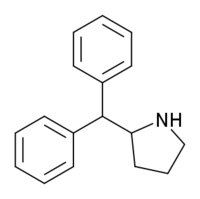
2-Diphenylmethylpyrrolidine
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.162.251 |
| Chemical and physical data | |
| Formula | C17H19N |
| Molar mass | 237.346 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
2-Diphenylmethylpyrrolidine (Desoxy-D2PM), also known as 2-benzhydrylpyrrolidine, is a stimulant psychoactive drug. It is the 4-dehydroxylated structural analog of diphenylprolinol (D2PM), and is also similar in structure to desoxypipradrol (2-DPMP), both of which act as norepinephrine-dopamine reuptake inhibitors (NDRIs). Like D2PM and 2-DPMP, Desoxy-D2PM is sold as a designer drug and has been used in the manufacture of legal highs. It has been marketed under the names A3A New Generation, A3A Methano, and Green Powder, and has been reported to cause hallucinations, violent behavior, dilated pupils, tachycardia, and high blood pressure.[1][2][3] Literature data suggest that it can produce the same psychotropic effects as other stimulants, but with a longer duration of action.[4]
Desoxy-D2PM has two enantiomers which are used industrially in their purified form as chiral derivatizing agents during chemical synthesis.[5]
As of 4 November 2010, the UK Home Office announced a ban on the importation of 2-diphenylmethylpyrrolidine, following a recommendation from the ACMD.[6] It was due to become a class B drug[7] on 28 March 2012,[8] but the bill was scrapped, due to the presence of two steroids included in the bill that were later recommended to remain uncontrolled.[9]
It was made a class B drug and placed in Schedule I on 13 June 2012.[10]
References
[edit]- ^ De Paoli G, Brandt SD, Pounder DJ (December 2011). "Analytical characterization and rapid determination of 2-(diphenylmethyl)pyrrolidine in blood and application to an internet product". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 879 (31): 3771–4. doi:10.1016/j.jchromb.2011.10.014. PMID 22055832.
- ^ "2-(Diphenylmethyl)pyrrolidine (desoxy-D2PM)" (in Italian). Dipartimento Politiche Antidroga, Rome. Archived from the original (pdf) on 27 February 2013. Retrieved 10 February 2012.
- ^ DC Shanie Nayar. "Green Powder called A3A Methano" (PDF). Archived from the original (PDF) on 27 February 2013. Retrieved 10 February 2012.
- ^ Coppola M, Mondola R (July 2012). "Research chemicals marketed as legal highs: the case of pipradrol derivatives". Toxicology Letters. 212 (1): 57–60. doi:10.1016/j.toxlet.2012.04.019. PMID 22564760.
- ^ Bertelsen S, Halland N, Bachmann S, Marigo M, Braunton A, Jørgensen KA (October 2005). "Organocatalytic asymmetric alpha-bromination of aldehydes and ketones". Chemical Communications (38): 4821–3. doi:10.1039/b509366j. PMID 16193126.
- ^ Import ban on psychoactive drug Archived 29 August 2012 at the Wayback Machine, UK Home Office
- ^ "The Misuse of Drugs Act 1971 (Amendment) Order 2012" (PDF). UK Home Office. 27 January 2012. Archived from the original on 1 January 2013. Retrieved 11 March 2012.
- ^ "Government accepts ACMD's advice to schedule D2PM, 2-DPMP and phenzepam" (PDF). UK Home Office. 27 January 2012. Archived from the original on 6 April 2012. Retrieved 11 March 2012.
- ^ "ACMD letter on further advice on the classification of two steroidal substances - February 2012" (PDF). UK Home Office. 14 February 2012. Archived from the original on 5 April 2012. Retrieved 18 March 2012.
- ^ "A Change to the Misuse of Drugs Act 1971: control of pipradrol-related compounds and phenazepam". UK Home Office. 7 June 2012. Archived from the original on 30 October 2012. Retrieved 30 July 2012.
