Iproniazid
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | ? |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Elimination half-life | ? |
| Excretion | ? |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.199 |
| Chemical and physical data | |
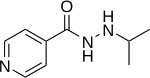
| Formula | C9H13N3O |
| Molar mass | 179.219 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Iproniazid (Marsilid, Rivivol, Euphozid, Iprazid, Ipronid, Ipronin) is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class.[1][2] It was discontinued in most of the world in the 1960s, but remained in use in France until fairly recently.[3]
Iproniazid was originally developed for the treatment of tuberculosis,[1] but in 1952, its antidepressant properties were discovered when researchers noted that patients given isoniazid became inappropriately happy.[1] Subsequently N-isopropyl addition led to development as an antidepressant and was approved for use in 1958.[1] It was withdrawn a few years later in 1961 due to a high incidence of hepatitis, and was replaced by less hepatotoxic drugs such as phenelzine and isocarboxazid.[1]
Although iproniazid was one of the first antidepressants ever marketed, amphetamine (marketed as Benzedrine from 1935, for "mild depression", amid other indications)[4] predates it; and frankincense has been marketed traditionally for millennia for, among other things, altering mood, although it was not until 2012 that one of the components of its smoke was found to have antidepressant effects in mice.[5] [6] [7]
See also
References
- ^ a b c d e Robert A. Maxwell; Shohreh B. Eckhardt (1990). Drug discovery. Humana Press. pp. 143–154. ISBN 0-89603-180-2. ISBN 9780896031807.
- ^ Fagervall I, Ross SB (April 1986). "Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors". Biochemical pharmacology. 35 (8): 1381–7. doi:10.1016/0006-2952(86)90285-6. PMID 2870717.
- ^ Maille F, Duvoux C, Cherqui D, Radier C, Zafrani ES, Dhumeaux D (October 1999). "[Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France?]". Gastroenterol. Clin. Biol. (in French). 23 (10): 1083–5. PMID 10592880.
- ^ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–96. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ^ Moussaieff, Arieh; Rimmerman, Neta; Bregman, Tatiana; Straiker, Alex; Felder, Christian C.; Shoham, Shai; Kashman, Yoel; Huang, Susan M.; Lee, Hyosang; Shohami, Esther; Mackie, Ken; Caterina, Michael J.; Walker, J. Michael; Fride, Ester; Mechoulam, Raphael (August 2008). "Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain". 22 (8). The FASEB Journal: 3024–3034. doi:10.1096/fj.07-101865. PMC 2493463. PMID 18492727.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: unflagged free DOI (link) - ^ Moussaieff, A; Gross, M; Nesher, E; Tikhonov, T; Yadid, G; Pinhasov, A (2012). "Incensole acetate reduces depressive-like behavior and modulates hippocampal BDNF and CRF expression of submissive animals". J Psychopharmacol. 26 (12). doi:10.1177/0269881112458729. PMID 23015543.
- ^ Drahl, Carmen (22 December 2008). "Frankincense And Myrrh". Chemical & Engineering News. 86 (51): 38. doi:10.1021/cen-v086n051.p038. ISSN 0009-2347.
