihood of developing pulmonary fibrosis following supplemental oxygen therapy, it has been questioned whether patients should take part in scuba diving following treatment with the drug.[15] Bleomycin has also been found to disrupt the sense of taste.[16]
Lifetime cumulative dose
Bleomycin should not exceed a lifetime cumulative dose greater than 400 units.[17] Pulmonary toxicities, most commonly presenting as pulmonary fibrosis, are associated with doses of bleomycin greater than 400 units.[17]
Mechanism of action
Bleomycin acts by induction of DNA strand breaks.[18] Some studies suggest bleomycin also inhibits incorporation of thymidine into DNA strands. DNA cleavage by bleomycin depends on oxygen and metal ions, at least in vitro. The exact mechanism of DNA strand scission is unresolved, but it has been suggested that bleomycin chelates metal ions (primarily iron), producing a pseudoenzyme that reacts with oxygen to produce superoxide and hydroxide free radicals that cleave DNA. An alternative hypothesis states that bleomycin may bind at specific sites in the DNA strand and induce scission by abstracting the hydrogen atom from the base, resulting in strand cleavage as the base undergoes a Criegee-type rearrangement, or forms an alkali-labile lesion.[19] In addition, these complexes also mediate lipid peroxidation and oxidation of other cellular molecules. Therefore, bleomycin is used in combination with doxorubicin in Hodgkins lymphoma, as they have additive and complementary effects on the DNA, since doxorubicin acts by intercalating between DNA strands, and also acts on topoisomerase II enzyme thus relaxing the topoisomerase complexes.[citation needed]
Biosynthesis
Bleomycin is a nonribosomal peptide that is a hybrid peptide-polyketide natural product. The peptide/polyketide/peptide backbone of the bleomycin aglycon is assembled by the bleomycin megasynthetase, which is made of both nonribosomal peptide synthetase (NRPS) and polyketide synthase (PKS) modules. Nonribosomal peptides and polyketides are synthesized from amino acids and short carboxylic acids by NRPSs and PKSs, respectively. These NRPSs and PKSs use similar strategies for the assembly of these two distinct classes of natural products. Both NRPs and type I PKSs are organized into modules. The structural variations of the resulting peptide and polyketide products are determined by the number and order of modules on each NRPS and PKS protein.[citation needed]
The biosynthesis of the bleomycin aglycon can be visualized in three stages:[citation needed]
- NRPS-mediated formation of P-3A from Ser, Asn, His, and Ala
- PKS-mediated elongation of P-3A by malonyl CoA and AdoMet to yield P-4
- NRPS-mediated elongation of P-4 by Thr to P-5 that is further elongated by β-Ala, Cys, and Cys to get P-6m.
On the basis of the bleomycin structure and the deduced functions of individual NRPS and PKS domains and modules, a linear model for the bleomycin megasynthetase-templated assembly of the bleomycin peptide/polyketide/peptide aglycon was proposed from nine amino acids and one acetate.[citation needed]
Biosynthesis of bleomycin is completed by glycosylation of the aglycones. Bleomycin naturally occurring-analogues have two to three sugar molecules, and DNA cleavage activities of these analogues have been assessed,[20][21] primarily by the plasmid relaxation and break light assays.
History
Bleomycin was first discovered in 1962 when the Japanese scientist Hamao Umezawa found anticancer activity while screening culture filtrates of Streptomyces verticillus. Umezawa published his discovery in 1966.[22] The drug was launched in Japan by Nippon Kayaku in 1969. In the US, bleomycin gained FDA approval in July 1973. It was initially marketed in the US by the Bristol-Myers Squibb precursor, Bristol Laboratories, under the brand name Blenoxane.
Research
Bleomycin is used in research to induce pulmonary fibrosis in mice.[23]
See also
- Flagellate pigmentation from bleomycin
- Pingyangmycin (Bleomycin A5)
References
- ^ a b "Bleomycin Use During Pregnancy". Drugs.com. 9 August 2019. Retrieved 16 February 2020.
- ^ a b c d e "Bleomycin- bleomycin sulfate injection, powder, lyophilized, for solution". DailyMed. 31 December 2019. Retrieved 16 February 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Bleo-Kyowa Powder for solution for injection - Summary of Product Characteristics (SmPC)". (emc). 31 August 2018. Archived from the original on 16 February 2020. Retrieved 16 February 2020.
- ^ a b c d e f g h i j k l m n o p q r "Bleomycin Sulfate". The American Society of Health-System Pharmacists. Archived from the original on 8 September 2015. Retrieved 1 August 2015.
- ^ Dipper A, Jones HE, Bhatnagar R, Preston NJ, Maskell N, Clive AO (April 2020). "Interventions for the management of malignant pleural effusions: a network meta-analysis". The Cochrane Database of Systematic Reviews. 2020 (4): CD010529. doi:10.1002/14651858.CD010529.pub3. PMC 7173736. PMID 32315458.
- ^ Sneader W (2005). Drug discovery : a history (Rev. and updated ed.). Chichester: Wiley. p. 312. ISBN 9780471899792. Archived from the original on 5 March 2016.
- ^ Phillips GO (2018). Innovation and Technology Transfer in Japan and Europe: Industry-Academic Interactions. Routledge. p. PT155. ISBN 9780429774546.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Shaw P, Agarwal R (2004). Shaw PH (ed.). "Pleurodesis for malignant pleural effusions". The Cochrane Database of Systematic Reviews (1): CD002916. doi:10.1002/14651858.CD002916.pub2. PMID 14973997. (Retracted, see doi:10.1002/14651858.cd002916.pub3)
- ^ Thomas R, Murray K, Lee YC (April 2018). "Treatment Approaches for Malignant Pleural Effusion". JAMA. 319 (14): 1507–1508. doi:10.1001/jama.2018.1323. PMID 29634827.
- ^ Kwok CS, Gibbs S, Bennett C, Holland R, Abbott R (September 2012). "Topical treatments for cutaneous warts". The Cochrane Database of Systematic Reviews. 9 (9): CD001781. doi:10.1002/14651858.CD001781.pub3. PMC 8101088. PMID 22972052.
- ^ Thompson M. "Bleomycin and Anaesthesia" (PDF). Anaesthesia Western Australia. Archived from the original (PDF) on 8 September 2017. Retrieved 8 September 2017.
- ^ Hoshino T, Okamoto M, Sakazaki Y, Kato S, Young HA, Aizawa H (December 2009). "Role of proinflammatory cytokines IL-18 and IL-1beta in bleomycin-induced lung injury in humans and mice". American Journal of Respiratory Cell and Molecular Biology. 41 (6): 661–670. doi:10.1165/rcmb.2008-0182OC. PMC 10283344. PMID 19265174.
- ^ Huls G, ten Bokkel Huinink D (February 2003). "Bleomycin and scuba diving: to dive or not to dive?". The Netherlands Journal of Medicine. 61 (2): 50–53. PMID 12735422.
- ^ Ackerman BH, Kasbekar N (1997). "Disturbances of taste and smell induced by drugs". Pharmacotherapy. 17 (3): 482–496. doi:10.1002/j.1875-9114.1997.tb03058.x. PMID 9165552. S2CID 12671326.
- ^ a b "bleomycin [TUSOM | Pharmwiki]". tmedweb.tulane.edu. Retrieved 2 February 2022.
- ^ Takimoto CH, Calvo E. "Principles of Oncologic Pharmacotherapy" Archived 15 May 2009 at the Wayback Machine in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach Archived 4 October 2013 at the Wayback Machine. 11 ed. 2008.
- ^ Hecht SM (January 2000). "Bleomycin: new perspectives on the mechanism of action". Journal of Natural Products. 63 (1): 158–168. doi:10.1021/np990549f. PMID 10650103.
- ^ Hindra, Yang D, Teng Q, Dong LB, Crnovčić I, Huang T, Ge H, Shen B (March 2017). "Genome Mining of Streptomyces mobaraensis DSM40847 as a Bleomycin Producer Providing a Biotechnology Platform To Engineer Designer Bleomycin Analogues". Organic Letters. 19 (6): 1386–1389. doi:10.1021/acs.orglett.7b00283. PMID 28256838.
- ^ Yang D, Hindra, Dong LB, Crnovcic I, Shen B (August 2017). "Engineered production and evaluation of 6'-deoxy-tallysomycin H-1 revealing new insights into the structure-activity relationship of the anticancer drug bleomycin". The Journal of Antibiotics. 71: 97–103. doi:10.1038/ja.2017.93. PMID 28831149. S2CID 33531845.
- ^ Umezawa H, Maeda K, Takeuchi T, Okami Y (September 1966). "New antibiotics, bleomycin A and B". The Journal of Antibiotics. 19 (5): 200–9. PMID 5953301.
- ^ Song N, Liu J, Shaheen S, Du L, Proctor M, Roman J, Yu J (August 2015). "Vagotomy attenuates bleomycin-induced pulmonary fibrosis in mice". Scientific Reports. 5: 13419. Bibcode:2015NatSR...513419S. doi:10.1038/srep13419. PMC 4542162. PMID 26289670.
In our studies, mice developed classic PF with structural alteration of the lung following intravenous bleomycin treatment
Further reading
- Claussen CA, Long EC (September 1999). "Nucleic Acid recognition by metal complexes of bleomycin". Chemical Reviews. 99 (9): 2797–2816. doi:10.1021/cr980449z. PMID 11749501.
- Shen B, Du L, Sanchez C, Edwards DJ, Chen M, Murrell JM (December 2001). "The biosynthetic gene cluster for the anticancer drug bleomycin from Streptomyces verticillus ATCC15003 as a model for hybrid peptide-polyketide natural product biosynthesis". Journal of Industrial Microbiology & Biotechnology. 27 (6): 378–385. doi:10.1038/sj.jim.7000194. PMID 11774003. S2CID 3022217.
External links
- "Bleomycin". Drug Information Portal. U.S. National Library of Medicine.
- "Bleomycin". European Medicines Agency (EMA). 17 September 2018.
