Ruxolitinib
 | |
| Clinical data | |
|---|---|
| Trade names | Jakafi, Jakavi, Opzelura |
| Other names | INCB018424, INC424 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612006 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 95%[10] |
| Protein binding | 97%[10] |
| Metabolism | Liver (mainly CYP3A4-mediated)[10] |
| Elimination half-life | 2.8-3 hours[10] |
| Excretion | Urine (74%), faeces (22%)[10] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
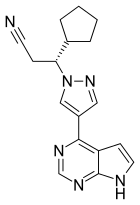
| Formula | C17H18N6 |
| Molar mass | 306.373 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ruxolitinib, sold under the brand name Jakafi among others, is a medication used for the treatment of intermediate or high-risk myelofibrosis,[6] a type of myeloproliferative neoplasm that affects the bone marrow;[11][12] polycythemia vera, when there has been an inadequate response to or intolerance of hydroxyurea;[6][13] and steroid-refractory acute graft-versus-host disease.[6] Ruxolitinib is a Janus kinase inhibitor.[6] It was developed and marketed by Incyte Corp in the US under the brand name Jakafi,[6] and by Novartis elsewhere in the world, under the brand name Jakavi.[14]
It was approved for medical use in the United States in 2011,[15] and in the European Union in 2012.[8] Ruxolitinib is the first FDA-approved pharmacologic treatment to address repigmentation in vitiligo patients.[16]
The crystal structure of ruxolitinib and of its dihydrate form are known.[17]
Medical uses
[edit]In the United States and the European Union, ruxolitinib is indicated for the treatment of disease-related splenomegaly or symptoms in adults with primary myelofibrosis (also known as chronic idiopathic myelofibrosis), post-polycythaemia-vera myelofibrosis, or post-essential thrombocythaemia myelofibrosis.[6][8] It is also indicated for the treatment of adults with polycythaemia vera who are resistant to or intolerant of hydroxyurea.[8] Ruxolitinib is also indicated for the treatment of steroid-refractory acute graft-versus-host disease in people who are twelve years of age and older,[6] and for the treatment of chronic graft-versus-host disease (cGVHD) after failure of one or two lines of systemic therapy in people twelve years of age and older.[6][8][18][19] It is commonly given as an oral tablet.[citation needed]
In the United States, ruxolitinib cream is indicated for the topical treatment of mild to moderate atopic dermatitis and vitiligo.[7] In the European Union, ruxolitinib cream is indicated for the treatment of non-segmental vitiligo with facial involvement in adults and adolescents from 12 years of age.[9]
Side effects
[edit]In myelofibrosis, the most common side effects include thrombocytopenia (low blood platelet counts), anaemia (low red blood cell counts), neutropenia (low levels of neutrophils), urinary tract infections (infection of the kidney, renal pelvis, ureter, bladder or urethra), bleeding, bruising, weight gain, hypercholesterolaemia (high blood cholesterol levels), dizziness, headache and raised liver enzyme levels.[8]
In polycythaemia vera, the most common side effects include anemia (low red blood cell counts) and thrombocytopenia (low blood platelet count), bleeding, bruising, hypercholesterolaemia (high blood cholesterol levels), hypertriglyceridemia (high blood fat levels), dizziness, raised liver enzyme levels and high blood pressure.[8]
In acute graft-versus-host disease, the most common hematologic adverse reactions include anemia, thrombocytopenia, and neutropenia.[6] The most common nonhematologic adverse reactions include infections and edema.[6]
Immunologic side effects have included herpes zoster (shingles) and case reports of opportunistic infections.[20] Metabolic side effects have included weight gain. Laboratory abnormalities have included alanine transaminase (ALT) abnormalities, aspartate transaminase (AST) abnormalities, and mildly elevated cholesterol levels.[6]
Mechanism of action
[edit]Ruxolitinib is a Janus kinase inhibitor (JAK inhibitor) with selectivity for subtypes JAK1 and JAK2.[21][22] Ruxolitinib inhibits dysregulated JAK signaling associated with myelofibrosis. JAK1 and JAK2 recruit signal transducers and activators of transcription (STATs) to cytokine receptors leading to modulation of gene expression.[6]
History
[edit]In March 2012, the phase III Controlled Myelofibrosis Study with Oral JAK Inhibitor-I (COMFORT-I) and COMFORT-II trials showed significant benefits by reducing spleen size and relieving debilitating symptoms.[23][24][25][26]
Society and culture
[edit]Legal status
[edit]In November 2011, ruxolitinib was approved by the U.S. Food and Drug Administration (FDA)[15] for the treatment of intermediate or high-risk myelofibrosis based on results of the COMFORT-I and COMFORT-II Trials.[27]
In 2014, it was approved in polycythemia vera when there has been an inadequate response to or intolerance of hydroxyurea, based on the RESPONSE trial.[28][13]
In May 2019, the indication for ruxolitinib was expanded in the US to include steroid-refractory acute graft-versus-host disease.[29] The indication was further expanded in the US in September 2021, for the treatment of chronic graft-versus-host disease (cGVHD) after failure of one or two lines of systemic therapy in people 12 years of age and older.[30]
In September 2021, ruxolitinib cream (sold under the brand name Opzelura) was approved for medical use in the United States[31] for the treatment of mild to moderate atopic dermatitis (AD).[32] It is the first topical Janus kinase inhibitor approved in the United States.[32]
In July 2022, ruxolitinib cream (sold under the brand name Opzelura) was approved for medical use in the United States for the treatment of vitiligo.[16][33]
On 23 February 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Opzelura, intended for the treatment of non-segmental vitiligo.[34] The applicant for this medicinal product is Incyte Biosciences Distribution B.V.[34]
Research
[edit]It is being investigated for plaque psoriasis,[21] alopecia areata,[35] relapsed diffuse large B-cell lymphoma, and peripheral T-cell lymphoma.[36]
In February 2016, a phase III trial for pancreatic cancer was terminated due to insufficient efficacy.[37]
Eight weeks-treatment with ruxolitinib blunted senescent cell-mediated inhibition of adipogenesis and increased insulin sensitivity in 22-month-old mice.[38]
As of September 2019, a clinical trial is in progress to evaluate "Treatment Free Remission After Combination Therapy With Ruxolitinib Plus Tyrosine Kinase Inhibitors".[39][full citation needed][needs update]
References
[edit]- ^ a b "JAKAVI (Novartis Pharmaceuticals Australia Pty LTD) | Therapeutic Goods Administration (TGA)". Archived from the original on 8 March 2023. Retrieved 8 March 2023.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "JAKAVI ruxolitinib (as phosphate) 5 mg tablet blister pack (198934)". Therapeutic Goods Administration (TGA). 27 May 2022. Archived from the original on 8 March 2023. Retrieved 12 July 2024.
- ^ "Jakavi Product information". Health Canada. 22 October 2009. Archived from the original on 8 March 2023. Retrieved 7 March 2023.
- ^ "Jakavi 10mg Tablets - Summary of Product Characteristics (SmPC)". (emc). 5 April 2022. Archived from the original on 8 March 2023. Retrieved 7 March 2023.
- ^ a b c d e f g h i j k l m "Jakafi- ruxolitinib tablet". DailyMed. 26 February 2020. Archived from the original on 3 November 2020. Retrieved 16 November 2020.
- ^ a b "Opzelura- ruxolitinib cream". DailyMed. Archived from the original on 1 November 2021. Retrieved 31 October 2021.
- ^ a b c d e f g "Jakavi EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 12 November 2020. Retrieved 16 November 2020. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b "Opzelura EPAR". European Medicines Agency. 20 April 2023. Archived from the original on 24 April 2023. Retrieved 23 April 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b c d e "Jakafi (ruxolitinib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 12 December 2018. Retrieved 16 February 2014.
- ^ Mesa RA, Yasothan U, Kirkpatrick P (February 2012). "Ruxolitinib". Nature Reviews. Drug Discovery. 11 (2): 103–4. doi:10.1038/nrd3652. PMID 22293561. S2CID 233195859.
- ^ Harrison C, Mesa R, Ross D, Mead A, Keohane C, Gotlib J, et al. (October 2013). "Practical management of patients with myelofibrosis receiving ruxolitinib". Expert Review of Hematology. 6 (5): 511–23. doi:10.1586/17474086.2013.827413. PMC 8201600. PMID 24083419. S2CID 5470231.
- ^ a b Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. (January 2015). "Ruxolitinib versus standard therapy for the treatment of polycythemia vera". The New England Journal of Medicine. 372 (5): 426–35. doi:10.1056/NEJMoa1409002. PMC 4358820. PMID 25629741.
- ^ "FDA Approves Jakafi® (Ruxolitinib) for the Treatment of Patients with Uncontrolled Polycythemia Vera". 4 December 2014. Archived from the original on 8 March 2023. Retrieved 8 March 2023.
- ^ a b "Drug Approval Package: Jakafi (ruxolitinib) Tablets NDA # 202192". Archived from the original on 20 December 2022. Retrieved 20 December 2022.
- ^ a b "FDA approves topical treatment". U.S. Food and Drug Administration (FDA) (Press release). 19 July 2022. Archived from the original on 20 December 2022. Retrieved 20 December 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Peng Z, Ye L (August 2024). "Comparison of the crystal structures of the JAK1/2 inhibitor ruxolitinib and its hydrate and phosphate". Acta Crystallographica Section C. 80 (Pt 8): 440–447. Bibcode:2024AcCrC..80..440P. doi:10.1107/S2053229624006740. PMID 39046815.
- ^ "FDA approves ruxolitinib for chronic graft-versus-host disease". U.S. Food and Drug Administration (FDA). 22 September 2021. Archived from the original on 23 September 2021. Retrieved 22 September 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "EU Commission Approval" (Press release). Novartis. 5 May 2022. Archived from the original on 6 July 2022. Retrieved 5 July 2022.
- ^ Wysham NG, Sullivan DR, Allada G (May 2013). "An opportunistic infection associated with ruxolitinib, a novel janus kinase 1,2 inhibitor". Chest. 143 (5): 1478–1479. doi:10.1378/chest.12-1604. PMC 5991580. PMID 23648912.
- ^ a b Mesa RA (June 2010). "Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis". IDrugs. 13 (6): 394–403. PMID 20506062.
- ^ Pardanani A, Tefferi A (March 2011). "Targeting myeloproliferative neoplasms with JAK inhibitors". Current Opinion in Hematology. 18 (2): 105–10. doi:10.1097/MOH.0b013e3283439964. PMID 21245760. S2CID 2059415.
- ^ Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. (March 2012). "JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis". The New England Journal of Medicine. 366 (9): 787–98. doi:10.1056/NEJMoa1110556. hdl:2158/605459. PMID 22375970.
- ^ Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. (March 2012). "A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis". The New England Journal of Medicine. 366 (9): 799–807. doi:10.1056/NEJMoa1110557. PMC 4822164. PMID 22375971.
- ^ Tefferi A (March 2012). "Challenges facing JAK inhibitor therapy for myeloproliferative neoplasms". The New England Journal of Medicine. 366 (9): 844–6. doi:10.1056/NEJMe1115119. PMID 22375977.
- ^ ASCO Annual Meeting 2011: JAK Inhibitor Ruxolitinib Demonstrates Significant Clinical Benefit in Myelofibrosis Archived 21 November 2011 at the Wayback Machine
- ^ "FDA Approves Incyte's Jakafi (ruxolitinib) for Patients with Myelofibrosis" (Press release). Incyte. Archived from the original on 24 June 2017. Retrieved 2 January 2012.
- ^ Kaminskas E (4 December 2014). "Supplemental FDA approval letter for Jakafi (ruxolitinib) tablets" (PDF). U.S. Food and Drug Administration. Archived (PDF) from the original on 11 April 2021. Retrieved 1 May 2016.
- ^ "FDA approves ruxolitinib for acute graft-versus-host disease". U.S. Food and Drug Administration. 24 May 2019. Archived from the original on 20 December 2022. Retrieved 20 December 2022.
- ^ "FDA approves ruxolitinib for chronic graft-versus-host disease". U.S. Food and Drug Administration. 31 January 2022. Archived from the original on 23 September 2021. Retrieved 20 December 2022.
- ^ "Drug Approval Package: OPZELURA". Archived from the original on 20 December 2022. Retrieved 20 December 2022.
- ^ a b "Incyte Announces U.S. FDA Approval of Opzelura (ruxolitinib) Cream, a Topical JAK Inhibitor, for the Treatment of Atopic Dermatitis (AD)". Incyte. 21 September 2021. Archived from the original on 22 September 2021. Retrieved 21 September 2021 – via Business Wire.
- ^ "Incyte Announces U.S. FDA Approval of Opzelura (ruxolitinib) Cream for the Treatment of Vitiligo". Incyte. 19 July 2022. Archived from the original on 19 July 2022. Retrieved 19 July 2022 – via Business Wire.
- ^ a b "Opzelura: Pending EC decision". European Medicines Agency (EMA). 24 February 2023. Archived from the original on 24 February 2023. Retrieved 24 February 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ Falto-Aizpurua L, Choudhary S, Tosti A (December 2014). "Emerging treatments in alopecia". Expert Opinion on Emerging Drugs. 19 (4): 545–56. doi:10.1517/14728214.2014.974550. PMID 25330928. S2CID 21604470.
- ^ Clinical trial number NCT01431209 for "Ruxolitinib Phosphate (Oral JAK Inhibitor INCB18424) in Treating Patients With Relapsed or Refractory Diffuse Large B-Cell or Peripheral T-Cell Non-Hodgkin Lymphoma" at ClinicalTrials.gov
- ^ House DW (February 2016). "Incyte bags late-stage development of Jakafi for solid tumors; shares down 10% premarket". Seeking Alpha. Archived from the original on 10 October 2016. Retrieved 11 February 2016.
- ^ Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, et al. (December 2015). "Targeting senescent cells enhances adipogenesis and metabolic function in old age". eLife. 4: e12997. doi:10.7554/eLife.12997. PMC 4758946. PMID 26687007.
- ^ Clinical trial number NCT03610971 for "Treatment Free Remission After Combination Therapy With Ruxolitinib Plus Tyrosine Kinase Inhibitors" at ClinicalTrials.gov
External links
[edit]- Clinical trial number NCT03112603 for "A Study of Ruxolitinib vs Best Available Therapy (BAT) in Patients With Steroid-refractory Chronic Graft vs. Host Disease (GvHD) After Bone Marrow (REACH3)" at ClinicalTrials.gov
- Clinical trial number NCT03745638 for "TRuE AD1 - An Efficacy and Safety Study of Ruxolitinib Cream in Adolescents and Adults With Atopic Dermatitis" at ClinicalTrials.gov
- Clinical trial number NCT03745651 for "TRuE AD2 - An Efficacy and Safety Study of Ruxolitinib Cream in Adolescents and Adults With Atopic Dermatitis" at ClinicalTrials.gov
