Chlordiazepoxide
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 5–30 hours (Active metabolite desmethyldiazepam 36-200 hours: other active metabolites include temazepam and oxazepam.) |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.337 |
| Chemical and physical data | |
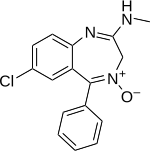
| Formula | C16H14ClN3O |
| Molar mass | 299.75 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Chlordiazepoxide (Template:Pron-en), is a sedative/hypnotic drug and benzodiazepine. It is marketed under the trade names AngirexKlopoxid, Librax (also contains clidinium bromide), Libritabs, Librium, Mesural, Multum, Novapam, Risolid, Silibrin, Sonimen and Tropium.
Chlordiazepoxide was the first benzodiazepine to be synthesised and the discovery of chlordiazepoxide was by pure chance.[1] Chlordiazepoxide and other benzodiazepines were initially accepted with widespread public approval but were followed with widespread public disapproval and recommendations for more restrictive medical guidelines for its use.[2]
Chlordiazepoxide has a medium to long half life but its active metabolite has a very long half life. The drug has amnestic, anxiolytic, hypnotic and skeletal muscle relaxant properties.[3]
History
Chlordiazepoxide (initially called methaminodiazepoxide) was the first benzodiazepine to be synthesised in the mid-1950s. Chlordiazepoxide was synthesised from work on a chemical dye, quinazolone-3-oxides. It was discovered by accident when in 1957 tests revealed that the compound had hypnotic, anxiolytic and muscle relaxant effects. Three years later chlordiazepoxide was marketed as a therapeutic benzodiazepine medication under the brand name Librium. Following chlordiazepoxide in 1963 diazepam hit the market under the brand name Valium followed by many further benzodiazepine compounds which were introduced over the subsequent years and decades.[4]
In 1959 it was used by over 2,000 physicians and more than 20,000 patients. It was described as "chemically and clinically different from any of the tranquilizers, psychic energizers or other psychotherapeutic drugs now available." During studies, chlordiazepoxide induced muscle relaxation and a quieting effect on laboratory animals like mice, rats, cats, and dogs. Fear and aggression were eliminated in much smaller doses than those necessary to produce hypnosis. Chlordiazepoxide is similar to phenobarbital in its anticonvulsant properties. However, it lacks the hypnotic effects of barbiturates. Animal tests were conducted in the Boston Zoo and the San Diego Zoo. Forty-two hospital patients admitted for acute and chronic alcoholism, and various psychoses and neuroses were treated with chlordiazepoxide. In a majority of the patients, anxiety, tension, and motor excitement were "effectively reduced." The most positive results were observed among alcoholic patients. It was reported that ulcers[disambiguation needed] and dermatologic problems, both of which involve emotional factors, were reduced by chlordiazepoxide.[5]
Chlordiazepoxide enabled the treatment of emotional disturbances without a loss of mental acuity or alertness. It assisted persons burdened by compulsive behavior like one that felt compelled to count the slats on venetian blinds upon entering a room.[6] In 1963, approval for use was given to diazepam (Valium), a "simplified" version of chlordiazepoxide, primarily to counteract anxiety symptoms. Sleep-related problems were treated with nitrazepam (Mogadon), which was introduced in 1965, temazepam (Restoril), which was introduced in 1969, and flurazepam (Dalmane), which was introduced in 1973.[7]
Indications
Chlordiazepoxide is indicated for the short term (2–4 weeks) treatment of anxiety which is severe and disabling or subjecting the person to unacceptable distress. It is also indicated as a treatment for the management of acute alcohol withdrawal syndrome.[8]
Contraindications
Use of chlordiazepoxide should be avoided in individuals with the following conditions:
- Myasthenia gravis
- Acute intoxication with alcohol, narcotics, or other psychoactive substances
- Ataxia
- Severe hypoventilation
- Acute narrow-angle glaucoma
- Severe liver deficiencies (hepatitis and liver cirrhosis decrease elimination by a factor of 2)
- Severe sleep apnea
- Hypersensitivity or allergy to any drug in the benzodiazepine class
Chlordiazepoxide is generally considered an inappropriate benzodiazepine for the elderly due to its long elimination half-life and the risks of accumulation.[9] Benzodiazepines require special precaution if used in the elderly, pregnancy, children, alcohol- or drug-dependent individuals and individuals with comorbid psychiatric disorders.[10]
Pregnancy
The research into the safety of benzodiazepines during pregnancy is limited and it is recommended that use of benzodiazepines during pregnancy should be based on whether the benefits outweigh the risks. If chlordiazepoxide is used during pregnancy the risks can be reduced via using the lowest effective dose and for the shortest time possible. Benzodiazepines should generally be avoided during the first trimester of pregnancy. Chlordiazepoxide and diazepam are considered to be among the safer benzodiazepines to use during pregnancy in comparison to other benzodiazepines. Possible adverse effects from benzodiazepine use during pregnancy include, abortion, malformation, intrauterine growth retardation, functional deficits, carcinogenesis and mutagenesis. Caution is also advised during breast feeding as chlordiazepoxide passes into breast milk.[11][12]
Side effects
Common side effects of chlordiazepoxide include:[13]
- Confusion
- Constipation
- Drowsiness
- Fainting
- Altered sex drive
- Liver problems
- Lack of muscle coordination
- Minor menstrual irregularities
- Nausea
- Skin rash or eruptions
- Swelling due to fluid retention
- Yellow eyes and skin
Chlordiazepoxide in laboratory mice studies impairs latent learning. Benzodiazepines impair learning and memory via their action on benzodiazepine receptors which causes a dysfunction in the cholinergic neuronal system in mice.[14] In tests of various benzodiazepine compounds, chlordiazepoxide was found to cause the most profound reduction in the turnover of 5HT (serotonin) in rats. Serotonin is closely involved in regulating mood and may be one of the causes of feelings of depression in rats using chlordiazepoxide or other benzodiazepines.[15]

Tolerance and dependence
Tolerance
Chronic use of benzodiazepines, such as chlordiazepoxide leads to the development of tolerance with a decrease in number of benzodiazepine binding sites in mouse forebrain.[16] The Committee of Review of Medicines who carried out an extensive review of benzodiazepines including chlordiazepoxide found and were in agreement with the Institute of Medicine (USA) and the conclusions of a study carried out by the White House Office of Drug Policy and the National Institute on Drug Abuse (USA) that there was little evidence that long term use of benzodiazepines were beneficial in the treatment of insomnia due to the development of tolerance. Benzodiazepines tended to lose their sleep promoting properties within 3–14 days of continuous use and in the treatment of anxiety the committee found that there was little convincing evidence that benzodiazepines retained efficacy in the treatment of anxiety after 4 months continuous use due to the development of tolerance.[17]
Dependence
Chlordiazepoxide can cause physical dependence, addiction and what is known as the benzodiazepine withdrawal syndrome. Withdrawal from chlordiazepoxide or other benzodiazepines often leads to withdrawal symptoms which are similar to those seen with alcohol and barbiturates. The higher the dose and the longer the drug is taken the greater the risk of experiencing unpleasant withdrawal symptoms. Withdrawal symptoms can however occur at standard dosages and also after short term use. Benzodiazepine treatment should be discontinued as soon as possible via a slow and gradual dose reduction regime.[18]
Chlordiazepoxide taken during pregnancy can cause a postnatal benzodiazepine withdrawal syndrome.[19]
Overdose
An individual who has consumed excess chlordiazepoxide may display some of the following symptoms:
- Somnolence (difficulty staying awake)
- Mental confusion
- Hypotension
- Hypoventilation
- Impaired motor functions
- Impaired reflexes
- Impaired coordination
- Impaired balance
- Dizziness
- Muscle weakness
- Coma
In animal models, the oral median lethal dose of chlordiazepoxide is 537 mg/kg.
Chlordiazepoxide is a drug which is very frequently involved in drug intoxication, including overdose.[20] Chlordiazepoxide overdose is considered a medical emergency and generally requires the immediate attention of medical personnel. The antidote for an overdose of chlordiazepoxide (or any other benzodiazepine) is flumazenil (Anexate).
Pharmacology
Chlordiazepoxide acts on benzodiazepine subreceptors of the main GABAA receptor and this results in an increased binding of the inhibitory neurotransmitter GABA to the GABAA receptor thereby producing inhibitory effects on the central nervous system and body similar to the effects of other benzodiazepines.[21] Chlordiazepoxide is anticonvulsant.[22] There is preferential storage of chlordiazepoxide in some organs including the heart of the neonate. Absorption by any administered route and the risk of accumulation is significantly increased in the neonate. The withdrawal of chlordiazepoxide during pregnancy and breast feeding is recommended, as chlordiazepoxide rapidly crosses the placenta and also is excreted in breast milk.[23] Chlordiazepoxide also decreases prolactin release in rats.[24] Benzodiazepines act via micromolar benzodiazepine binding sites as Ca2+ channel blockers and significantly inhibit depolarization-sensitive Calcium uptake in animal nerve terminal preparations.[25] Chlordiazepoxide inhibits acetylcholine release in mouse hippocampal synaptosomes in vivo. This has been found by measuring sodium-dependent high affinity choline uptake in vitro after pretreatment of the mice in vivo with chlordiazepoxide. This may play a role in chlordiazepoxide's anticonvulsant properties.[26]
Pharmacokinetics
Chlordiazepoxide is a long acting benzodiazepine drug. The half life of Chlordiazepoxide is 5 – 30 hours but has an active benzodiazepine metabolite (desmethyldiazepam) which has a half life of 36 – 200 hours.[27] The half life of chlordiazepoxide increases significantly in the elderly which may result in prolonged action as well as accumulation of the drug during repeated administration. Delayed body clearance of the long half life active metabolite also occurs in those over 60 years of age which further prolongs the effects of the drugs with additional accumulation after repeated dosing.[28]
Interactions
Some of the major interactions involving Chlordiazepoxide are listed below.[29]
- ACE inhibitors, Adrenergic neurone blockers, Angiotensin II receptor antagonists, Beta blockers, Calcium channel blockers, Clonidine, Diazoxide, Diuretics, Hydralazine, Methyldopa, Minoxidil, Nitrates, Sodium Nitroprusside - enhanced hypotensive effect
- Alcohol, barbiturates, opiates, antihistamines, antipsychotics - increased sedative effect in combination with benzodiazapines.
- Cimetidine - metabolism of benzodiazepines inhibited by cimetidine (increased plasma concentration)
- Disulfiram - metabolism of benzodiazepines inhibited by disulfiram (increased sedative effects)
- Fluvoxamine - plasma concentration of some benzodiazepines increased by fluvoxamine
- Levodopa - benzodiazepines possibly antagonise effects of levodopa
- Moxonidine - sedative effects possibly increased when benzodiazepines given with moxonidine
- Olanzapine - increased risk of hypotension, bradycardia and respiratory depression when parenteral benzodiazepines given with intramuscular olanzapine
- Phenytoin - benzodiazepines possibly increase or decrease plasma concentration of phenytoin
- Rifampicin - metabolism of benzodiazepines possibly accelerated by rifampicin (reduced plasma concentration)
- Sodium Oxybate - benzodiazepines enhance effects of sodium oxybate (avoid concomitant use)
Recreational use
Dr. Carl F. Essig of the Addiction Research Center of the National Institute of Mental Health spoke at a symposium on drug abuse at an annual meeting of the American Association for the Advancement of Science, in December 1963. He named meprobamate, glutethimide, ethinamate, ethchlorvynol, methyprylon and chlordiazepoxide as drugs whose usefulness can hardly be questioned. However, Essig labeled these newer products as drugs of addiction, like barbiturates, whose habit-forming qualities were more widely known. He mentioned a 90-day study of chlordiazepoxide, which concluded that the automobile accident rate among 68 users was 10 times higher than normal. Participants' daily dosage ranged from 5 to 100 milligrams.[30]
Chlordiazepoxide is a drug of potential misuse and is frequently detected in urine samples of drug users who have not been prescribed the drug.[31] Chlordiazepoxide in animal studies has been shown to increase reward seeking behaviours which may suggest an increased risk of addictive behavioural patterns.[32] In addition chlordiazepoxide has been shown to be able to substitute for the behavioural effects of barbiturates in a primate study.[33]
Legal status
Internationally, chlordiazepoxide is a Schedule IV controlled drug under the Convention on Psychotropic Substances.[1]
Toxicity
Animal
Laboratory tests assessing the toxicity of chlordiazepoxide, nitrazepam and diazepam on mice spermatozoa found that chlordiazepoxide produced toxicities in sperm including abnormalities involving both the shape and size of the sperm head. Nitrazepam however caused more profound abnormalities than chlordiazepoxide.[34]
Availability
Chlordiazepoxide is available in 5 mg, 10 mg and 25 mg strengths.
Trade names
Chlordiazepoxide is available er the following trade names in Englbenzodiazepines.org.uk/benzodiazepine-names.html| title=Benzodiazepine Names| accessdate=2008
- Novapam
- Tropium
Combination Drugs
- Librax - chlordiazepoxide and clidinium
- Apo-Chlorax - chloridazepoxide and clidinium
- Limbitrol - chlordiazepoxide and amitriptyline
- Clindex - chloridazepoxide and clidinium
Chemistry
Chlordiazepoxide is synthesized from 2-amino-5-chlorobenzophenone. This
is reacted in the usual manner with hydroxylamine, forming 2-amino-5-chlorobenzophenone
oxide, which upon reaction with chloroacetic acid chloride in acetic acid
easily cyclizes to 6-chloro-2-chloromethyl-4-phenylquinazolin-3-oxide. Reacting this with a primary amine, methylamine in particular, leads to an interesting rearrangement (with a ring expansion), and the reaction product turns out to be 7-chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepin-4-oxide. An analogous rearrangement with a ring expansion also proceeds upon reaction with alkaline or alkoxides; however, it should be noted that with dialkylamines, the reaction forms the expected substitution products, 2-dialkylaminomethyl derivatives of 6-chloro-
4-phenylquinazolin-3-oxide.

- L.H. Sternbach, U.S. patent 2,893,992 (1959).
- G. Muller, J. Warnant, DE 1096363 (1959).
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/jo01063a034, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/jo01063a034instead.
See also
- Alcohol withdrawal syndrome
- Long term effects of benzodiazepines
- Benzodiazepine withdrawal syndrome
- Benzodiazepine dependence
- Benzodiazepine
References
- ^ Ban, TA. (2006). "The role of serendipity in drug discovery". Dialogues Clin Neurosci. 8 (3): 335–44. PMID 17117615.
- ^ Marshall, KP.; Georgievskava, Z.; Georgievsky, I. (2009). "Social reactions to Valium and Prozac: a cultural lag perspective of drug diffusion and adoption". Res Social Adm Pharm. 5 (2): 94–107. doi:10.1016/j.sapharm.2008.06.005. PMID 19524858.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Liljequist R (1979). "Effects on learning and memory of 2-week treatments with chlordiazepoxide lactam, N-desmethyldiazepam, oxazepam and methyloxazepam, alone or in combination with alcohol". Int Pharmacopsychiatry. 14 (4): 190–8. PMID 42628.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Cooper, Jack R (January 15, 1996). The Complete Story of the Benzodiazepines (in Eng) (seventh ed.). USA: Oxford University Press. ISBN 0195103998. Retrieved 7 Apr 2008.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unrecognized language (link) - ^ New York Times (28 February 1960). "Help For Mental Ills (Reports on Tests of Synthetic Drug Say The Results are Positive)". USA: New York Times. p. E9.
- ^ New York Times (28 August 1960). "Makers Worried On Tranquilizers". USA: New York Times. p. F1.
{{cite news}}: Unknown parameter|curly=ignored (help) - ^ Sternbach LH (1972). "The discovery of librium". Agents Actions. 2 (4): 193–6. doi:10.1007/BF01965860. PMID 4557348.
- ^ British National Formulary (April 8, 2008). "CHLORDIAZEPOXIDE HYDROCHLORIDE". BNF.org. Retrieved 8 April 2008.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Liu, GG.; Christensen, DB. (2002). "The continuing challenge of inappropriate prescribing in the elderly: an update of the evidence". J Am Pharm Assoc (Wash). 42 (6): 847–57. doi:10.1331/108658002762063682. PMID 12482007.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ Authier, N.; Balayssac, D.; Sautereau, M.; Zangarelli, A.; Courty, P.; Somogyi, AA.; Vennat, B.; Llorca, PM.; Eschalier, A. (2009). "Benzodiazepine dependence: focus on withdrawal syndrome". Ann Pharm Fr. 67 (6): 408–13. doi:10.1016/j.pharma.2009.07.001. PMID 19900604.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Iqbal, MM.; Aneja, A.; Fremont, WP. (2003). "Effects of chlordiazepoxide (Librium) during pregnancy and lactation". Conn Med. 67 (5): 259–62. PMID 12802839.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Iqbal, MM.; Sobhan, T.; Ryals, T. (2002). "Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant". Psychiatr Serv. 53 (1): 39–49. doi:10.1176/appi.ps.53.1.39. PMID 11773648.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ drugs. "Chlordiazepoxide patient advice including side effects". drugs.com. Retrieved April 7, 2008.
- ^ Nabeshima T (1990). "Effects of benzodiazepines on passive avoidance response and latent learning in mice: relationship to benzodiazepine receptors and the cholinergic neuronal system". J Pharmacol Exp Ther. 255 (2): 789–94. PMID 2173758.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Antkiewicz-Michaluk L (1975). "Influence of benzodiazepines on turnover of serotonin in cerebral structures in normal and aggressive rats". Arch Immunol Ther Exp (Warsz). 23 (6): 763–7. PMID 1241268.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Crawley JN (1982). "Chronic clonazepam administration induces benzodiazepine receptor subsensitivity". Neuropharmacology. 21 (1): 85–9. doi:10.1016/0028-3908(82)90216-7. PMID 6278355.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Committee on the Review of Medicines (March 29, 1980). "Systematic review of the benzodiazepines. Guidelines for data sheets on diazepam, chlordiazepoxide, medazepam, clorazepate, lorazepam, oxazepam, temazepam, triazolam, nitrazepam, and flurazepam. Committee on the Review of Medicines". Br Med J. 280 (6218): 910–2. doi:10.1136/bmj.280.6218.910. PMC 1601049. PMID 7388368.
- ^ MacKinnon GL (1982). "Benzodiazepine withdrawal syndrome: a literature review and evaluation". The American journal of drug and alcohol abuse. 9 (1): 19–33. doi:10.3109/00952998209002608. PMID 6133446.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Moretti M (1982). "[Fetal defects caused by the passive consumption of drugs]". Pediatr Med Chir. 4 (5): 481–90. PMID 6985425.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Zevzikovas A (2002). "[Analysis of benzodiazepine derivative mixture by gas-liquid chromatography]". Medicina (Kaunas). 38 (3): 316–20. PMID 12474705.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Skerritt JH (May 6, 1983). "Enhancement of GABA binding by benzodiazepines and related anxiolytics". Eur J Pharmacol. 89 (3–4): 193–8. doi:10.1016/0014-2999(83)90494-6. PMID 6135616.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Chweh AY (February 25, 1985). "Effect of GABA agonists on the neurotoxicity and anticonvulsant activity of benzodiazepines". Life Sci. 36 (8): 737–44. doi:10.1016/0024-3205(85)90193-6. PMID 2983169.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Olive G (1977). "Pharmacologic bases of use of benzodiazepines in peréinatal medicine". Arch Fr Pediatr. 34 (1): 74–89. PMID 851373.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Grandison L (1982). "Suppression of prolactin secretion by benzodiazepines in vivo". Neuroendocrinology. 34 (5): 369–73. doi:10.1159/000123330. PMID 6979001.
- ^ Taft WC (1984). "Micromolar-affinity benzodiazepine receptors regulate voltage-sensitive calcium channels in nerve terminal preparations" (PDF). Proc Natl Acad Sci USA (PDF). 81 (10): 3118–22. doi:10.1073/pnas.81.10.3118. PMC 345232. PMID 6328498.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Miller JA (1985). "Effects of anticonvulsants in vivo on high affinity choline uptake in vitro in mouse hippocampal synaptosomes". Br J Pharmacol. 84 (1): 19–25. PMC 1987204. PMID 3978310.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Ashton CH. (2007). "BENZODIAZEPINE EQUIVALENCY TABLE". Retrieved September 23, 2007.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Vozeh S. (November 21, 1981). "[Pharmacokinetic of benzodiazepines in old age]". Schweiz Med Wochenschr. 111 (47): 1789–93. PMID 6118950.
- ^ British National Formulary. "Chlordiazepoxide interactions". BNF. Retrieved 07 A p r 2008.
{{cite web}}: Check date values in:|accessdate=(help); Cite has empty unknown parameter:|dateformat=(help) - ^ New York Times (30 December 1963). "Warning Is Issued On Tranquilizers". USA: New York Times. p. 23.
{{cite news}}: Unknown parameter|curly=ignored (help) - ^ Garretty DJ (1997). "Benzodiazepine misuse by drug addicts". Annals of clinical biochemistry. 34 (Pt 1): 68–73. PMID 9022890.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Thiébot MH (1985). "Benzodiazepines reduce the tolerance to reward delay in rats". Psychopharmacology (Berl). 86 (1–2): 147–52. doi:10.1007/BF00431700. PMID 2862657.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Woolverton WL, Nader MA (1995). "Effects of several benzodiazepines, alone and in combination with flumazenil, in rhesus monkeys trained to discriminate pentobarbital from saline". Psychopharmacology (Berl.). 122 (3): 230–6. doi:10.1007/BF02246544. PMID 8748392.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kar RN (1983). "Induction of sperm head abnormalities in mice by three tranquilizers". Cytobios. 36 (141): 45–51. PMID 6132780.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)
