Anthony J. Arduengo
Anthony J. Arduengo, III | |
|---|---|
 | |
| Born | 1952 |
| Nationality | United States |
| Alma mater | Georgia Institute of Technology |
| Known for | Unusual Valency, Carbene Chemistry |
| Awards | Alexander von Humboldt Prize; Fellow - American Association for the Advancement of Science; ICMGC Gold Medal for Excellence in Main-Group Element Chemistry |
| Scientific career | |
| Fields | Inorganic chemistry, Organic chemistry, Unusual Valency |
| Institutions | University of Alabama; Technische Universität - Braunschweig; DuPont Central Research; University of Illinois |
| Doctoral advisor | E.M. Burgess |
Anthony Joseph Arduengo, III is the Saxon Professor of Chemistry at the University of Alabama and an adjunct professor at the Institute for Inorganic Chemistry of Braunschweig University of Technology in Germany. He is notable for his work on chemical compounds with unusual valency, especially in the field of stable carbene research.
Early life
Anthony "Bo" Arduengo was born in 1952 in Tampa, Florida.[1] He grew up in the Atlanta, Georgia area. His father was a pressman and mechanic with the Atlanta Journal-Constitution and instilled his son with an interest and skill for all things mechanical and scientific. By the age of 16, he and his father had built his first car from miscellaneous parts.[2] The car was registered as street-legal and road-worthy. With some re-engineering, the car was later fitted to run on alternate fuels including alcohol and hydrogen (which would foretell Arduengo's professional research involvement with President Bush's 2003 National Hydrogen Fuel Initiative (HFI)[3] and United States Department of Energy's Chemical Hydrogen Storage Program by more than 30 years).
Education
Arduengo attended Bouldercrest and Meadowview Elementary Schools, and Walker High School.[4] In 1969 he left high school with enrollment in Georgia Tech's Joint Enrollment Program for High School Students (JEPHS).[5] He obtained his BSc (1974, cum laude) and his PhD (1976) at Georgia Tech, advised by Edward M. Burgess.[6] That made him an academic descendant of Justus von Liebig.[4] As an undergraduate at Georgia Tech, Arduengo's research activities began in the laboratory of Professor Charles L. Liotta. He was awarded NSF undergraduate fellowships in 1972 & 1973 when he had moved to research in the Burgess group.[4]
As an undergraduate, Arduengo was a member of the Georgia Tech Band and served as Executive Officer and Captain for that organization. In 1971 he was inducted into the Iota chapter[7] of ΚΚΨ. In 1972 he was tapped by the Alpha Eta Circle[8] of ΟΔΚ; later serving as Secretary and President for the local Circle.
Career
Arduengo was a research scientist at DuPont from 1976 to 1977 and from 1984 to 1998, and assistant professor at the University of Illinois from 1977 to 1984.[4] He is currently the Saxon Chair of Chemistry at the University of Alabama and holds a position as adjunct professor at the Technische Universität in Braunschweig, Germany.[4]
In DuPont's Central Research and Development Department, Arduengo began his career in the Chemical Sciences Section (1977 & on return in 1984). In 1988 he was appointed Research Leader. A move into the Polymer Science section of CR&D in 1991 was accompanied by promotion to Group Leader. His final position with DuPont was as Research Fellow which he attained in 1995. The award of an Alexander von Humboldt Senior Research Prize in 1996 began Arduengo's transition back into academe. The one year Humboldt award was spent in Braunschweig, Germany at the Technical University. On return to DuPont, Arduengo maintained a guest Professor appointment in Braunschweig, and in 1999 also made the transition to academe in the U.S. with his assumption of the Saxon Chair in Chemistry at The University of Alabama in Tuscaloosa.[4]
Research
Graduate Research at Georgia Tech
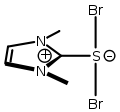
Arduengo's research interests focus largely on the chemistry of new or unusual bonding arrangements, and unusual valency. As a graduate student in the Burgess group, his research involved organo-main group element chemistry, specifically, thiocarbonyl ylides, and low-coordinate hypervalent sulfur compounds.[6][9][10][11]
-
Thiocarbonyl ylide from Arduengo's Ph.D. dissertation. external viewer.
-
Hypervalent sulfuranide from Arduengo's Ph.D. dissertation. external viewer.
DuPont 1977
In 1977 when he joined DuPont, Arduengo became a member of the exploratory chemistry group of Howard Simmons in CR&D.[4] His first research project involved trimethylsilyl esters of inorganic acids as reagents for organic synthesis.
University of Illinois 1978–1984
At Illinois Arduengo examined more broadly the areas of organo-main group element chemistry, and molecules containing unusual valency. His first publications involving the chemistry of electron-deficient carbenes occurred during this period.[12] This work with electron deficient carbenes led to the first structure determinations on a nitrile ylide[13] and a carbonyl ylide.[14] His later work with carbene chemistry would become his most recognized contribution to the field of chemistry (vide infra). During the Illinois years Arduengo had a close collaboration with his colleague J.C. Martin who was a physical-organic chemist also working on organo-main group element chemistry and hypervalency. Many of the technical discussions between Martin and Arduengo would take place over lunch (the choice of restaurants often influenced by the quality of the napkins for writing chemical structures).[15] To facilitate discussions about unusual molecular structures and bonding for main-group element centers, Martin and Arduengo devised the N-X-L nomenclature system.[16][17][18] The synthesis and characterization of the first compound with a planar T-shaped, 10-electron 3-coordinate bonding arrangement at a phosphorus atom, ADPO,[19] was also accomplished by the Arduengo group at Illinois and paved the way for a range of novel main-group element chemistry (including the discovery of edge inversion) on his return to DuPont. The final Illinois research extended the newly discovered ADPO chemistry to the arsenic analog (ADAsO).[20]
-
Diazotetrakis(trifluoro methyl)cyclopentadiene from Arduengo and Janulis. external viewer.
-
Nitrilium ylide from Arduengo and Janulis. external viewer.
-
Carbonyl ylide from Arduengo and Janulis. external viewer.
-
The original 10-P-3 ADPO from Arduengo and Culley. external viewer.
-
ADAsO from Arduengo and Culley. external viewer.
DuPont 1984–1999
On returning to DuPont in 1984, Arduengo resumed a position in CR&D and continued the research into the recently discovered ADPO molecule and related structures. This line of research proved to be extremely fruitful and resulted in a steady string of publications on new and unusual bonding arrangements.[21][22] The ADPO related chemistry provided a basis for the discovery of a new inverson process, edge inverson, which was fully characterized and modelled by the collaborative work of Arduengo and David A. Dixon at DuPont.[23] Additionally, the DuPont team provided experimental verification on the new inversion pathway at 3-coordinate phosphorus centers[24] and a 4-coordinate germanium molecule.[25]
Arduengo's work with DuPont also involved a number of applied projects including the flexible polyimide film, Kapton-ZT, that is widely used in electronics for flexible printed circuits, connections, and insulation.[26] Arduengo's research at DuPont often coalesced with his other hobbies outside the laboratory; for example with sports cars (cf. photo in the summary box above). He contributed to development of low VOC automotive coatings by devising catalysts for a novel cross-linking chemistry used by DuPont Performance Coatings in next-generation low-VOC paints.[27][28][29] Eventually, DuPont waterborne performance coatings would be used by Lotus on their Elise and Exige models.[30][31] Arduengo's effort on the industrial-scale syntheses[32][33][34] of the catalysts for the paints on which he worked would launch his re-entry into the area of carbene chemistry, but this time it was to be nucleophilic rather than electrophilic carbenes.[1][35] The observation that the catalyst syntheses were well tolerant of varied reaction conditions and substituents led Arduengo to postulate that the imidazol-2-ylidenes that were intermediates in the syntheses had to be far more stable than the then conventional wisdom would allow.[35][36]
As Arduengo's involvement in the automotive coatings program came to an end, he submitted a proposal to the management in CR&D to isolate these apparently stable carbenes and study their chemistry. The proposal was soundly declined with the admonition that he should have certainly known better than to make such a suggestion in light of the long history of carbene chemistry that firmly established them as reactive intermediates that could not be isolated as stable entities.[1][35][36] However, Arduengo (already well-aware of the history) had the starting materials on hand for the chemistry and decided to proceed with the experiments.[36] "Arduengo's gamble paid off. In 1991, more than 150 years after the first attempt ..."[37] a stable crystalline carbene was isolated and characterized in laboratories at DuPont.[38] After the first successful reaction to produce a stable carbene, Arduengo won the support of DuPont management[36] and research in this area continued. Carbenes bearing a variety of substituent groups were prepared and characterized.[39][40] The saturated imidazolin-2-ylidenes that were extensively investigated by Hans-Werner Wanzlick thirty years earlier (without isolation) were now also shown to be stable enough to isolate with appropriate substituents at nitrogen.[41] An air-stable carbene was produced.[42] The chemistry was extended to include thiazol-2-ylidenes (conjectured to exist in 1957 as a reactive intermediate in the vitamin B1 catalytic cycle, but not isolated for 40 years).[43] The imidazol-2-ylidenes were extensively characterized by their NMR properties,[44] photo-electron spectroscopy,[45] and exact experimental electron density mapping by X-ray and neutron diffraction techniques.[46]
The Arduengo group's characterization of stable carbenes was complemented by a wide ranging exploration of their chemistry. This new chemistry included carbene reactions with numerous element centers including iodine,[47][48] aluminum,[49] copper,[50] silver,[50] magnesium,[51] zinc,[51] germanium,[52] nickel,[53] platinum,[53] lanthanides,[54] and hydrogen in the form a bis(carbene)-proton complex.[55] Arduengo's research from 1996 also reflects his interaction with his host for his Alexander von Humboldt Research Prize, Professor Reinhard Schmutzler. His carbene chemistry conducted from Braunschweig included reactivity studies of imidazol-2-ylidenes with fluorinated inorganic compounds. New structures included carbene•phenyltetrafluorophosphorane,[56] carbene•PF5,[57] carbene•AsF5,[57] carbene•SbF5,[57] and carbene•BF3[57] adducts. Arduengo's final work on carbenes at DuPont included synthesis and characterization of carbene·alkaline earth metal,[58] carbene•antimony,[59] carbene•cadmium,[60] and carbene•lithium[61] adducts. Reactions of carbenes with phosphinidenes[62][63] were also reported from Arduengo's laboratory in addition to insertion reactions of imidazolin-2-ylidenes.[64]
In 1998, Arduengo and coworkers carefully re-examined the earlier attempts to produce stable carbenes in Wanzlick's laboratory in light of the knowledge and experience gained from the recent successful experiments at DuPont.[65] Although the majority of Wanzlick's work on the saturated imidazolin-2-ylidenes would have been expected to yield dimers in the absence of bulky substituents on nitrogen, a single (unsaturated) imidazol-2-ylidene, 1,3,4,5-tetraphenylimidazol-2-ylidene, remained as an example of a carbene that could (should) have been isolable. The re-examination of Wanzlick's original procedure[66] identified some key experimental features that would have hindered the original researchers. With these problems corrected, the DuPont scientists were able to isolate the target carbene and fully characterize it including an X-ray structure determination. In a tribute to Hans-Werner Wanzlick, these results were published under the title "1,3,4,5-Tetraphenylimidazol-2-ylidene: The Realization of Wanzlick's Dream."[65]
-
Antimony analog of ADPO (ADSbO) synthesized at DuPont by Arduengo and Stewart.[67] external viewer.
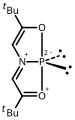
-
Nine coordinate bismuth complex prepared by Arduengo and Stewart.[68] external viewer.
-
Saturated ADPO analog used by Arduengo, Dixon, and Roe to verify Edge Inversion at 3-coordinate phosphorus.[24]
-
Germanium compound used by Arduengo, Dixon, and Roe to verify Edge Inversion at 4-coordinate centers.[25] external viewer.
-
First stable crystalline carbene prepared at DuPont.[38] external viewer.
-
First fully characterized imidazolin-2-ylidene (saturated) carbene isolated at DuPont.[41] external viewer.
-
Air-stable carbene.[42] external viewer
-
Stable small perdeuterocarbene used for exact electron density mapping experiments.[46] external viewer.
-
Wanzlick's original tetraphenylimidazol-2-ylidene isolated and characterized at DuPont.[65] external viewer.
University of Alabama 1999–present
At the University of Alabama research from Arduengo's laboratory has focused on enhancements to the basic structure of the imidazol-2-ylidenes through substituent effects leading to novel compounds like a cyclopentadienyl fused imidazol-2-ylidene.[69][70][71][72] Research into the unusual valency in diphosphacyclobutane-2,4-diyls has been reported from the Arduengo group in collaboration with Professors Masaaki Yoshifuji and Shigekazu Ito.[73][74][75][76][77][78][79] Arduengo also directs research programs into Chemical Hydrogen Storage and nonlinear optical materials.[4]
Awards
- Charles M. Knight Lectureship, University of Akron, April 2013.[80]
- Fellow of the American Association for the Advancement of Science, October 2007
- Walter J. Chute Lectureship 1999–2000.[81]
- Gold Medal for "Excellence in Main Group Chemistry Research" from The International Council on Main Group Chemistry, 1996
- Alexander von Humboldt Senior Research Prize, 1996
- NSF Undergraduate Fellowship, 1972, 1973
References
- ^ a b c A. J. Arduengo (1976). "Looking for Stable Carbenes: The Difficulty in Starting Anew". Accounts of Chemical Research. 32 (11): 913–921. doi:10.1021/ar980126p.
- ^ Talon (Walker High School paper), Vol. V, No. 1. September, 1968.
- ^ HFI - Hydrogen Fuel Initiative. Retrieved 2010-10-04.
- ^ a b c d e f g h Anthony J. Arduengo, III - personal home page. Retrieved 2010-10-03.
- ^ JEPHS Archived September 5, 2010, at the Wayback Machine - Georgia Tech's Joint Enrollment Program. Retrieved 2010-10-04.
- ^ a b Anthony Joseph Arduengo (1976), The synthesis, structure and chemistry of substituent-perturbed thione S-methylides and S,S-dihalothiones Ph.D. Thesis, Georgia Institute of Technology. Online catalog entry. Retrieved 2009-12-04.
- ^ Iota Chapter Georgia Tech's Iota Chapter of ΚΚΨ. Retrieved 2013-02-08.
- ^ Alpha Eta Circle - Georgia Tech's Alpha Eta Circle of ΟΔΚ. Retrieved 2013-02-08.
- ^ A. J. Arduengo; E. M. Burgess (1976). "Syntheses and reactions of substituent stabilized thione methylides". J. Am. Chem. Soc. 98 (16): 1520–1521. doi:10.1021/ja00432a056.
- ^ A. J. Arduengo; E. M. Burgess (1976). "The structure of a substituent stabilized thione methylide". J. Am. Chem. Soc. 98 (16): 1521–1523. doi:10.1021/ja00432a057.
- ^ A. J. Arduengo; E. M. Burgess (1977). "Tricoordinate hypervalent sulfur compounds". J. Am. Chem. Soc. 99 (2): 2376–2377. doi:10.1021/ja00449a078.
- ^ E. P. Janulis; A. J. Arduengo (1983). "Diazotetrakis(trifluoromethyl)cyclopentadiene and ylides of electronegative elements". J. Am. Chem. Soc. 105 (11): 3563–3567. doi:10.1021/ja00349a032.
- ^ E. P. Janulis; S. R. Wilson; A. J. Arduengo (1984). "The synthesis and structure of a stabilized nitrilium ylide". Tetrahedron Letters. 25: 405–408. doi:10.1016/S0040-4039(00)99896-4.
- ^ E. P. Janulis; A. J. Arduengo (1983). "carbonyl ylide". J. Am. Chem. Soc. 105 (18): 5929–5930. doi:10.1021/ja00356a044.
- ^ A.J. Arduengo "From Hypervalent Compounds to Hypovalent Carbenes," J.C. Martin Symposium: From σ-Constants to σ-Aromaticity (Vanderbilt University, May, 1992)
- ^ C. W. Perkins; J. C. Martin; A. J. Arduengo; W. Lau; A. Alegria; J. K. Kochi (1980). "An electrically neutral σ-sulfuranyl radical from the homolysis of a perester with neighboring sulfenyl sulfur: 9-S-3 species". J. Am. Chem. Soc. 102 (26): 7753–7759. doi:10.1021/ja00546a019.
- ^ J. C. Martin (1983). ""Frozen" transition states: pentavalent carbon et al.". Science. 221 (4610): 509–514. doi:10.1126/science.221.4610.509. PMID 17830935.
- ^ K. Akiba; Y. Yamamoto (1988). "Chemistry of hypervalent organic compounds. Fundamental aspects of hypervalent organic compounds. Characteristic features of structure and reactivity of hypervalent organic compounds of main group elements". Kikan Kagaku Sosetsu. 34: 9–39.
- ^ S. A. Culley; A. J. Arduengo (1984). "Synthesis and structure of the first 10-P-3 species". J. Am. Chem. Soc. 106 (4): 1164–1165. doi:10.1021/ja00316a084.
- ^ S. A. Culley; A. J. Arduengo (1985). "Synthesis and Structure of the First 10-As-3 Species". J. Am. Chem. Soc. 107 (4): 1089–1090. doi:10.1021/ja00290a072.
- ^ A. J. Arduengo; C. A. Stewart; F. Davidson; D. A. Dixon; J. Y. Becker; S. A. Culley; M. B. Mizen (1987). "The synthesis, structure, and chemistry of 10-Pn-3 systems: tricoordinate hypervalent pnictogen compounds". J. Am. Chem. Soc. 109 (3): 627–647. doi:10.1021/ja00237a001.
- ^ A. J. Arduengo; C. A. Stewart (1994). "Low coordinate hypervalent phosphorus". Chemical Reviews. 94 (5): 1215–1237. doi:10.1021/cr00029a003.
- ^ D. A. Dixon; A. J. Arduengo; T. Fukunaga (1986). "A new inversion process at Group VA (Group 15) elements. Edge inversion through a planar T-shaped structure". J. Am. Chem. Soc. 108 (9): 2461–2462. doi:10.1021/ja00269a063.
- ^ a b A. J. Arduengo; D. A. Dixon; D. C. Roe (1986). "Direct determination of the barrier to edge inversion at trivalent phosphorus: verification of the edge inversion mechanism". J. Am. Chem. Soc. 108 (9): 6821–6823. doi:10.1021/ja00281a070.
- ^ a b A. J. Arduengo; D. A. Dixon; D. C. Roe; M. Kline (1988). "Edge inversion barrier at a four-coordinate main group IV center". J. Am. Chem. Soc. 110 (13): 4437–4438. doi:10.1021/ja00221a067.
- ^ US patent 5272194, A. J. Arduengo, Y. C. Ray, "Process for Preparing a Strengthened Polyimide Film Containing Organometallic Compounds for Improving Adhesion", issued 1993-12-21, assigned to E. I. du Pont de Nemours and Company, Inc.
- ^ US patent 5034464, A. J. Arduengo,, "Amine-Borane Adduct Curing Agents for Epoxy/Anhydride Resins", issued 1991-07-23, assigned to E. I. du Pont de Nemours and Company, Inc.
- ^ US patent 5084542, A. J. Arduengo, P. H. Corcoran, "Epoxy/Isocyanate Crosslinked Coatings Containing 1,3-Disubstituted Imidazole-2-thione Catalysts", issued 1991-01-28, assigned to E. I. du Pont de Nemours and Company, Inc.
- ^ US patent 5091498, A. J. Arduengo, R. J. Barsotti, P. H. Corcoran, "Curable compositions containing 1,3-dialkylimidazole-2-thione catalysts", issued 1993-02-25, assigned to E. I. du Pont de Nemours and Company, Inc.
- ^ R. W. Yearich (2004). "Sports Rainbow of Waterborne Finish Paints from DPC". DuPont Refinisher News. Fall (342): 4. Retrieved July 3, 2015.
- ^ C. A. Sawyer (2004). "Developing the Lotus Elise Series 2". Automotive Design and Production. 6. Retrieved June 27, 2015.
- ^ US patent 5144032, A. J. Arduengo, "Preparation of Tertiary Amine-Borane Adducts", issued 1992-09-01, assigned to E. I. du Pont de Nemours and Company, Inc.
- ^ US patent 5104993, A. J. Arduengo, "1,3-Dialkylimidazole-2-thione Catalyst and Method for Making Same", issued 1992-04-14, assigned to E. I. du Pont de Nemours and Company, Inc.
- ^ US patent 5182405, A. J. Arduengo, "Preparation of 1,3- Disubstituted Imidazolium Salts", issued 1993-01-26, assigned to E. I. du Pont de Nemours and Company, Inc.
- ^ a b c Anthony J. Arduengo, III, Roland Krafczyk (1998) "Auf der Suche nach Stabilen Carbenen". Chemie in Unserer Zeit, volume 32, number 1, pages 6–14. doi:10.1002/ciuz.19980320103
- ^ a b c d A.J. Arduengo “Cars to Carbenes: A Personal Account of Georgia Tech – Molding Futures One at a Time,” Georgia Tech's 100 Years of Chemistry Symposium (Atlanta, Georgia, April 19, 2007)
- ^ Cristina Luiggi (2009). "Taming Carbon's Wild Side". Seed Magazine (November 30, 2009). Retrieved October 6, 2010.
- ^ a b A. J. Arduengo; R. L. Harlow; M. Kline (1991). "A stable crystalline carbene". J. Am. Chem. Soc. 113 (1): 361–363. doi:10.1021/ja00001a054.
- ^ a b A. J. Arduengo; H. V. R. Dias; R. L. Harlow; M. Kline (1992). "Electronic stabilization of nucleophilic carbenes". J. Am. Chem. Soc. 114 (14): 5530–5534. doi:10.1021/ja00040a007.
- ^ A. J. Arduengo; R. Krafczyk; R. Schmutzler; H. A. Craig; J. R. Goerlich; W. J. Marshall; M. Unverzagt (1992). "Imidazolylidenes, imidazolinylidenes and imidazolidines". Tetrahedron. 55 (51): 14523–14534. doi:10.1016/S0040-4020(99)00927-8.
- ^ a b A. J. Arduengo; J. R. Goerlich; W. J. Marshall (1995). "stable diaminocarbene". J. Am. Chem. Soc. 117 (44): 11027–11028. doi:10.1021/ja00149a034.
- ^ a b A. J. Arduengo; F. Davidson; H. V. R. Dias; J. R. Goerlich; D. Khasnis; W. J. Marshall; T. K. Prakasha (1997). "An Air Stable Carbene and Mixed Carbene "Dimers"". J. Am. Chem. Soc. 119 (52): 12742–12749. doi:10.1021/ja973241o.
- ^ a b A. J. Arduengo; J. R. Goerlich; W. J. Marshall (1997). "A Stable Thiazol-2-ylidene and Its Dimer". Liebigs Annalen. 1997 (2): 365–374. doi:10.1002/jlac.199719970213.
- ^ A. J. Arduengo; D. A. Dixon; K. K. Kumashiro; C. Lee; W. P. Power; K. W. Zilm (1994). "Chemical Shielding Tensor of a Carbene". J. Am. Chem. Soc. 116 (14): 6361–6367. doi:10.1021/ja00093a041.
- ^ A. J. Arduengo; H. Bock; H. Chen; M. Denk; D. A. Dixon; J. C. Green; W. A. Herrmann; N. L. Jones; M. Wagner; R. West (1994). "Photoelectron Spectroscopy of a Carbene/Silylene/Germylene Series". J. Am. Chem. Soc. 116 (15): 6641–6649. doi:10.1021/ja00094a020.
- ^ a b A. J. Arduengo; H. V. Rasika Dias; D. A. Dixon; R. L. Harlow; W. T. Klooster; T. F. Koetzle (1994). "Electron Distribution in a Stable Carbene". J. Am. Chem. Soc. 116 (15): 6812–6822. doi:10.1021/ja00094a040.
- ^ A. J. Arduengo; M. Kline; J. C. Calabrese; F. Davidson (1991). "Synthesis of a reverse ylide from a nucleophilic carbene". J. Am. Chem. Soc. 113 (25): 3625–3626. doi:10.1021/ja00025a063.
{{cite journal}}: External link in|title= - ^ A. J. Arduengo; M. Tamm; J. C. Calabrese (1994). "A Bis(carbene) Adduct of Iodine(1+)". J. Am. Chem. Soc. 116 (8): 3625–3626. doi:10.1021/ja00087a069.
{{cite journal}}: External link in|title= - ^ A. J. Arduengo; M. Kline; J. C. Calabrese; F. Davidson (1992). "A stable carbene-alane adduct". J. Am. Chem. Soc. 114 (24): 9724–9725. doi:10.1021/ja00050a098.
{{cite journal}}: External link in|title= - ^ a b A. J. Arduengo; H. V. R. Dias; J. C. Calabrese; F. Davidson (1993). "Homoleptic carbene-silver(I) and carbene-copper(I) complexes". Organometallics. 12 (9): 3405–3409. doi:10.1021/om00033a009.
{{cite journal}}: External link in|title= - ^ a b A. J. Arduengo; H. V. R. Dias; F. Davidson; R. L. Harlow (1993). "Carbene adducts of magnesium and zinc". Journal of Organometallic Chemistry. 462 (1–2): 13–18. doi:10.1016/0022-328X(93)83336-T.
{{cite journal}}: External link in|title= - ^ A. J. Arduengo; H. V. R. Dias; J. C. Calabrese; F. Davidson (1993). "A carbene germanium diiodide adduct: model of the non-least-motion pathway for dimerization of singlet carbenes". Inorganic Chemistry. 32 (9): 1541–1542. doi:10.1021/ic00061a004.
{{cite journal}}: External link in|title= - ^ a b A. J. Arduengo; S. F. Gamper; J. C. Calabrese; F. Davidson (1994). "Low-Coordinate Carbene Complexes of Nickel(0) and Platinum(0)". J. Am. Chem. Soc. 116 (10): 4391–4394. doi:10.1021/ja00089a029.
{{cite journal}}: External link in|title= - ^ A. J. Arduengo; M. Tamm; S. J. McLain; J. C. Calabrese; F. Davidson; W. J. Marshall (1994). "Carbene-Lanthanide Complexes". J. Am. Chem. Soc. 116 (17): 7927–7928. doi:10.1021/ja00096a072.
- ^ A. J. Arduengo; S. F. Gamper; M. Tamm; J. C. Calabrese; F. Davidson; H. A. Craig (1995). "A Bis(carbene)-Proton Complex: Structure of a C-H-C Hydrogen Bond". J. Am. Chem. Soc. 117 (1): 572–573. doi:10.1021/ja00106a082.
- ^ A. J. Arduengo; R. Krafczyk; W. J. Marshall; R. Schmutzler (1997). "A Carbene−Phosphorus(V) Adduct". J. Am. Chem. Soc. 119 (14): 3381–3382. doi:10.1021/ja964094h.
- ^ a b c d A. J. Arduengo; F. Davidson; R. Krafczyk; W. J. Marshall; R. Schmutzler (1997). "Carbene Complexes of Pnictogen Pentafluorides and Boron Trifluoride". Monatshefte für Chemie. 131 (3): 251–265. doi:10.1007/s007060070101.
- ^ A. J. Arduengo; F. Davidson; R. Krafczyk; W. J. Marshall; M. Tamm (1999). "Adducts of Carbenes with Group II and XII Metallocenes". Organometallics. 17 (15): 3375–3382. doi:10.1021/om980438w.
- ^ A. J. Arduengo; F. Davidson; R. Krafczyk; W. J. Marshall; R. Schmutzler (1999). "A Tris(trifluoromethyl)antimony Adduct of a Nucleophilic Carbene: Geometric Distortions in Carbene Adducts". Z. Anorg. Allg. Chem. 625 (11): 1813–1817. doi:10.1002/(SICI)1521-3749(199911)625:11<1813::AID-ZAAC1813>3.0.CO;2-C.
{{cite journal}}: External link in|title= - ^ A. J. Arduengo; J. R. Goerlich; F. Davidson; W. J. Marshall (1999). "Carbene Adducts of Dimethylcadmium" (PDF). Z. Naturforsch. B. 54 (11): 1350–1356. doi:10.1515/znb-1999-1102.
- ^ A. J. Arduengo; M. Tamm; J. C. Calabrese; F. Davidson; W. J. Marshall (1999). "Carbene-Lithium Interactions". Chemistry Letters. 28 (10): 1021–1022. doi:10.1246/cl.1999.1021.
- ^ A. J. Arduengo; H. V. R. Dias; J. C. Calabrese (1997). "A Carbene•Phosphinidene Adduct: "Phosphaalkene"". Chemistry Letters. 26 (2): 143–144. doi:10.1246/cl.1997.143.
- ^ A. J. Arduengo; J. C. Calabrese; A. H. Cowley; H. V. R. Dias; J. R. Goerlich; W. J. Marshall; B. Riegel (1997). "Carbene−Pnictinidene Adducts". Inorganic Chemistry. 36 (10): 2151–2158. doi:10.1021/ic970174qh.
- ^ A. J. Arduengo; J. C. Calabrese; F. Davidson; H. V. R. Dias; J. R. Goerlich; R. Krafczyk; W. J. Marshall; M. Tamm; R. Schmutzler (1999). "C-H Insertion Reactions of Nucleophilic Carbenes". Helv. Chim. Acta. 82 (12): 2348–2364. doi:10.1002/(SICI)1522-2675(19991215)82:12<2348::AID-HLCA2348>3.0.CO;2-M.
- ^ a b c A. J. Arduengo; J. R. Goerlich; R. Krafczyk; W. J. Marshall (1998). "1,3,4,5-Tetraphenylimidazol-2-ylidene: The Realization of Wanzlick's Dream". Angewandte Chemie, Int. Ed. Engl. 37 (13–14): 1963–1965. doi:10.1002/(SICI)1521-3773(19980803)37:13/14<1963::AID-ANIE1963>3.0.CO;2-M.
- ^ H.-J. Schönherr; H.-W. Wanzlick (1970). "Chemie nucleophiler Carbene, XVIII 1.3.4.5-Tetraphenyl-imidazoliumperchlorat". Liebigs Annalen. 731 (1): 176–179. doi:10.1002/jlac.19707310121.
- ^ C. A. Stewart; R. L. Harlow; A. J. Arduengo (1985). "Chemistry and structure of the first 10-Sb-3 species". J. Am. Chem. Soc. 107 (19): 5543–5544. doi:10.1021/ja00305a046.
- ^ C. A. Stewart; J. C. Calabrese; A. J. Arduengo (1985). "Synthesis and structure of the first 20-Bi-9 system: a discrete nine-coordinate 20-electron bismuth". J. Am. Chem. Soc. 107 (11): 3397–3398. doi:10.1021/ja00297a084.
- ^ A. J. Arduengo; T. P. Bannenberg; D. Tapu; W. J. Marshall (2005). "Heteroferrocene: The Synthesis of Bis[(3a,4,5,6,6a-η)-1,3,4,5,6-pentamethylcyclopenta[d]imidazo-2-thionoyl]iron(II)". Chemistry Letters. 34 (7): 1010–1011. doi:10.1246/cl.2005.1010.
- ^ A. J. Arduengo; T. P. Bannenberg; D. Tapu; W. J. Marshall (2005). "A zwitterionic cyclopentadienyl annulated imidazolium salt". Tetrahedron Letters. 46 (40): 6847–6850. doi:10.1016/j.tetlet.2005.08.018.
- ^ "Metallocene-Fused Imidazol-2-ylidene and Its Mercury Complex". Angewandte Chemie, Int. Ed. Engl. 44 (44): 7240–7244. 2005. doi:10.1002/anie.200502814.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "A Bimetallic Complex Containing a Cyclopentadienyl-Annulated Imidazol-2-ylidene". Journal of the American Chemical Society. 127 (47): 16400–16401. 2005. doi:10.1021/ja055565f. PMID 16305219.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ M. Yoshifuji; A. J. Arduengo; T. A. Konovalova; L. D. Kispert; M. Kikuchi; S. Ito (2006). "Oxidation of 1,3-Diphosphacyclobutane-2,4-diyl with Ammoniumyl Antimonate and EPR Study of the Corresponding Cation Radical". Chemistry Letters. 35 (10): 1136–1137. doi:10.1246/cl.2006.1136.
- ^ M. Yoshifuji; A. J. Arduengo; S. Ito (2008). "Studies on Stable 1,3-Diphosphacyclobutane-2,4-diyls". Phosphorus, Sulfur, and Silicon and the Related Elements. 183 (2 & 3): 335–339. doi:10.1080/10426500701734588.
- ^ S. Ito; J. Miura; N. Morita; M. Yoshifuji; A. J. Arduengo (2009). "Catenation of 1,3-Diphosphacyclobutane-2,4-diyl Units Having 2,4,6-Tri-tert-butylphenyl Protecting Groups and a P-sec-Butyl Group in the Ring". Z. Anorg. Allg. Chem. 635 (3): 488–495. doi:10.1002/zaac.200801265.
- ^ S. Ito; J. Miura; N. Morita; M. Yoshifuji; A. J. Arduengo (2009). "Modeling the Direct Activation of Dihydrogen by a P2C2 Cyclic Biradical: Formation of a Cyclic Bis(P−H λ5-phosphorane)". Inorganic Chemistry. 48 (17): 8063–8065. doi:10.1021/ic901072z.
- ^ S. Ito; J. Miura; N. Morita; M. Yoshifuji; A. J. Arduengo (2010). "Synthesis and properties of oligo(biradicals) composed of 1,3-diphosphacyclobutane-2,4-diyl units and benzyl-type linkers". Heteroatom Chemistry. 21 (6): 404–411. doi:10.1002/hc.20625. ISSN 1098-1071.
- ^ S. Ito; J. Miura; N. Morita; M. Yoshifuji; A. J. Arduengo (2010). "Diverse reactions of sterically-protected 1,3-diphosphacyclobutane-2,4-diyls with hydride". Dalton Transactions. 39 (35): 8281–8287. doi:10.1039/c0dt00532k.
- ^ S. Ito; J. Miura; N. Morita; M. Yoshifuji; A. J. Arduengo (2010). "Synthesis and physicochemical properties of stable 1,3-diphosphacyclobutane-2,4-diyls bearing sulfanyl groups". Comptes Rendus Chimie. 13 (8–9): 1180–1184. doi:10.1016/j.crci.2010.04.007.
- ^ Charles M. Knight Lecture series Web Page - Retrieved Oct. 20, 2016.
- ^ Walter J. Chute Lecture Series Web Page - Retrieved Oct. 20, 2016.
External links
 Media related to Anthony Joseph Arduengo III at Wikimedia Commons
Media related to Anthony Joseph Arduengo III at Wikimedia Commons- Saxon Professor of Chemistry, Anthony J. Arduengo, III
- Anthony J Arduengo's Personal Web Pages
- Chemical & Engineering News interview with Anthony J. Arduengo, III, October 2012
- Alabama's Arduengo Lecturship
- Georgia Tech's Arduengo Scholarship
- Living people
- 1952 births
- American chemists
- Organic chemists
- Inorganic chemists
- Georgia Institute of Technology alumni
- DuPont people
- University of Illinois at Urbana–Champaign faculty
- University of Alabama faculty
- Alexander von Humboldt Fellows
- Fellows of the American Association for the Advancement of Science






![Antimony analog of ADPO (ADSbO) synthesized at DuPont by Arduengo and Stewart.[67] external viewer.](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e0/AJA_ADSbO.svg/75px-AJA_ADSbO.svg.png)
![Nine coordinate bismuth complex prepared by Arduengo and Stewart.[68] external viewer.](http://upload.wikimedia.org/wikipedia/commons/thumb/1/1e/AJA_20-Bi-9.svg/120px-AJA_20-Bi-9.svg.png)
![Saturated ADPO analog used by Arduengo, Dixon, and Roe to verify Edge Inversion at 3-coordinate phosphorus.[24]](http://upload.wikimedia.org/wikipedia/commons/thumb/7/7a/AJA_satADPO-EdgeInv.svg/120px-AJA_satADPO-EdgeInv.svg.png)
![Germanium compound used by Arduengo, Dixon, and Roe to verify Edge Inversion at 4-coordinate centers.[25] external viewer.](http://upload.wikimedia.org/wikipedia/commons/thumb/5/5f/AJA_Ge-EdgeInv.svg/102px-AJA_Ge-EdgeInv.svg.png)
![Stable thiazol-2-ylidene (vitamin B1 analog).[43] external viewer.](http://upload.wikimedia.org/wikipedia/commons/thumb/c/ce/AJA_3-26-diip-45-dimethylthiazol-2-ylidene.svg/114px-AJA_3-26-diip-45-dimethylthiazol-2-ylidene.svg.png)
![Air-stable carbene.[42] external viewer](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d3/AJA_13-dimesityl-45-dichloroimidazol-2-ylidene.svg/120px-AJA_13-dimesityl-45-dichloroimidazol-2-ylidene.svg.png)
![Stable small perdeuterocarbene used for exact electron density mapping experiments.[46] external viewer.](http://upload.wikimedia.org/wikipedia/commons/thumb/9/90/AJA_1345-tetramethylimidazol-2-ylidene-d12.svg/120px-AJA_1345-tetramethylimidazol-2-ylidene-d12.svg.png)
![Wanzlick's original tetraphenylimidazol-2-ylidene isolated and characterized at DuPont.[65] external viewer.](http://upload.wikimedia.org/wikipedia/commons/thumb/9/95/AJA_1345-tetraphenylimidazol-2-ylidene.svg/120px-AJA_1345-tetraphenylimidazol-2-ylidene.svg.png)

