Bovidae
| Bovidae | |
|---|---|

| |
| Example Bovidae (clockwise from top left) – addax, domestic cattle, gazelle, impala, wildebeest, and mouflon | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Artiodactyla |
| Superfamily: | Bovoidea |
| Family: | Bovidae Gray, 1821 |
| Subfamilies | |
|
Aepycerotinae (1 genus) | |
The Bovidae comprise the biological family of cloven-hoofed, ruminant vertebrates that includes bison, African buffalo, water buffalo, antelopes, sheep, goats, muskoxen, and domestic cattle. A member of this family is called a bovid. With 143 extant species and 300 known extinct species, the family Bovidae consists of eight major subfamilies apart from the disputed Peleinae and Pantholopinae. The family evolved 20 million years ago, in the early Miocene.
The bovids show great variation in size and pelage colouration. Excepting some domesticated forms, all male bovids have two or more horns, and in many species, females possess horns, too. The size and shape of the horns vary greatly, but the basic structure is always one or more pairs of simple bony protrusions without branches, often having a spiral, twisted or fluted form, each covered in a permanent sheath of keratin. Most bovids bear 30 to 32 teeth.
Most bovids are diurnal. Social activity and feeding usually peak during dawn and dusk. Bovids typically rest before dawn, during midday, and after dark. They have various methods of social organisation and social behaviour, which are classified into solitary and gregarious behaviour. Bovids use different forms of vocal, olfactory, and tangible communication. Most species alternately feed and ruminate throughout the day. While small bovids forage in dense and closed habitat, larger species feed on high-fiber vegetation in open grasslands. Most bovids are polygynous. Mature bovids mate at least once a year and smaller species may even mate twice. In some species, neonate bovids remain hidden for a week to two months, regularly nursed by their mothers; in other species, neonates are followers, accompanying their dams, rather than tending to remain hidden.
The greatest diversities of bovids occur in Africa. The maximum concentration of species is in the savannas of eastern Africa. Other bovid species also occur in Europe, Asia, and North America. Bovidae includes three of the five domesticated mammals whose use has spread outside their original ranges, namely cattle, sheep, and goats. Dairy products such as milk, butter, and cheese are manufactured largely from domestic cattle. Bovids also provide leather, meat, and wool.
Etymology
The name "Bovidae" was given by the British zoologist John Edward Gray in 1821.[1] The word "Bovidae" is the combination of the prefix bov- (originating from Latin bos, "ox", through Late Latin bovinus) and the suffix -idae.[2]
Taxonomy
The family Bovidae is placed in the order Artiodactyla (which includes the even-toed ungulates). It includes 143 extant species, accounting for nearly 55% of the ungulates, and 300 known extinct species.[3]
Molecular studies have supported monophyly in the family Bovidae (a group of organisms comprises an ancestral species and all their descendants).[4][5] The number of subfamilies in Bovidae is disputed, with suggestions of as many as ten and as few as two subfamilies.[5] However, molecular, morphological and fossil evidence indicates the existence of eight distinct subfamilies : Aepycerotinae (consisting of just the impala), Alcelaphinae (bontebok, hartebeest, wildebeest and relatives), Antilopinae (several antelopes, gazelles, and relatives), Bovinae (cattle, buffaloes, bison and other antelopes), Caprinae (goats, sheep, ibex, serows and relatives), Cephalophinae (duikers), Hippotraginae (addax, oryx and relatives) and Reduncinae (reedbuck and kob antelopes). In addition, three extinct subfamilies are known: Hypsodontinae (mid-Miocene), Oiocerinae (Turolian) and the subfamily Tethytraginae, which contains Tethytragus (mid-Miocene).[6][7]
In 1992, Alan W. Gentry of the Natural History Museum, London divided the eight major subfamilies of Bovidae into two major clades on the basis of their evolutionary history: the Boodontia, which comprised only the Bovinae, and the Aegodontia, which consisted of the rest of the subfamilies. Boodonts have somewhat primitive teeth, resembling those of oxen, whereas aegodonts have more advanced teeth like those of goats.[8]
A controversy exists about the recognition of Peleinae and Patholopinae, comprising the genera Pelea and Pantholops respectively, as subfamilies. In 2000, American biologist George Schaller and palaeontologist Elisabeth Vrba suggested the inclusion of Pelea in Reduncinae,[9] though the grey rhebok, the sole species of Pelea, is highly different from kobs and reduncines in morphology.[10] Pantholops, earlier classified in the Antilopinae, was later placed in its own subfamily, Pantholopinae. However, molecular and morphological analysis supports the inclusion of Pantholops in Caprinae.[11]
Below is a cladogram based on Gatesy et al. (1997) and Gentry et al. (1997)
| Bovidae |
| |||||||||||||||||||||||||||||||||||||||||||||
Evolution
Early Miocene and before

In the early Miocene, bovids began diverging from the cervids (deer) and giraffids. The earliest bovids, whose presence in Africa and Eurasia in the latter part of early Miocene (20 Mya) has been ascertained, were small animals, somewhat similar to modern gazelles, and probably lived in woodland environments.[12] Eotragus, the earliest known bovid, weighed 18 kg (40 lb) and was nearly the same in size as the Thompson's gazelle.[13] Early in their evolutionary history, the bovids split into two main clades: Boodontia (of Eurasian origin) and Aegodontia (of African origin). This early split between Boodontia and Aegodontia has been attributed to the continental divide between these land masses. When these continents were later rejoined, this barrier was removed, and either group expanded into the territory of the other.[14] The tribes Bovini and Tragelaphini diverged in the early Miocene.[15] Bovids are known to have reached the Americas in the Pleistocene by crossing the Bering land bridge.[13]
The present genera of Alcelaphinae appeared in the Pliocene. The extinct Alcelaphine genus Paramularius, which was the same in size as the hartebeest, is believed to have come into being in the Pliocene, but became extinct in the middle Pleistocene.[5] Several genera of Hippotraginae are known since the Pliocene and Pleistocene. This subfamily appears to have diverged from the Alcelaphinae in the latter part of early Miocene.[15] The Bovinae are believed to have diverged from the rest of the Bovidae in the early Miocene.[16] The Boselaphini became extinct in Africa in the early Pliocene; their latest fossils were excavated in Langebaanweg (South Africa) and Lothagam (Kenya).[17]
Middle Miocene
The middle Miocene marked the spread of the bovids into China and the Indian subcontinent.[13] According to Vrba, the radiation of the subfamily Alcelaphinae began in the latter part of middle Miocene.[5] The Caprinae tribes probably diverged in the early middle Miocene. The Caprini emerged in the middle Miocene, and seem to have been replaced by other bovids and cervids in Eurasia.[18] The earliest fossils of the antilopines are from the middle Miocene, though studies show the existence of the subfamily from the early Miocene. Speciation occurred in the tribe Antilopini during the middle or upper Miocene, mainly in Eurasia. Tribe Neotragini seems to have appeared in Africa by the end of Miocene, and had become widespread by the Pliocene.[15]
Late Miocene
By the late Miocene, around 10 Mya, the bovids rapidly diversified, leading to the creation of 70 new genera.[13] This late Miocene radiation was partly because many bovids became adapted to more open, grassland habitats.[12] The Aepycerotinae first appeared in the late Miocene, and no significant difference in the sizes of the primitive and modern impala has been noted.[19] Fossils of ovibovines, a tribe of Caprinae, in Africa date back to the late Miocene.[15] The earliest Hippotragine fossils date back to the late Miocene, and were excavated from sites such as Lothagam and Awash Valley.[15] The first African fossils of Reduncinae date back to 6-7 Mya.[20] Reduncinae and Peleinae probably diverged in the mid-Miocene.[5]
Characteristics

All bovids have the similar basic form - a snout with a blunt end, one or more pairs of horns (generally present on males) immediately after the oval or pointed ears, a distinct neck and limbs, and a tail varying in length and bushiness among the species.[21] Most bovids exhibit sexual dimorphism, with males usually larger as well as heavier than females. Sexual dimorphism is more prominent in medium- to large-sized bovids. All bovids have four toes on each foot – they walk on the central two (the hooves), while the outer two (the dewclaws) are much smaller and rarely touch the ground.[3]
The bovids show great variation in size: the gaur can weigh as much as 1,000 kg (2,200 lb) and stands 2–3 m (6.6–9.8 ft) high at the shoulder.[22] The water buffalo can be even heavier, and weigh 1,200 kg (2,600 lb), though it is shorter than the gaur, being at most 2 m (6.6 ft) tall.[23] The royal antelope, in sharp contrast, is only 25 cm (9.8 in) tall and weighs at most 3 kg (6.6 lb).[24] The klipspringer, another small antelope, stands 45–60 cm (18–24 in) at the shoulder and weighs just 10–20 kg (22–44 lb).[25]
Differences occur in pelage colouration, ranging from a pale white (as in the Arabian oryx)[26] to black (as in the black wildebeest).[27] However, only the intermediate shades, such as brown and reddish brown (as in the reedbuck), are commonly observed.[28] In several species, females and juveniles exhibit a light-coloured coat, while those of males darken with age. As in the wildebeest, the coat may be marked with prominent or faint stripes. In some species such as the addax, the coat colour can vary by the season.[29] Scent glands and sebaceous glands are often present.[21]

Some species, such as the gemsbok, sable antelope, and Grant's gazelle, are camouflaged with strongly disruptive facial markings that conceal the highly recognisable eye.[31] Many species, such as gazelles, may be made to look flat, and hence to blend into the background, by countershading.[32] The outlines of many bovids are broken up with bold disruptive colouration, the strongly contrasting patterns helping to delay recognition by predators.[33] However, all the Hippotraginae (including the gemsbok) have pale bodies and faces with conspicuous markings. The zoologist Tim Caro describes this as difficult to explain, but given that the species are diurnal, he suggests that the markings may function in communication. Strongly contrasting leg colouration is common only in the Bovidae, where for example Bos, Ovis, bontebok and gemsbok have white stockings. Again, communication is the likely function.[30]
Excepting some domesticated forms, all male bovids have horns, and in many species, females, too, possess horns. The size and shape of the horns vary greatly, but the basic structure is a pair of simple bony protrusions without branches, often having a spiral, twisted, or fluted form, each covered in a permanent sheath of keratin. Although horns occur in a single pair on almost all bovid species, there are exceptions such as the four-horned antelope[34] and the Jacob sheep.[35][36] The unique horn structure is the only unambiguous morphological feature of bovids that distinguishes them from other pecorans.[37][38] A high correlation exists between horn morphology and fighting behaviour of the individual. For instance, long horns are intended for wrestling and fencing, whereas curved horns are used in ramming.[39] Males with horns directed inwards are monogamous and solitary, while those with horns directed outwards tend to be polygynous. These results were independent of body size.[40]
Male horn development has been linked to sexual selection,[41][42] Horns are small spikes in the monogamous duikers and other small antelopes, whereas in the polygynous, they are large and elaborately formed (for example in a spiral structure, as in the giant eland). Thus, to some extent, horns depict the degree of competition among males in a species.[28] However, the presence of horns in females is likely due to natural selection.[41][43] The horns of females are usually smaller than those of males, and are sometimes of a different shape. The horns of female bovids are believed to have evolved for defence against predators or to express territoriality, as nonterritorial females, which are able to use crypsis for predator defence, often do not have horns.[43] Females possess horns only in half of the bovid genera, and females in these genera are heavier than those in the rest. Females use horns mainly for stabbing.[44]

Anatomy
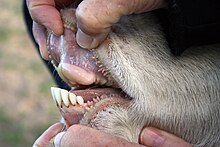
In bovids, the third and fourth metapodials are combined into the cannon bone. The ulna and fibula are reduced, and fused with the radius and tibia, respectively. Long scapulae are present, whereas the clavicles are absent. Being ruminants, the stomach is composed of four chambers: the rumen (80%), the omasum, the reticulum, and the abomasum. The ciliates and bacteria of the rumen ferment the complex cellulose into simpler fatty acids, which are then absorbed through the rumen wall. Bovids have a long small intestine; the length of the small intestine in cattle is 29–49 m (95–161 ft). Body temperature fluctuates through the day; for instance, in goats the temperature can change slightly from nearly 37 °C (99 °F) in the early morning to 40 °C (104 °F) in the afternoon. Temperature is regulated through sweating in cattle, whereas goats use panting for the same. The right lung, consisting of four to five lobes, is around 1.5 times larger than the left, which has three lobes.[3][21]
Dentition
Most bovids bear 30 to 32 teeth.[28] While the upper incisors are absent, the upper canines are either reduced or absent. Instead of the upper incisors, bovids have a thick and tough layer of tissue, called the dental pad, that provides a surface to grip grasses and foliage. They are hypsodont and selenodont, since the molars and premolars are low-crowned and crescent-shaped cusps. The lower incisors and canines project forward. The incisors are followed by a long toothless gap, known as the diastema.[45] The general dental formula for bovids is 0.0.2-3.33.1.3.3. Most members of the family are herbivorous, but most duikers are omnivorous. Like other ruminants, bovids have four-chambered stomachs, which allow them to digest plant material, such as grass, that cannot be used by many other animals. Ruminants (and some others like kangaroos, rabbits, and termites) are able to use micro-organisms living in their guts to break down cellulose by fermentation.[3]
Ecology and behaviour

The bovids have various methods of social organisation and social behaviour, which are classified into solitary and gregarious behaviour. Further, these types may each be divided into territorial and nonterritorial behaviour.[28] Small bovids such as the klipspringer, oribi, and steenbok are generally solitary and territorial. They hold small territories into which other members of the species are not allowed to enter. These antelopes form monogamous pairs. Many species such as the dik-dik use pheromone secretions from the preorbital glands and sometimes dung, as well, to mark their territories.[46] The offspring disperse at the time of adolescence, and males need must acquire territories prior to mating.[3] The bushbuck is the only bovid that is both solitary and not territorial. This antelope hardly displays aggression, and tends to isolate itself or form loose herds, though in a favourable habitat, several bushbuck may be found quite close to one another.[47]
Excluding the cephalophines (duikers), tragelaphines (spiral-horned antelopes) and the neotragines, most African bovids are gregarious and territorial. Males are forced to disperse on attaining sexual maturity, and must form their own territories, while females are not required to do so. Males that do not hold territories form bachelor herds. Competition takes place among males to acquire dominance, and fights tend to be more rigorous in limited rutting seasons. With the exception of migratory males, males generally hold the same territory throughout their lives.[28] In the waterbuck, some male individuals, known as "satellite males", may be allowed into the territories of other males and have to wait till the owner grows old so they may acquire his territory.[48] Lek mating, where males gather together and competitively display to potential mates, is known to exist among topis, kobs, and lechwes.[49] The tragelaphines, cattle, sheep, and goats are gregarious and not territorial. In these species, males must gain absolute dominance over all other males, and fights are not confined to territories. Males, therefore, spend years in body growth.[28]
Activity

Most bovids are diurnal, although a few such as the buffalo, bushbuck, reedbuck, and grysbok are exceptions. Social activity and feeding usually peak during dawn and dusk. The bovids usually rest before dawn, during midday, and after dark. Grooming is usually by licking with the tongue. Rarely do antelopes roll in mud or dust. Wildebeest and buffalo usually wallow in mud, whereas the hartebeest and topi rub their heads and horns in mud and then smear it over their bodies. Bovids use different forms of vocal, olfactory, and tangible communication. These involve varied postures of neck, head, horns, hair, legs, and ears to convey sexual excitement, emotional state, or alarm. One such expression is the flehmen response. Bovids usually stand motionless, with the head high and an intent stare, when they sense danger. Some like the impala, kudu, and eland can even leap to heights of a few feet.[28] Bovids may roar or grunt to caution others and warn off predators.[3] Bovids such as gazelles stot or pronk in response to predators, making high leaps on stiff legs, indicating honestly both that the predator has been seen, and that the stotting individual is strong and not worth chasing.[50]

In the mating season, rutting males bellow to make their presence known to females. Muskoxen roar during male-male fights, and male saigas force air through their noses, producing a roar to deter rival males and attract females. Mothers also use vocal communication to locate their calves if they get separated. During fights over dominance, males tend to display themselves in an erect posture with a level muzzle.[51][52]
Fighting techniques differ amongst the bovid families and also depend on their build. While the hartebeest fight on knees, others usually fight on all fours. Gazelles of various sizes use different methods of combat. Gazelles usually box, and in serious fights may clash and fence, consisting of hard blows from short range. Ibex, goat and sheep males stand upright and clash into each other downwards. Wildebeest use powerful head butting in aggressive clashes. If horns become entangled, the opponents move in a circular manner to unlock them. Muskoxen will ram into each other at high speeds. As a rule, only two bovids of equal build and level of defence engage in a fight, which is intended to determine the superior of the two. Individuals that are evidently inferior to others would rather flee than fight; for example, immature males do not fight with the mature bulls. Generally, bovids direct their attacks on the opponent's head rather than its body. The S-shaped horns, such as those on the impala, have various sections that help in ramming, holding, and stabbing. Serious fights leading to injury are rare.[28][51][53]
Diet

Most bovids alternately feed and ruminate throughout the day. While those that feed on concentrate feed and digest in short intervals, the roughage feeders take longer intervals. Only small species such as the duiker browse for a few hours during day or night.[28] Feeding habits are related to body size; while small bovids forage in dense and closed habitat, larger species feed upon high-fiber vegetation in open grasslands. Subfamilies exhibit different feeding strategies. While Bovinae species graze extensively on fresh grass and diffused forage, Cephalophinae species (with the exception of Sylvicapra) primarily consume fruits.[3] Reduncinae and Hippotraginae species depend on unstable food sources, but the latter are specially adapted to arid areas. Members of Caprinae, being flexible feeders, forage even in areas with low productivity. Tribes Alcelaphini, Hippotragini, and Reduncini have high proportions of monocots in their diets. On the contrary, Tragelaphini and Neotragini (with the exception of Ourebia) feed extensively on dicots.[54] No conspicuous relationship exists between body size and consumption of monocots.[55]
Sexuality and reproduction

Most bovids are polygynous. In a few species, individuals are monogamous, resulting in minimal male-male aggression and reduced selection for large body size in males. Thus, sexual dimorphism is almost absent. Females may be slightly larger than males, possibly due to competition among females for the acquisition of territories. This is the case in duikers and other small bovids.[56][57] The time taken for the attainment of sexual maturity by either sex varies broadly among bovids. Sexual maturity may even precede or follow mating. For instance, the impala males, though sexually mature by a year, can mate only after four years of age.[58] On the contrary barbary sheep females may give birth to offspring even before they have gained sexual maturity.[59] The delay in male sexual maturation is more visible in sexually dimorphic species, particularly the reduncines, probably due to competition among males.[3] For instance, the blue wildebeest females become capable of reproduction within a year or two of birth, while the males become mature only when four years old.[27]
All bovids mate at least once a year, and smaller species may even mate twice. Mating seasons occur typically during the rainy months for most bovids. As such, breeding might peak twice in the equatorial regions. The sheep and goats exhibit remarkable seasonality of reproduction, in the determination of which the annual cycle of daily photoperiod plays a pivotal role. Other factors that have a significant influence on this cycle include the temperature of the surroundings, nutritional status, social interactions, the date of parturition and the lactation period. A study of this phenomenon concluded that goats and sheep are short-day breeders. Mating in most sheep breeds begins in summer or early autumn.[60] Mating in sheep is also affected by melatonin, that advances the onset of the breeding season;[61] and thyroxine, that terminates the breeding season.[62] Estrus lasts for at most a day in bovids, with the exception of bovines and tragelaphines. Except for the hartebeest and the topi, all bovids can detect estrus in females by testing the urine using the vomeronasal organ.[28] Once the male is assured that the female is in estrus, he begins courtship displays; these displays vary greatly from the elaborate marches among gregarious species to the fervent licking of female genitalia among solitary species. Females, initially not receptive, ultimately mates with the male which has achieved dominance over others. Receptiveness is expressed by permission for mounting by the male and setting aside the tail by the female. Copulation generally takes a few seconds.[28][56]
Gestational period varies among bovids - while duiker gestation ranges from 120 to 150 days, gestation in African buffalo ranges from 300 to 330 days. Usually, a single offspring is born (twins are less frequent), and it is able to stand and run by itself within an hour of birth. In monogamous species, males assist in defending their young, but that is not the case in polygynous species. Most newborn calves remain hidden for a week to two months, regularly nursed by their mothers. In some bovid species, the neonates start following about their mothers immediately or within a few days, as in the impala.[58] Different bovids have different strategies for the defence of juveniles. For instance, while wildebeest mothers solely defend their young, buffaloes exhibit collective defence. Weaning might occur as early as two months (as in royal antelope) or as late as a year (as in muskox).[56][57]
Lifespan
Most wild bovids live for 10 to 15 years. Larger species tend to live longer;[3] for instance, American bison can live up to 25 years and gaur up to 30 years. The mean lifespan of domesticated individuals is nearly ten years. For example, domesticated goats have an average lifespan of 12 years. Usually males, mainly in polygynous species, have shorter lifespans than females. This can be attributed to several reasons: early dispersal of young males, aggressive male-male fights, vulnerability to predation (particularly when males are less agile, as in kudu), and malnutrition (being large in size, the male body has high nutritional requirements which may not be satisfied).[63][64] Richard Despard Estes suggested that females mimic male secondary sexual characteristics like horns to protect their male offspring from dominant males. This feature seems to have been strongly selected to prevent male mortality and imbalanced sex ratios due to attacks by aggressive males and forced dispersal of young males during adolescence.[65]
Distribution

Most of the diverse bovid species occur in Africa. The maximum concentration is in the savannas of eastern Africa. Depending on their feeding habits, several species have radiated over large stretches of land, and hence several variations in dental and limb morphology are observed. Duikers inhabit the equatorial rainforests, sitatunga, and lechwe occur near swamps, eland inhabit grasslands, springbok and oryx occur in deserts, bongo and anoa live in dense forests, and mountain goats and takin live at high altitudes.[28] A few bovid species also occur in Europe, Asia, and North America. Sheep and goats are found primarily in Eurasia, though the Barbary sheep and the ibex form part of the African fauna. The muskox is confined to the arctic tundra. Several bovid species have been domesticated by human beings. The domestication of goats and sheep began 10 thousand years ago, while cattle were domesticated about 7.5 thousand years ago.[3][56]
Interaction with humans
Domesticated animals

The domestication of bovids has contributed to shifting the dependence of human beings from hunting and gathering to agriculture. The Bovidae includes three of the five large domesticated herbivores whose use has spread outside their original ranges, namely cattle, sheep, and goats; all are from Eurasia, and are now found across the world. The other two species are the horse and pig. Other large bovids that have been domesticated but which remain within the ranges of their wild ancestors are the water buffalo (from the Indian water buffalo), domestic yak (from the wild yak), zebu (from the Indian aurochs), gayal (from the gaur) and Bali cattle (from the banteng).[56] Some antelopes have been domesticated including the oryxes, addax, elands and the extinct bubal hartebeest. In Ancient Egypt oryxes, addaxes and bubal hartebeests are depicted in carved walls.
The earliest evidence of cattle domestication is from 8000 BC, suggesting that the process began in Cyprus and the Euphrates basin.[66]
Animal products

Dairy products such as milk, butter, ghee, yoghurt, buttermilk and cheese are manufactured largely from domestic cattle, though the milk of sheep, goat, yak, and buffalo is also used in some parts of the world and for gourmet products. For example, buffalo milk is used to make mozzarella in Italy and gulab jamun dessert in India,[67] while sheep milk is used to make blue Roquefort cheese in France.[68] Beef is an excellent source of zinc, selenium, phosphorus, iron, and B vitamins.[69] Bison meat is lower in fat and cholesterol than beef, but has a higher protein content.[70]
Bovid leather is tough and durable, with the additional advantage that it can be made into leathers of varying thicknesses - from soft clothing leather to hard shoe leather. While goat and cattle leather have a wide variety of use, sheepskin is suited only for clothing purposes.[71] Wool from Merino hoggets is the finest and most valuable. Merino wool is 3–5 in (7.6–12.7 cm) long and very soft. Coarse wools, being durable and resistant to pilling, are used for making tough garments[72] and carpets.

Bone meal is an important fertilizer rich in calcium, phosphorus, and nitrogen, effective in removing soil acidity.[73] Bovid horns have been used as drinking vessels since antiquity.[74]
In human culture
Bovidae have featured in stories since at least the time of Aesop's fables from Ancient Greece around 600 BC. Fables by Aesop include The Crow and the Sheep, The Frog and the Ox, and The Wolf and the Lamb.[75] The mythological creature Chimera, depicted as a lion, with the head of a goat arising from its back, and a tail that might end with a snake's head, was one of the offspring of Typhon and Echidna and a sibling of such monsters as Cerberus and the Lernaean Hydra.[76] The sheep, synonymous with the goat in Chinese mythology, is the eighth animal of the Chinese zodiac, and a symbol of filial piety.[77]
Notes
References
- ^ Grubb, P. (2005). "Family Bovidae". In Wilson, D.E.; Reeder, D.M (eds.). Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Johns Hopkins University Press. pp. 637–722. ISBN 978-0-8018-8221-0. OCLC 62265494.
- ^ "Bovidae". Merriam-Webster online dictionary. Retrieved 7 October 2014.
- ^ a b c d e f g h i j Gomez, W.; Patterson, T. A.; Swinton, J.; Berini, J. "Bovidae: antelopes, cattle, gazelles, goats, sheep, and relatives". Animal Diversity Web. University of Michigan Museum of Zoology. Retrieved 7 October 2014.
- ^ Gatesy, J.; Amato, G.; Vrba, E.; Schaller, G. (1997). "A cladistic analysis of mitochondrial ribosomal DNA from the Bovidae". Molecular Phylogenetics and Evolution. 7 (3): 303–19. doi:10.1006/mpev.1997.0402. PMID 9187090.
- ^ a b c d e Fernández, M. H.; Vrba, E. S. (2005). "A complete estimate of the phylogenetic relationships in Ruminantia: a dated species-level supertree of the extant ruminants". Biological Reviews. 80 (2): 269–302. doi:10.1017/S1464793104006670. PMID 15921052.
- ^ Harrison, T. (2011). Paleontology and Geology of Laetoli Human Evolution in Context. Dordrecht: Springer. pp. 363–465. ISBN 978-9048-199-624.
- ^ Demiguel, D.; Sánchez, I. M.; Alba, D. M.; Galindo, J.; Robles, J. M.; Moyà-Solà, S. (2012). "First evidence of Azanza and Morales, 1994 (Ruminantia, Bovidae), in the Miocene of the Vallès-Penedès Basin (Spain)". Journal of Vertebrate Paleontology. 32 (6): 1457–62. doi:10.1080/02724634.2012.696082.
- ^ Harrison, T. (1997). Neogene Paleontology of the Manonga Valley, Tanzania : A Window into the Evolutionary History of East Africa. New York: Plenum Press. p. 113. ISBN 978-0-306-45471-4.
- ^ Vrba, E. S.; Schaller, G. (2000). Antelopes, Deer, and Relatives : Fossil Record, Behavioral Ecology, Systematics, and Conservation. New Haven: Yale University Press. ISBN 978-0300-081-428.
- ^ Grubb, P. (2005). "Order Artiodactyla". In Wilson, D.E.; Reeder, D.M (eds.). Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Johns Hopkins University Press. p. 719. ISBN 978-0-8018-8221-0. OCLC 62265494.
- ^ Grubb, P. (2005). "Order Artiodactyla". In Wilson, D.E.; Reeder, D.M (eds.). Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Johns Hopkins University Press. p. 699. ISBN 978-0-8018-8221-0. OCLC 62265494.
- ^ a b Savage, R.J.G.; Long, M.R. (1986). Mammal Evolution: an illustrated guide. New York: Facts on File. pp. 232–5. ISBN 978-0-8160-1194-0.
- ^ a b c d Prothero, D. R.; Schoch, R. M. (2002). Horns, Tusks, and Flippers : the Evolution of Hoofed Mammals. Baltimore: Johns Hopkins University Press. pp. 87–90. ISBN 978-0-8018-7135-1.
- ^ Hassanin, D.; Douzery, E.J. (1999). "The tribal radiation of the family Bovidae (Artiodactyla) and the evolution of the mitochondrial cytochrome b gene" (PDF). Molecular Phylogenetics and Evolution. 13 (2): 227–43. doi:10.1006/mpev.1999.0619. PMID 10603253. Archived from the original (PDF) on 2011-07-20.
- ^ a b c d e Gilbert, W. H.; Asfaw, B. (2008). Homo Erectus : Pleistocene Evidence from the Middle Awash, Ethiopia. Berkeley: University of California Press. pp. 45–84. ISBN 978-0-520-25120-5.
- ^ "A multi-calibrated mitochondrial phylogeny of extant Bovidae (Artiodactyla, Ruminantia) and the importance of the fossil record to systematics". BMC Evolutionary Biology. 13: 166. August 2013. doi:10.1186/1471-2148-13-16. PMC 3607928. PMID 23339550.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Geraads, D.; El Boughabi, S.; Zouhri, S. (2012). "A new caprin bovid (Mammalia) from the late Miocene of Morocco". Palaeontologica Africana (47): 19–24. ISSN 0078-8554.
- ^ Kingdon, J. (1989). East African Mammals: An Atlas of Evolution in Africa (Volume III, Part C). Chicago: University of Chicago press. pp. 1–33. ISBN 978-0-226-43724-8.
- ^ Stanley, S. M.; Eldredge, N. (1984). "Evolutionary Pattern and Process in the Sister-Group Alcelaphini-Aepycerotini (Mammalia: Bovidae)". Living Fossils. Springer. pp. 62–79. ISBN 978-1461-382-737.
- ^ Vrba, E. S.; Burckle, L. H.; Partridge, T. C.; Denton, G. H. (1995). Paleoclimate and Evolution, with Emphasis on Human Origins. New Haven: Yale University Press. pp. 24–5. ISBN 978-0300-063-486.
- ^ a b c Walton, D.W. (1989). Fauna of Australia (Volume 1B). Canberra: Australian Government Publication Service. pp. 1–14. ISBN 978-0644-060-561.
- ^ Lundrigan, B.; Zachariah, T. "Bos frontalis, Gaur". Animal Diversity Web. University of Michigan Museum of Zoology. Retrieved 8 October 2014.
- ^ Roth, J. "Bubalus bubalis, Water buffalo". Animal Diversity Web. University of Michigan Museum of Zoology. Retrieved 8 October 2014.
- ^ Huffman, B. "Royal antelope". Ultimate Ungulate. Archived from the original on 16 December 2014. Retrieved 8 October 2014.
- ^ Hildyard, A. (2001). Endangered Wildlife and Plants of the World. New York: Marshall Cavendish. pp. 769–70. ISBN 978-0-7614-7200-1.
- ^ "Oryx leucoryx" at the Encyclopedia of Life
- ^ a b Lundrigan, B.; Bidlingmeyer, J. (2000). "Connochaetes gnou: black wildebeest". Animal Diversity Web. University of Michigan. Retrieved 2013-08-21.
- ^ a b c d e f g h i j k l Estes, R. D. (2004). The Behavior Guide to African Mammals : Including Hoofed Mammals, Carnivores, Primates (4th ed.). Berkeley: University of California Press. pp. 7–25. ISBN 978-0-520-08085-0.
- ^ Krausman, P.R.; Casey, A.L. (2012). "Addax nasomaculatus". Mammalian Species. 807: 1–4. doi:10.1644/807.1.
- ^ a b Caro, Tim (2009). "Contrasting colouration in terrestrial mammals". Philosophical Transactions of the Royal Society B. 364 (1516): 537–548. doi:10.1098/rstb.2008.0221. PMC 2674080. PMID 18990666.
- ^ Cott, H. B. (1940). Adaptive Coloration in Animals. London: Methuen. pp. 88 and plate 25.
- ^ Kiltie, R.A. (January 1998). "Countershading: Universally deceptive or deceptively universal?". Trends in Ecology & Evolution. 3 (1): 21–23. doi:10.1016/0169-5347(88)90079-1. PMID 21227055.
- ^ Cott, H. B. (1940). Adaptive Coloration in Animals. London: Methuen. p. 53.
- ^ Leslie, D. M.; Sharma, K. (25 September 2009). "Tetracerus quadricornis (Artiodactyla: Bovidae)". Mammalian Species. 843: 1–11. doi:10.1644/843.1.
- ^ E.C., Mungall (2007). Exotic Animal Field Guide : Nonnative Hoofed Mammals in the United States (1st ed.). College Station: Texas A&M University Press. p. 197. ISBN 978-1-58544-555-4.
- ^ American Livestock Breeds Conservancy (2009). "Jacob Sheep". Pittsboro, North Carolina: American Livestock Breeds Conservancy. Retrieved 2011-05-05.
- ^ Bibi, F.; Bukhsianidze, M.; Gentry, A.; Geraads, D.; Kostopoulos, D.; Vrba, E. (2009). "The fossil record and evolution of Bovidae: state of the field". Palaeontologia Electronica. 12 (3): 10A.
- ^ Gatesy, J.; Yelon, D.; DeSalle, R.; Vrba, E. (1992). "Phylogeny of the Bovidae (Artiodactyla, Mammalia), based on mitochondrial ribosomal DNA sequences". Molecular Biology and Evolution. 9 (3): 433–446. doi:10.1093/oxfordjournals.molbev.a040734. PMID 1584013.
- ^ Lundrigan, B. (1996). "Morphology of horns and fighting behavior in the family Bovidae". Journal of Mammalogy. 77 (2): 462–75. doi:10.2307/1382822. JSTOR 1382822.
- ^ Caro, T. M.; Graham, C. M.; Stoner, C. J.; Flores, M. M. (2003). "Correlates of horn and antler shape in bovids and cervids". Behavioral Ecology and Sociobiology. 55 (1): 32–41. doi:10.1007/s00265-003-0672-6.
- ^ a b Bro-Jørgensen, J. (2007). "The intensity of sexual selection predicts weapon size in male bovids". Evolution. 61 (6): 1316–1326. doi:10.1111/j.1558-5646.2007.00111.x. PMID 17542842.
- ^ Ezenwa, V. (2008). "Horns honestly advertise parasite infection in male and female African buffalo". Animal Behaviour. 75 (6): 2013–21. doi:10.1016/j.anbehav.2007.12.013.
- ^ a b Stankowich, T.; Caro, T. (2009). "Evolution of weaponry in female bovids". Proceedings of the Royal Society B: Biological Sciences. 276 (1677): 4329–34. doi:10.1098/rspb.2009.1256. PMC 2817105. PMID 19759035.
- ^ Packer, C. (1983). "Sexual Dimorphism: The Horns of African Antelopes". Science. 221 (4616): 1191–3. Bibcode:1983Sci...221.1191P. doi:10.1126/science.221.4616.1191. PMID 17811523.
- ^ Janis, C.; Jarman, P. (1984). Macdonald, D. (ed.). The Encyclopedia of Mammals. New York: Facts on File. pp. 498–9. ISBN 978-0-87196-871-5.
- ^ Wyatt, T. D. (2003). Pheromones and Animal Behaviour: Communication by Smell and Taste. Cambridge: Cambridge University Press. p. 97. ISBN 978-0-521-48526-5.
- ^ Ciszek, D. "Bushbuck". Animal Diversity Web. University of Michigan Museum of Zoology. Retrieved 28 October 2014.
- ^ T. L., Newell. "Waterbuck". Animal Diversity Web. University of Michigan Museum of Zoology. Retrieved 28 October 2014.
- ^ Lott, Dale F. (1991). Intraspecific Variation in the Social Systems of Wild Vertebrates. Cambridge University Press. pp. 37. ISBN 978-0521370240.
- ^ Bigalke, R.C. (1972). "Observations on the behaviour and feeding habits of the springbok Antidorcas marsupialis". Zoologica Africana. 7 (1): 333–359. doi:10.1080/00445096.1972.11447448.
- ^ a b Parker, S.P. (1990). Grzimek's Encyclopedia of Mammals (Volume 5) (1st ed.). New York: McGraw-Hill Publishing. pp. 288–324, 338–9, 354–5, 432–3, 444–5, 460–1, 482–3. ISBN 9780079095084.
- ^ Czaplewski, N. J.; Ryan, J. M.; Vaughan, T. A. (2011). Mammalogy (5th ed.). Sudbury: Jones and Bartlett Publishers. ISBN 9780763762995.
- ^ Post, E.; Forchhammer, M. C (July 2008). "Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch". Philosophical Transactions of the Royal Society B: Biological Sciences. 363 (1501): 2367–2373. doi:10.1098/rstb.2007.2207. PMC 2606787. PMID 18006410.
- ^ Gagnon, M.; Chew, A.E. (May 2000). "Dietary preferences in extant African Bovidae". Journal of Mammalogy. 81 (2): 490–511. doi:10.1644/1545-1542(2000)081<0490:DPIEAB>2.0.CO;2.
- ^ Sponheimer, M.; Lee-Thorp, J.A.; DeRuiter, D.J.; Smith, J.M.; van der Merwe, N.J.; Reed, K.; Grant, C.C.; Ayliffe, L.K.; Robinson, T.F. (2003). "Diets of Southern African Bovidae: Stable Isotope Evidence". Journal of Mammalogy. 84 (2): 471–9. doi:10.1644/1545-1542(2003)084<0471:DOSABS>2.0.CO;2.
- ^ a b c d e Feldhamer, George A.; Drickamer, Lee C.; Vessey, Stephen H.; Merritt, Joseph F.; Krajewski, Carey (2007). Mammalogy: Adaptation, Diversity, Ecology. Johns Hopkins University Press. pp. 519–522. ISBN 978-0-8018-8695-9.
- ^ a b Krebs, J.R.; Davies, N.B. (1997). Behavioural Ecology: An Evolutionary Approach (4th ed.). Wiley-Blackwell. ISBN 9780865427310.
- ^ a b Estes, R. D. (2004). The Behavior Guide to African Mammals : Including Hoofed Mammals, Carnivores, Primates (4th ed.). Berkeley: University of California Press. pp. 158–66. ISBN 978-0-520-08085-0.
- ^ Gray, Gary G.; Simpson, C. David (1980). "Ammotragus lervia". Mammalian Species. 1980 (144): 1–7. doi:10.2307/3504009. JSTOR 3504009.
- ^ Rosa, H.J.D.; Bryant, M.J. (2003). "Seasonality of reproduction in sheep". Small Ruminant Research. 48 (3): 155–71. doi:10.1016/S0921-4488(03)00038-5.
- ^ Chemineau, P.; Pelletier, J.; Guérin, Y.; Colas, G.; Ravault, J.P.; Touré, G.; Almeida, G.; Thimonier, J.; Ortavant, R. (1988). "Photoperiodic and melatonin treatments for the control of seasonal reproduction in sheep and goats". Reproduction, Nutrition, Development. 28 (2B): 409–22. doi:10.1051/rnd:19880307. PMID 3413339.
- ^ Prendergast, B. J.; Mosinger, B.; Kolattukudy, P. E.; Nelson, R. J. (2002). "Hypothalamic gene expression in reproductively photoresponsive and photorefractory Siberian hamsters". Proceedings of the National Academy of Sciences. 99 (25): 16291–6. Bibcode:2002PNAS...9916291P. doi:10.1073/pnas.232490799. PMC 138604. PMID 12456888.
- ^ Owen-Smith, N. (1993). "Comparative mortality rates of male and female kudus: the costs of sexual size dimorphism". Journal of Animal Ecology. 62 (3): 428–40. doi:10.2307/5192. JSTOR 5192.
- ^ Toigo, C.; Gaillard, J.M. (2003). "Causes of Sex-Biased Adult Survival in Ungulates: Sexual Size Dimorphism, Mating Tactic or Environment Harshness?". Oikos. 101 (2): 376–84. doi:10.1034/j.1600-0706.2003.12073.x.
- ^ Estes, R.D. (1991). "The significance of horns and other male secondary sexual characters in female bovids". Applied Animal Behaviour Science. 29 (1–4): 403–51. doi:10.1016/0168-1591(91)90264-X.
- ^ Zeder, M. A. (2006). Documenting Domestication : New Genetic and Archaeological Paradigms. Berkeley, California: University of California Press. p. 317. ISBN 978-0520246386.
- ^ Phelan, Benjamin; Phelan, Benjamin (24 July 2013). "Others' Milk". Slate.com. Retrieved 10 October 2014.
- ^ Hughes, Tom; Hughes, Meredith Sayles (2005). Gastronomie!: Food Museums and Heritage Sites of France. Bunker Hill Publishing. p. 19. ISBN 978-1-59373-029-1.
- ^ "Beef, lean organic". WHFoods. 18 October 2004. Retrieved 1 April 2015.
- ^ "| National Bison Association". Bisoncentral.com. Archived from the original on January 20, 2011. Retrieved 1 April 2015.
- ^ Veldmeijer, A. J.; Harris, S. (2014). Why Leather?: The Material and Cultural Dimensions of Leather. Sidestone Press. pp. 31–6. ISBN 9789088902611.
- ^ "Merino Sheep in Australia". Archived from the original on 2006-11-05. Retrieved 1 April 2015.
- ^ Kolay, A. K. (2007). Manures and fertilizers. New Delhi: Atlantic Publications. p. 98. ISBN 978-8126908103.
- ^ Vi, E. (1975). "Germanic glass drinking horns". Journal of Glass Studies. 17: 74–87.
- ^ "Aesop's Fables". Aesop's Fables. Retrieved 10 October 2014.
- ^ Peck. "Entry:Chimaera". Retrieved 31 March 2015.
- ^ Eberhard, W. A Dictionary of Chinese Symbols: Hidden Symbols in Chinese Life and Thought. London: Routledge. ISBN 978-0-415-00228-8.
External links
- . Encyclopædia Britannica (11th ed.). 1911.
- . Collier's New Encyclopedia. 1921.






