RTI-177
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H23ClN2O |
| Molar mass | 378.90 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
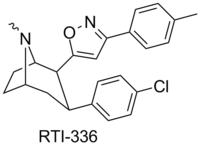
RTI(-4229)-177 (2β-(3-phenylisoxazol-5-yl)-3β-(4-chlorophenyl)tropane, β-CPPIT) is a synthetic stimulant drug from the phenyltropane family, which acts as a DRI with micromolar affinity for the SERT.[1] RTI-177 has an unusually long duration of action of 20 hours or more, substantially longer than the related compound RTI-336 from which it differs in molecular structure only by the absence of a p-methyl group.[2]
"the nonselective monoamine transporter inhibitor RTI-126 and the DAT-selective inhibitors RTI-150 and RTI-336 both had a faster rate of onset (30 min) and a short duration of action (4h). In contrast, the nonselective monoamine transporter inhibitor RTI-112 had a slower rate of onset (30–60 min) and a longer duration of action (10h). The DAT-selective inhibitors RTI-171 and RTI-177 also had slower rates of onset (30–120 min), but RTI-171 had a short duration of action (2.5 h) while RTI-177 had a very long duration of action (20 h)."[3]
Update
[edit]Comparison of six MAT inhibitors
[edit]| RTI | X | R | [3H]CFT | [3H]Nisoxetine | [3H]Paroxetine |
|---|---|---|---|---|---|
| Coc | — | — | 89.1 | 3298 (1986) | 1045 (45) |
| 177 | Cl | phenyl | 1.28 | 504 (304) | 2420 (220) |
| 176 | Me | phenyl | 1.58 | 398 (239) | 5110 (465) |
| 354 | Me | ethyl | 1.62 | 299 (180) | 6400 (582) |
| 336 | Cl | p-cresyl | 4.09 | 1714 (1033) | 5741 (522) |
| 386 | Me | p-anisoyl | 3.93 | 756 (450) | 4027 (380) |
In the Lindsey paper, RTI-177 was wrongly considered to be a dual inhibitor of the NET, although this was later found out to be incorrect.[citation needed]
"In acute toxicity studies in male rats, 3β-(4-chlorophenyl)-2β-[3-(4’-methylphenyl)isoxazol-5-yl]tropane (RTI-336) possessed an LD50 of 180 mg/kg after oral administration, compared with 49 mg/kg for RTI-177 (unpublished results, Howell 2005; Table 9). These results suggested that RTI-336 was a better candidate than RTI-177 for further preclinical development."[2]
Also the potency of the heterocyclic compounds is not as great as would be predicted based on in vitro test results.
References
[edit]- ^ Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, et al. (June 2004). "Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 309 (3): 959–69. doi:10.1124/jpet.103.060293. PMID 14982963. S2CID 39794215. Archived from the original (PDF) on 2010-06-11.
- ^ a b Carroll FI, Howard JL, Howell LL, Fox BS, Kuhar MJ (March 2006). "Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse". The AAPS Journal. 8 (1): E196-203. doi:10.1208/aapsj080124. PMC 2751440. PMID 16584128.
- ^ Kimmel HL, O'Connor JA, Carroll FI, Howell LL (January 2007). "Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys". Pharmacology, Biochemistry, and Behavior. 86 (1): 45–54. doi:10.1016/j.pbb.2006.12.006. PMC 1850383. PMID 17258302.
