Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
April 20
Bleach and chlorine
So what happens if you mix Clorox bleach with chlorine? Albacore (talk) 02:21, 20 April 2012 (UTC)
- Chlorine + water + high pH = Clorox bleach. If you add more chlorine, your just going to get more concentrated bleach. See Hypochlorous acid. --Jayron32 02:28, 20 April 2012 (UTC)
- Correction: If you add more chlorine, you're just going to get more concentrated bleach. See Hypochlorous acid. 84.209.89.214 (talk) 03:17, 25 April 2012 (UTC)
- Thanks. I have this smell coming from my laundry room, smells like chlorine and bleach but stronger; I'm trying to find out what this is. Albacore (talk) 02:37, 20 April 2012 (UTC)
- Please note that sodium hypochlorite and chlorine are two different chemicals - chlorine gas is an authentic chemical weapon from the bad old days. Hopefully you have not mixed your bleach with chlorine, but that leaves open the question of whether you mixed it with something else, and if so what.
- Also note that Wikipedia cannot give medical advice, in particular, advice about whether your laundry room is dangerous to you or not. Wnt (talk) 18:52, 20 April 2012 (UTC)
- They're different, but they're found in equilibrium in solution, as per Jayron. Mixing bleach with chlorine would just give stronger bleach. The smell from bleach is mostly chlorine gas. Chlorine gas is an irritant, but it's not particularily dangerous at low concentrations in well ventilated areas. See Chlorine bombings in Iraq: most of the deaths came from the blasts, the actual chemical is readily dispersed, and really doesn't make a good weapon. Buddy431 (talk) 18:03, 22 April 2012 (UTC)
- My grandmother sometimes combined chlorine bleach with other products such as Drano to clean toilets or unclog drains, liberating large amounts of chlorine (I do not recommend this). The exact chemical equation is left as an exercise for others. Liquid bleach seems always poised to liberate chlorine, given the slightest provocation. Edison (talk) 03:36, 23 April 2012 (UTC)
- Actually, Drano is a lye-containing (basic) drain cleaner, which should not produce chlorine gas with bleach, but other drain cleaners are indeed acids and could do so. Wnt (talk) 00:35, 24 April 2012 (UTC)
- My grandmother sometimes combined chlorine bleach with other products such as Drano to clean toilets or unclog drains, liberating large amounts of chlorine (I do not recommend this). The exact chemical equation is left as an exercise for others. Liquid bleach seems always poised to liberate chlorine, given the slightest provocation. Edison (talk) 03:36, 23 April 2012 (UTC)
- They're different, but they're found in equilibrium in solution, as per Jayron. Mixing bleach with chlorine would just give stronger bleach. The smell from bleach is mostly chlorine gas. Chlorine gas is an irritant, but it's not particularily dangerous at low concentrations in well ventilated areas. See Chlorine bombings in Iraq: most of the deaths came from the blasts, the actual chemical is readily dispersed, and really doesn't make a good weapon. Buddy431 (talk) 18:03, 22 April 2012 (UTC)
- Thanks. I have this smell coming from my laundry room, smells like chlorine and bleach but stronger; I'm trying to find out what this is. Albacore (talk) 02:37, 20 April 2012 (UTC)
Mechanics problem
I tried this problem and I couldn't get the answer at the back of the book. I use 0.31 as the x in F=kx, but the solution manual uses 1.31. I don't know how they do that. Can anyone explain this to me? Thanks. Problem: http://i41.tinypic.com/i384f6.png --116.71.13.116 (talk) 04:45, 20 April 2012 (UTC)
- The springs are intially stretched 1 foot therefore side rope intial tension = 30 Lb.
- When centre rope pulled down 1 foot, by pythagorus the additional spring stretch is 5^0.5 - 2.
- Therefore final side rope tension is 30 + 30(5^0.5 - 2) = 30(5^0.5 - 1).
- Tension on centre rope is then 2 x 30(5^0.5 - 1) / 5^0.5 = 33.167 Lb.
- Wickwack60.228.237.42 (talk) 05:28, 20 April 2012 (UTC)
- Why would the center rope be pulled down 1 foot? The ropes BA and DA increase in length by 0.3 feet when θ=30 (after solving triangle). So by this, I deduced that the springs would extend by 0.3 feet as well. --119.155.36.194 (talk) 08:11, 20 April 2012 (UTC)
- You are told that the spring is already tensioned by stretching 1 foot so you have to add this to x. I agree the rope is not pulled down one foot, it is pulled down √2 feet, but you don't need to calculate this. Wickwack is also mistaken in calculation of final tension on the rope. The forces form a 60° triangle so the magnitude of the downward force is going to equal the tension in the spring. SpinningSpark 10:28, 20 April 2012 (UTC)
- Why would the center rope be pulled down 1 foot? The ropes BA and DA increase in length by 0.3 feet when θ=30 (after solving triangle). So by this, I deduced that the springs would extend by 0.3 feet as well. --119.155.36.194 (talk) 08:11, 20 April 2012 (UTC)
- Poster 119.155.36.194 has indeed spotted my mistake (I was just seeing if you all are awake, as the saying goes...). The springs get stretched from the initial 1 foot to 1 + (2/sin(30))-2. so the final tension is 30(4/√3 - 1) = 30 x 1.3094... close to the 1.31 that the IP said was in the answer. So it appears that the IP's error was that he/she ignored the initial tension. The tension in the centre rope is twice 30(4/√3 -1) x sin(30), i.e., 30(4/√3 - 1). Spinningspark is correct in saying the tension in the centre rope is equal to that of the springs, but this isn't a 60-60-60 triangle. You don't indeed need to claculate the how much the centre rope is pulled down, but it is 2/√3 Wickwack60.228.237.42 (talk) 10:48, 20 April 2012 (UTC)
- No..., the forces do form an equilateral triangle (if they are all going to be equal they have to). The angle made by the centre of the rope is 120° and the force from the springs must act along them. The weight produces a force straight down so the angle to both ropes must be 120° as well. Translating the forces to a balanced triangle (do they still ue parallelograms in colleges) cannot fail to form an equilateral triangle. SpinningSpark 12:59, 20 April 2012 (UTC)
Ah, yes, I see now that I forgot to consider the 1 foot that the spring was already stretched. Thanks! --119.155.49.170 (talk) 11:02, 20 April 2012 (UTC)
Hail
I came across this sentence in the hail article. Seems to be some sort of vandalism but I can determine what the original would have been; graupel perhaps? I thought folk here would be more likely to know than on the Help desk. "Hail is most frequently formed in the interior of continents within the mid-latitudes of Earth, with hail [sic] generally confined to higher elevations within the tropics."--Shantavira|feed me 12:04, 20 April 2012 (UTC)
- I don't think it is vandalism, it was inserted way back in 2009 with this edit. What exactly do you think is wrong with it? SpinningSpark 12:34, 20 April 2012 (UTC)
- I suspect that it's the second use of 'hail', which is in my view a little clumsy - it could be rewritten as "Hail is most frequently formed in the interior of continents within the mid-latitudes of Earth, but is generally confined to higher elevations within the tropics." Mikenorton (talk) 12:38, 20 April 2012 (UTC)
- That's still pretty unclear. I'd hate to be answering an exam question based on that. I reckon it could be interpreted in several ways. Not sure what the "but" is really doing there. HiLo48 (talk) 12:43, 20 April 2012 (UTC)
- Actually 'mid-latitudes' is a problem, I was interpreting that to be anywhere from about 60°N to 60°S, so the reference to the tropics being confined to higher altitudes would be a special case, hence the 'but'. If however it means the zone from 60° to 30° N or S as in our article, then it should be 'and' rather than 'but' - my mistake I think. In which case "Hail is most frequently formed in the interior of continents within the mid-latitudes of Earth and within the tropics, where it is generally confined to higher elevations." Any clearer? Mikenorton (talk) 13:00, 20 April 2012 (UTC)
- I don't see how anyone can say that hail is "generally confined to higher elevations within the tropics". We've just had hail showers on two consecutive days in Birmingham (UK), which is a long way from any tropics.--Shantavira|feed me 13:05, 20 April 2012 (UTC)
- Try this: "In the mid-latitudes, hail forms near the interiors of continents, while in the tropics, it tends to be confined to high elevations." Does that work? Better use of parallel construction helps aid comprehension. --Jayron32 13:08, 20 April 2012 (UTC)
- I was about to say something similar myself, so instead I have boldly pu it in the article. SpinningSpark 13:12, 20 April 2012 (UTC)
- I'm with Shantavira and still a bit puzzled; here in London, there was a light hail shower about 10 minutes ago. The wind direction is South West[1], which means that it's coming from the Atlantic, not the "interiors of continents". So demonstrably untrue I'd say, even if it is OR. Alansplodge (talk) 17:32, 20 April 2012 (UTC)
- I think that the key phrase in the original text was 'most frequently', not excluding the type of weather we're experiencing in our temperate maritime climate at the moment - unfortunately the source that backs up this statement in the article is currently a deadlink. Mikenorton (talk) 17:42, 20 April 2012 (UTC)
- Ok, "most frequently" helps. Alansplodge (talk) 19:46, 20 April 2012 (UTC)
- I think that the key phrase in the original text was 'most frequently', not excluding the type of weather we're experiencing in our temperate maritime climate at the moment - unfortunately the source that backs up this statement in the article is currently a deadlink. Mikenorton (talk) 17:42, 20 April 2012 (UTC)
- I'm with Shantavira and still a bit puzzled; here in London, there was a light hail shower about 10 minutes ago. The wind direction is South West[1], which means that it's coming from the Atlantic, not the "interiors of continents". So demonstrably untrue I'd say, even if it is OR. Alansplodge (talk) 17:32, 20 April 2012 (UTC)
- I was about to say something similar myself, so instead I have boldly pu it in the article. SpinningSpark 13:12, 20 April 2012 (UTC)
- Try this: "In the mid-latitudes, hail forms near the interiors of continents, while in the tropics, it tends to be confined to high elevations." Does that work? Better use of parallel construction helps aid comprehension. --Jayron32 13:08, 20 April 2012 (UTC)
- I don't see how anyone can say that hail is "generally confined to higher elevations within the tropics". We've just had hail showers on two consecutive days in Birmingham (UK), which is a long way from any tropics.--Shantavira|feed me 13:05, 20 April 2012 (UTC)
- Actually 'mid-latitudes' is a problem, I was interpreting that to be anywhere from about 60°N to 60°S, so the reference to the tropics being confined to higher altitudes would be a special case, hence the 'but'. If however it means the zone from 60° to 30° N or S as in our article, then it should be 'and' rather than 'but' - my mistake I think. In which case "Hail is most frequently formed in the interior of continents within the mid-latitudes of Earth and within the tropics, where it is generally confined to higher elevations." Any clearer? Mikenorton (talk) 13:00, 20 April 2012 (UTC)
- That's still pretty unclear. I'd hate to be answering an exam question based on that. I reckon it could be interpreted in several ways. Not sure what the "but" is really doing there. HiLo48 (talk) 12:43, 20 April 2012 (UTC)
- I suspect that it's the second use of 'hail', which is in my view a little clumsy - it could be rewritten as "Hail is most frequently formed in the interior of continents within the mid-latitudes of Earth, but is generally confined to higher elevations within the tropics." Mikenorton (talk) 12:38, 20 April 2012 (UTC)
As a meteorologist, I believe I can clear this up: The sentence is trying to say that "Hail is most frequent towards the center of mid-latitude continents, and at high elevations in the tropics." However I do not believe this is correct, and I will look to improve the wording of the article. I suspect (and will look for references to back up) that smaller hail (0.25 inches (0.64 cm) to 1 inch (2.5 cm) diameter) is most common in the mid-latitudes regardless of proximity to water. However, very large hail (2 inches (5.1 cm) or greater diameter) occurs almost exclusively over Bangladesh/eastern India and in the Central United States, which are (not coincidently) the same areas where the most violent tornadoes typically form. This link has a good US map of very large hail occurrences. -RunningOnBrains(talk) 20:02, 20 April 2012 (UTC)
- Thanks RunningOnBrains, it could certainly do with some help. I'm in Melbourne, Australia, latitude around 38⁰, and at sea level. In any year we get several instances of small hail, and occasional events with golfball size. HiLo48 (talk) 21:30, 20 April 2012 (UTC)
- South Africa also gets large haistones as can be seen in this video. It must happen frequently enough for there to be specialist hail damage repair businesses for cars. SpinningSpark 22:27, 20 April 2012 (UTC)
- , Yes, RunningOnBrains needs to check his stats. In Perth, Western Australia, a long way from USA or India, we occaisonally get hail over 2 cm, sometimes well over 2 cm. You sometimes see cars with small dents all over them, because they were left outside when a large hail hailstorm occurred and the owners hadn't got them repaired. My house (as with hundreds of others) was damaged last year from hail about 10 to 15 cm. Keit121.215.149.247 (talk) 00:57, 21 April 2012 (UTC)
- Note that I said 2 inches, not centimeters. 2 cm hail is fairly common. And I'll agree, maybe I should say 2+ inch (5+cm) hail is extremely uncommon outside of these areas, but not unheard of. Unfortunately there is no complete worldwide climatology, but I've found various sources for various areas. Southwest Germany had only 9 such events from 1900–2009, and New South Wales has had a few such events, most notably the 1947 and 1999 Sydney hailstorms. But some areas in the central US can expect hail of such sizes at any given location once every year or two.-RunningOnBrains(talk) 01:13, 21 April 2012 (UTC)
regrading r-e model of a BJT
In the input portion of a simple r-e model of a transistor, there is essentially a diode. During AC analysis, an AC signal source, if considered, will alternate the direction of current flow. This means that current will flow in one direction for one half cycle and in another for the second half cycle. But a diode will allow current flow in one direction only. So how does an AC signal work with an r-e model? — Preceding unsigned comment added by 210.4.65.52 (talk) 12:51, 20 April 2012 (UTC)
- What do you mean by r-e model? It does not seem to be this which is what I would have assumed if you had not mentioned diodes. Transistors in amplifler circuits are usually modelled with linear components only. SpinningSpark 13:06, 20 April 2012 (UTC)
- The IP does mean the re model this. The reason why the base emittor diode is not considered to be rectifying, is because this is a small signal model - the DC bias is of far larger magnitude than the AC signal and thus can hold the base-emittor diode biased on at all times. The signal will of course modulate the input resistance (modelled as re x beta) to a degree - the model assumes this modulation is negligible. This question is essentially a re-written version of the IP's last question about AC signal & DC bias in the same BJT circuit. The IP needs to go back and study basic loop & mesh circuits, & superposition of voltages & currents until he thoroughly understands them, as I previously recommended. Keit58.169.237.250 (talk) 14:21, 20 April 2012 (UTC)
Environmental effects of a plastic bag ban
Last time I asked this, my question was not answered properly so I'll try to clarify some things first. The question remains: What are the possible long-term positive and negative effects of a plastic bag ban? This time, I'll clarify what my definition of a "plastic bag" ban is, partially based on the ban currently in place in my hometown. In a "plastic bag ban", at first, the use of plastic bags and styrofoam will be prohibited for dry goods, and instead people will be encouraged to bring their own bags, in addition to the use of paper bags. A total ban on plastic bags (and presumably styrofoams) for wet goods follows after 6 months. So my question now is, what will be the long-term environmental effects of such a ban? In the long term, will it be for the better or for the worse? Obviously, it will encourage the use of more environmentally-friendly containers, but I read somewhere that more paper bags means more trees are cut, and more water is used, which would in the long-term actually harm the environment. Is this true? Narutolovehinata5 tccsdnew 14:28, 20 April 2012 (UTC)
- Paper is heavier than plastic, so there would also be a significant increase in carbon emissions via transportation of bags. That is, it takes more trucks to move the same amount of bags, which is a significant environmental disadvantage to using paper. The "cutting down of trees" is usually a red herring as paper production depends on tree farming, that is companies that own large tracts of land have a strong financial incentive to not just clear trees and leave barren land; it devalues the land. Instead, land owners who are involved in growing trees for lumber and paper production want to maintain the value of their land, no less so than any other farmer, and thus will plant trees to continue to keep the land productive. In simpler terms, just as a cabbage farmer doesn't grow a single crop of cabbage and leave his land fallow for eternity after that, a tree farmer doesn't just produce one crop of trees and leave the land fallow forever. In terms of number of trees, paper production in a properly managed economy will be roughly "tree neutral". --Jayron32 14:51, 20 April 2012 (UTC)
- Except that increased use of paper bags mean more land has to be put into wood pulp production reducing the amount of natural forest or food crop farmland. Rmhermen (talk) 15:13, 20 April 2012 (UTC)
- From http://www.dcnr.state.pa.us/forestry/farmbill/pdfs/assessment.pdf : "The certified public forest base provides a sound foundation for certified product production and marketing; without it, Pennsylvania’s solid wood products would be only a niche market and certified paper production would likely become infeasible." And if you've ever seen what these orcs do to your favorite wilderness getaway, the lack of degradation of plastic bags in a landfill no longer seems such a high priority. Wnt (talk) 16:32, 20 April 2012 (UTC)
- I live in Taiwan and it's been probably a decade since the island-wide ban of free plastic bags. If you want one, pay about US$0.07 for it. Since probably also ten years ago, the county government decided to get rid of ALL STREET-SIDE TRASH CANS so people are forced to bring their garbage home or reduce their garbage productions. In my county, the per-bag garbage collection fees are in their 4th year, I guess. These draconian rules really work well. I have to say.
- My city enforces a US$0.05 charge per plastic bag, but that's not what OP is talking about at all. Philippine is banning all plastic bags, not just free plastic bags. It's a ~$200 USD fine for the first offense, not just a couple of cents. 142.150.237.18 (talk) 18:34, 20 April 2012 (UTC)
- I live in Taiwan and it's been probably a decade since the island-wide ban of free plastic bags. If you want one, pay about US$0.07 for it. Since probably also ten years ago, the county government decided to get rid of ALL STREET-SIDE TRASH CANS so people are forced to bring their garbage home or reduce their garbage productions. In my county, the per-bag garbage collection fees are in their 4th year, I guess. These draconian rules really work well. I have to say.
- From http://www.dcnr.state.pa.us/forestry/farmbill/pdfs/assessment.pdf : "The certified public forest base provides a sound foundation for certified product production and marketing; without it, Pennsylvania’s solid wood products would be only a niche market and certified paper production would likely become infeasible." And if you've ever seen what these orcs do to your favorite wilderness getaway, the lack of degradation of plastic bags in a landfill no longer seems such a high priority. Wnt (talk) 16:32, 20 April 2012 (UTC)
- Except that increased use of paper bags mean more land has to be put into wood pulp production reducing the amount of natural forest or food crop farmland. Rmhermen (talk) 15:13, 20 April 2012 (UTC)
- Long before the plastic bag ban, I carry my back pack almost every day. I put everything in my XXL back pack each time I go shopping for food. This is not the end of the world. As a result, the plastic bag ban does not change my lifestyle too much. Anyway, most of the people now carry their bags. Paper bags are supposedly expensive here. We have to import paper from Scandinavian countries. -- Toytoy (talk) 18:02, 20 April 2012 (UTC)
- I'm amazed that eliminating trash cans would work. In the U.S. it is a challenge to get people to not litter even if a trash can is ten steps away... Wnt (talk) 18:11, 20 April 2012 (UTC)
- The question presupposes that the only options are disposable plastic bags and disposable paper bags. As Toytoy mentions, there are other options. Back in my undergrad days, I always took a backpack to the grocery store—not to save the planet, but because it was the only way I had to carry stuff on my bicycle. Today I live in a jurisdiction that charges for plastic bags, so I bring reusable bags with me when I go shopping. (I also have a couple of compact, lightweight bags that I can stuff in my pocket or bag when I'm out and about, so I'm never without some sort of bag.) I note that the reusable bags generally have much more comfortable handles than the disposable plastic bags (and may also include shoulder loops as well as regular handles), which makes the walk home from the store much more pleasant. TenOfAllTrades(talk) 18:51, 20 April 2012 (UTC)
- Backpacks ARE a good alternative. What's not good are those other seemingly woven bags sold by supermarkets for repeat usage. I don't believe they're made from natural fibres anyway. They wear out and get thrown away too straight to landfill. And we have to pay for them! In my household the older style plastic bags always have at least one more life, if only for cleaning up dog poo, although I note that supermarkets are happy to sell me plastic bags for that purpose too. I'm very cynical about all these "improvements". HiLo48 (talk) 21:23, 20 April 2012 (UTC)
- The other issue is that the plastic bags should go straight back to a recycling bin at the market, so it's really just an issue that people spend the energy to remelt and recast them for aesthetics and ease of dispensing. Wnt (talk) 02:14, 21 April 2012 (UTC)
- Backpacks ARE a good alternative. What's not good are those other seemingly woven bags sold by supermarkets for repeat usage. I don't believe they're made from natural fibres anyway. They wear out and get thrown away too straight to landfill. And we have to pay for them! In my household the older style plastic bags always have at least one more life, if only for cleaning up dog poo, although I note that supermarkets are happy to sell me plastic bags for that purpose too. I'm very cynical about all these "improvements". HiLo48 (talk) 21:23, 20 April 2012 (UTC)
How many people alive today were born before the year 1900?
If we don't look at official birth records, but instead estimate this from statistics about population sizes and life expectancies of all the countries of the World, what is the approximate figure for this number? Also, what is the probability for zero people being alive who were born before 1900, as a function of time? So, e.g. when can we be 99% sure that no one is alive who was born before the year 1900? Count Iblis (talk) 17:28, 20 April 2012 (UTC)
- For your second question, Wikipedia has decided that someone is likely to have died by their 115th birthday (see WP:BDP). Therefore, under this assumption, 2015 would be the answer. We also have the list of Oldest people, which shows that no-one has ever (verifiably) lived past 123. Therefore, 2023 is another potential answer. - Cucumber Mike (talk) 18:00, 20 April 2012 (UTC)
- If you don't mind stats on people born in or before 1902, SUPERCENTENARIANS is the website for you. Apparently there are 67 people known, who can be proved to be 110 or older. The distribution map shows that they all seem to live in North America, Europe or Japan. Alansplodge (talk) 20:00, 20 April 2012 (UTC)
- Note: This may be a record-keeping-related bias: I'm sure that reliable birth records are not available outside the west prior to the mid-20th century, and in some parts of Africa and southeast Asia are likely still not available. -RunningOnBrains(talk) 20:07, 20 April 2012 (UTC)
- According to our List of living supercentenarians, there are currently 25 living people verified as having been born before 1900. If the youngest of them, Grace Jones (no, not that one), were to live as long as the oldest ever verified person, Jeanne Calment, she would, by my reckoning, die on 20 May 2022. Ghmyrtle (talk) 20:19, 20 April 2012 (UTC)
If there are a few dozen such people in the US, Europe and Japan, could there be a few hundred in total? Count Iblis (talk) 15:13, 21 April 2012 (UTC)
Inside of a hen it's too ... whatever ... to breathe
- the egg was incubated in the hen for 21 days and then hatched inside the hen.
Now, how does the egg get its own air inside the hen's body? -- Toytoy (talk) 17:48, 20 April 2012 (UTC)
- Unfortunately, we don't know where exactly it stopped - the answer could be something trivial, like one end exposed to the air. Also, remember the oxygen requirements of eggs are relatively small; indeed, some birds even bury their eggs. [2] The episode just goes to show how evolutionarily labile live birth really is - though archaic textbooks made it sound like it was some kind of advance toward humanity, really, going back to live birth is more of a degeneration that can readily take hold in almost any lineage. Wnt (talk) 18:09, 20 April 2012 (UTC)
- There is a big difference between ovoviviparity, which is what unintentionally happened here, and live births in placental mammals. The placenta is the result of a long line of evolutionary advances. Ovoviviparity is a relatively simple step from the regular laying of eggs (and was, I think, one of the first steps towards the placenta). --Tango (talk) 13:18, 21 April 2012 (UTC)
- True. Wnt (talk) 23:31, 23 April 2012 (UTC)
- There is a big difference between ovoviviparity, which is what unintentionally happened here, and live births in placental mammals. The placenta is the result of a long line of evolutionary advances. Ovoviviparity is a relatively simple step from the regular laying of eggs (and was, I think, one of the first steps towards the placenta). --Tango (talk) 13:18, 21 April 2012 (UTC)
Does water ice get colder or is it temperature static?
I've been wondering about the coldness of water ice. Of course I know it freezes at 32°F/0°C but can it get colder than that or is it stuck at that temperature? For example, could you make ice much colder, maybe by letting it sit in some liquid nitrogen, and then because it was much colder, you could pour hot coffee over it and only a little would melt while cooling it down quickly, so you wouldn't get watery ice coffee (as they do at my local bodega when you order ice coffee (yuck).--108.54.17.230 (talk) 22:34, 20 April 2012 (UTC)
- Yes, you can make ice as cold as your equipment allows. However, if it's too cold, it make actually freeze the coffee, and also becomes dangerous to handle. StuRat (talk) 22:40, 20 April 2012 (UTC)
- A mixture of ice and water will, after a time, equilibrate at 0C. There are conditions where you can supercool liquid water. See supercooling. --Jayron32 22:53, 20 April 2012 (UTC)
- That would depend on the mixture, and the temperature of the surrounding environment. StuRat (talk) 00:06, 21 April 2012 (UTC)
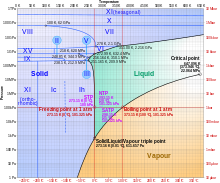
- You can make ice as cold as your freezer will get, but the cooling it produces does not change as much as you might think. The latent heat of melting for water is high -- if I have my numbers straight, it takes as much energy to change a quantity of water from 0°C (frozen) to 0°C (liquid) as it does to raise the temperature of the same quantity of water by 90°C without changing its state. Looie496 (talk) 23:00, 20 April 2012 (UTC)
- You can cool ice to any arbitrary temperature above absolute zero. However, at very low temperatures, or very high pressures there are a number of further phase (matter) changes. SpinningSpark 00:28, 21 April 2012 (UTC)
- At reasonable pressures, this is how it works: At temperatures not too much lower than 0 C, the specific heat of ice is approx 2.1 Joules per gram per centigrade. In other words, from 0 C (freeze point) for every 2.1 J /g heat you remove from ice by refigeration, you lower it's temperature by 1 degree C. You can, if you have good enough refrigeration keep on doing this until you get to absolute zero, -273 C, and by then you will have removed 5.2 kJ/mol. However, as you get it colder and colder, the specific heat reduces, tending to zero at absolute zero. Kiet121.215.149.247 (talk) 00:40, 21 April 2012 (UTC)
- If you've got liquid nitrogen, why not cool the coffee directly with that? Then you won't dilute it at all. --Trovatore (talk) 00:45, 21 April 2012 (UTC)
- Because you'd shatter the cup. Also, you may want continuous cooling, so you have iced coffee for an hour, rather than frozen coffee at first, and room temp coffee later. StuRat (talk) 00:49, 21 April 2012 (UTC)
- Bubble it through the coffee before you put it in the cup. Then you can add ice if you want for later cooling, but the dilution effect will be much less because the coffee is already cold. --Trovatore (talk) 00:55, 21 April 2012 (UTC)
- Because you'd shatter the cup. Also, you may want continuous cooling, so you have iced coffee for an hour, rather than frozen coffee at first, and room temp coffee later. StuRat (talk) 00:49, 21 April 2012 (UTC)
I think the question asks if you can have ice and water co-existing at temperatures different from 0°C. Of course, you can consider water not in thermal equilibrium with the ice or even supercooled water. But when they are in thermal equilibrium with each other, then you can change the temperature of the ice-water mixture by changing the pressure. Count Iblis (talk) 01:36, 21 April 2012 (UTC)
- This would be moving along the melt line in the pressure-temperature phase diagram. However, this is of no practical significance, as the temperature change over a pressure range of from the triple point (612 Pa) to 10 Mpa (ie from 0.006 atmospheres to ~100 atmospheres) the temperature change is only a fraction of a degree. Keit60.230.226.13 (talk) 03:02, 21 April 2012 (UTC)
- Still, note that triple point with ice and ice III at 251 K - there's a narrow range of high pressures where pure water is liquid at under -20 C. This might be relevant for finding deep oceans in places like Enceladus (moon), though of course dissolved salts may affect this even more. Wnt (talk) 19:41, 21 April 2012 (UTC)
How can Mars acquire to more substantial atmosphere over sun's brightening
This article said when Mars gets warmed up by sun's brightening by 10 to 20 percent it can actually create a greenhouse effect which creates a more substantial atmosphere. That is quite weird because Mars have lost so much atmosphere over billion years by solar wind, I don't know how can it get its atmosphere back. I heard is because of frozen gases beneath the soil and at the poles is acts like a thick ice sheet. Mars don't have magnetic field or magnetosphere, then what makes Mars get its atmosphere back? --69.228.132.91 (talk) 23:35, 20 April 2012 (UTC)
- I can see how warming would temporarily increase the thickness of Mar's atmosphere, but I agree that, over the long term, the solar wind would blow it away in the absence of a strong magnetic field. However, that "long term" might be millions of years, so not something we humans need be much concerned with. StuRat (talk) 23:43, 20 April 2012 (UTC)
- Indeed, if you converted all the frozen CO2 in the Martian ice caps to a gas, you would achieve a significant fraction of Earth's atmospheric pressure (I'm away from my work papers so I couldn't tell you how much, but I'd conservatively say at least 10% of Earth's atmospheric pressure, up from its current 0.6%). This is not counting the suspected underground reserves of CO2 and water vapor. The loss of atmosphere would occur due to thermal escape, but I believe StuRat is correct to say that it would occur over a process of millions to tens of millions of years. This leaves more than enough time for a sufficiently advanced civilization to import atmosphere from other sources, like comets and such. -RunningOnBrains(talk) 02:09, 21 April 2012 (UTC)
- Much of Mars's former atmosphere (and hydrosphere) is still contained in its rocks and soil, not as separate ices, but chemically combined with other elements to form solid minerals, such as carbonates and oxides. These combinatory processes occur all the time on Earth, but Earth's active tectonics cause the minerals to be subducted and decomposed, and the gasses are recycled back into our atmosphere via volcanic processes.
- Because Mars is smaller than Earth, it (supposedly) cooled quicker and its tectonics (if it ever had any) and volcanism (which it most certainly had), shut down a few billion years ago, so the gasses remain locked up. If one could release enough of them to form an atmosphere comparable in density (if not composition) to Earth's, it has been estimated – by, for example, Martyn J. Fogg – that it would last for at least tens of thousands of years before requiring further renewal.
- Fogg has suggested using thermonuclear devices buried in major carbonate deposits to achieve this ("in one go", to quote the film), with the drawback that their installation would be difficult to achieve before significant colonisation, but their use disruptive to existing colonists. Another suggestion is to steer a sizeable comet so as to impact the planet and contribute (much of) its frozen gasses and liquids: again, one would not want to be on the planet when this occurred. {The poster formerly known as 87.81.230.195} 90.197.66.64 (talk) 08:35, 21 April 2012 (UTC)
Europa's atmosphere
how can Europa, Jupiter's moon get an atmosphere? It have a weak scale heights which isn't even sufficient. Does Jupiter have a magnetic fields or is it the volatiles can hang on to keep Europa an atmosphere.--69.228.132.91 (talk) 23:35, 20 April 2012 (UTC)
- I broke this question off as it's own section. StuRat (talk) 23:40, 20 April 2012 (UTC)
- Have you read Europa_(moon)#Atmosphere? SmartSE (talk) 00:12, 21 April 2012 (UTC)
- Because all solids have a vapour pressure, meaning that they sublime and reverse-sublime into an equilibrium state; and gravity limits the rate at which a tenuous atmosphere can escape; gravity captures solar wind and any other interplanetary gasses permeating space; tidal forces experienced by the moon increases the production of vapours. Every astronomical body has an atmosphere, however thin. Mercury has an atmosphere of sodium vapour. Plasmic Physics (talk) 06:04, 21 April 2012 (UTC)
- Planets are always losing or gaining atmosphere from external sources, this produces a net effect. If the vacuum around the planet is high enough, the planet may eventually evaporate away into a diffuse cloud, which may take longer than the age of the universe. If the vaccuum is low enough, the planet may grow. This does not take into account contributions from collisions with foreign bodies. Gravity does not stop the evaporation of a planet, it can only alter its rate. This is the same process by which a black hole evaporates, only difference is that the atmosphere of a black hole is composed of Hawking radiation. Plasmic Physics (talk) 06:22, 21 April 2012 (UTC)
- To answer the original question: Europa can obtain an atmosphere by gravitationally collecting volatiles evaporating from Jupiter, the other moons, from solar wind, and other volatiles permiating space. Plasmic Physics (talk) 06:28, 21 April 2012 (UTC)
- In the heat death scenario of the universe, it is expected that eventually everything will diffuse completely into a ever thining cloud of something with very high entropy. Plasmic Physics (talk) 06:32, 21 April 2012 (UTC)
- Solar wind also has the potential to remove atmosphere. Plasmic Physics (talk) 09:15, 21 April 2012 (UTC)
- Have you read Europa_(moon)#Atmosphere? SmartSE (talk) 00:12, 21 April 2012 (UTC)
Why Earth dies Mars survives
Dr. Smith's calculation shows when sun hits RGB, once it reaches Earth's orbit at 1 AU, when sun loses one-fifth of its mass, then Earth's orbit gets sucked it to sun. My astronomy teacher told me once sun passes Earth's orbit, then Earth is gone completely. if Earth gets sucked in once sun crosses Earth's orbit, how can Mars and the outer planets escape to higher orbit. Where will Mars and the outer planets expand to a higher orbit, at the tip of RGB, or when sun expands at gradual degree between 5 and 7.5 billion years Mars and outer planets will escape. And about astroengineer to nudge Earth's orbit so it may be saved, does nudging Earth's orbit by flying asteroids work for Earth only, or it can also work with Mercury and Venus along with it together. If we move Earth orbit further by astroengineer asteroid nudging can we also move Mercury and Venus along with it together?--69.228.132.91 (talk) 23:58, 20 April 2012 (UTC)
- Formation and evolution of the Solar System has information about the future of the solar system. Speculation about what fantastical astroengineering magic may save the earth is outside of the scope of this Reference desk. --Jayron32 00:22, 21 April 2012 (UTC)
- Oh, just because the technology is unknown doesn't mean we can't say anything scientific about it. Consider that the potential energy is -GMm/r, where G is 6.67E-11 m3kg-1s-2, M is the mass of the Sun (1.99E30 kg) and m is the mass of the Earth (5.98E24 kg) and r is 1.50E11 m at Earth orbit, 2.28E11 m at Mars orbit. So the difference in potential energy is (79.4E43 kg m3s-2)(1/1.50E11 m - 1/2.28E11 m = 0.228E-11m-1) = 18.1E32 kg m2s-2 or 1.81 x 1033 joules. This is about 4 trillion times the Annual global energy consumption of 0.5 x 1021 joules, which for today does seem beyond the reach of humanity, except perhaps in its government deficits. Still, given a significant fraction of a billion years, with an energy budget that might be thousands if not millions of times larger? Only the future can tell. Wnt (talk) 02:09, 21 April 2012 (UTC) Note: I have been thoroughly leeted by Gr8xoz below, who points out half this energy comes from the orbital speed and much of the energy could be stolen from some other planet (Jupiter) with clever orbital mechanics. Wnt (talk) 19:19, 21 April 2012 (UTC)
- Who's to say that anything resembling a human will even exist. That's a long time for evolution to just quit working... --Jayron32 03:25, 21 April 2012 (UTC)
- My guess would be the penal system. Life means life, you know. Wnt (talk) 15:12, 21 April 2012 (UTC)
- I can't see why anyone would want to keep Earth out of the Sun when by that time we probably will have hundreds of thousands of self-sufficient space stations with artificial gravity and asteroid fragment hull shielding, will have colonized the entire galaxy, and maybe even other galaxies. Mars and multiple moons of Jupiter and Saturn will likely be terraformed by then, too. 71.212.237.20 (talk) 03:45, 21 April 2012 (UTC)
- Sentimental value? 203.27.72.5 (talk) 06:36, 21 April 2012 (UTC)
- Conservation Areas?--Shantavira|feed me 08:40, 21 April 2012 (UTC)
- This is such a long time into the future that there will probably be no-one who even remembers or wants to conserve the origin of humans from time immemorial, but, if "we" (our descendants) are still in existence and remember our origin, then technology might have advanced so far that we could encase the Earth in a radiation and meteor shield, provide a temporary artificial energy source, and move it to a new sun that would be stable for millions of years more. Dbfirs 13:25, 21 April 2012 (UTC)
- Conservation Areas?--Shantavira|feed me 08:40, 21 April 2012 (UTC)
- Sentimental value? 203.27.72.5 (talk) 06:36, 21 April 2012 (UTC)
- Actually it is a loot easier to move the Earth than the potential energy difference indicates. (Given that you have a few million of our current years available.) Half the energy comes from the decreases of orbital speed but even 1.81 x 1033/2 joules is rather much energy. In order to do this we will need to use different mechanisms that amplifies our actions. Let say we put 16 Psyche in a cycler orbit between Earth and Jupiter so that it exchanges momentum between Earth and Jupiter. Psyche is about 4 x 10-6 earth masses so it needs in the order of magnitude about 250 000 orbits to change the orbit of the Earth a significant part of the distance to Jupiter. Each orbit takes about 10 years so the operation will take a few million years, given that the expansion of the Sun are process over billions (1e9) of years this is very fast. The next question is how to move Psyche in to the cycler orbit, the energy needed are on the the scale of 1.81 x 1033 x 4 x 10-6 Joules, around 1 x 10 28 Joules. This is still to much to just use ordinary rockets but we can move other smaller objects in to colliding orbits and in order to do that we can use even smaller objects.
- It seems that the way to do this depends more on good telescopes and computers than on massive rockets. If we know about more objects to chose from and can plan the collisions for longer times then we can use smaller rockets. To me it seems like we could build the equipment needed to get started within our lifetime.
- I see no reason the same could not be done with the other planets in the inner solar system.
- According to Stability_of_the_Solar_System#Laskar & Gastineau it is not clear that dramatic changes in the orbit of Earth will not happen without intervention. The development of the solar system seems to bee very sensitive, they tried 2500 simulations that each differed by a meter in the initial position of Mercury. In one of them Mars collided with Earth. Gr8xoz (talk) 16:25, 21 April 2012 (UTC)
- Who's to say that anything resembling a human will even exist. That's a long time for evolution to just quit working... --Jayron32 03:25, 21 April 2012 (UTC)
- Oh, just because the technology is unknown doesn't mean we can't say anything scientific about it. Consider that the potential energy is -GMm/r, where G is 6.67E-11 m3kg-1s-2, M is the mass of the Sun (1.99E30 kg) and m is the mass of the Earth (5.98E24 kg) and r is 1.50E11 m at Earth orbit, 2.28E11 m at Mars orbit. So the difference in potential energy is (79.4E43 kg m3s-2)(1/1.50E11 m - 1/2.28E11 m = 0.228E-11m-1) = 18.1E32 kg m2s-2 or 1.81 x 1033 joules. This is about 4 trillion times the Annual global energy consumption of 0.5 x 1021 joules, which for today does seem beyond the reach of humanity, except perhaps in its government deficits. Still, given a significant fraction of a billion years, with an energy budget that might be thousands if not millions of times larger? Only the future can tell. Wnt (talk) 02:09, 21 April 2012 (UTC) Note: I have been thoroughly leeted by Gr8xoz below, who points out half this energy comes from the orbital speed and much of the energy could be stolen from some other planet (Jupiter) with clever orbital mechanics. Wnt (talk) 19:19, 21 April 2012 (UTC)
April 21
Is US or UK ahead in commercial transportation fuel synthesis from CO2?
Of http://dotyenergy.com in the US and http://airfuelsynthesis.com in the UK, which company is closest to mass production of carbon neutral transportation fuel from carbon dioxide? Which has the most capitalization? What other companies are working in this field? Is http://rentechinc.com/pdu.php? 71.212.237.20 (talk) 00:46, 21 April 2012 (UTC)
- Why care? To turn CO2 into fuel, you have to split it: 2CO2 → 2 x CO and O2, or co2 → C and O2. To do that, you have to add the heat of formation. That doesn't necesarily mean you have to raise it to a high temperature - but the energy must be supplied in the process somehow. When you burn/consume fuel, you get the heat of formation back. So, to turn CO2 into fuel, you need an energy source (i.e., fuel to be consumed) equal to the energy you get out of the fuel you make. That is pointless. Ratbone60.230.208.219 (talk) 11:41, 21 April 2012 (UTC)
- It's useless as a source of energy, but quite useful as a kind of battery of energy. 88.9.107.123 (talk) 14:47, 21 April 2012 (UTC)
- The point is that if the energy comes from wind, hydropower, or solar, then it's carbon neutral. 71.212.237.20 (talk) 17:22, 21 April 2012 (UTC)
- If wind or hydro, yes, but the ouput is electrical, so just distribute it on the grid. Solar is NOT carbon nuetral if photovoltaic, nothing like it. To make the panels takes considerable energy. Solar power via heating a fluid that spins a turbine is theoretically better, but it seems that nobody has come up with a reliable maintenance free and efficent device yet. Ratbone121.215.58.238 (talk) 01:08, 22 April 2012 (UTC)
- If solar panels (or wind turbines, or hydroelectric stations) did not produce more energy than it takes to manufacture and install them, nobody would purchase them. 71.212.237.20 (talk) 03:22, 22 April 2012 (UTC)
- Well, until heavy government subsidies, here in Australia at any rate, only nutters and remote area folks who had no alternative, did buy solar panels. The energy required to make the panels is why they are so expensive. Wind power and hydro is totally different. Hydro has been mainstrean power in favourable areas since the elctricity industry started. Ratbone121.215.58.238 (talk) 03:42, 22 April 2012 (UTC)
- Manufacturing solar cells is very energy intensive due to the zone refining that is required. I wonder if you could set up a zone refining solar cell factory in the desert where you have plenty of sand for raw materials and use an array of mirrors to heat the ingot... 203.27.72.5 (talk) 07:21, 22 April 2012 (UTC)
- There's a hole in the bucket, dear Liza, dear Liza, there's a hole....http://www.youtube.com/watch?v=ElLpKewnxp4&feature=related Ratbone58.170.172.202 (talk) 09:57, 22 April 2012 (UTC)
- Solar cells pay for themselves in 4 to 10 years. Wind turbines pay for themselves in 6 to 8 months. I don't know what the figure is for hydroelectric plants. Again, the point is that any renewable source producing transportation fuel will make it carbon neutral. Using the same CO2 feedstock processes for plastic lumber represents an essentially permanent economical means of carbon sequestration which has the advantage of reducing deforestation. 71.212.237.20 (talk) 19:03, 22 April 2012 (UTC)
- The only way you can get anything like a payback period as low as 4 to 10 years is either a)solar power is subsidised by the Govt, b)it will be used in a remote area, or c)you are in a western country and purchasing cheap Chinese-made solar panels made with their low cost electricity sourced from their coal fired power stations using coal mined with their low cost labour. Having the coal burnt in a counry not your own is NOT environmentally responsible. Ratbone60.230.215.57 (talk) 01:30, 23 April 2012 (UTC)
- Solar cells pay for themselves in 4 to 10 years. Wind turbines pay for themselves in 6 to 8 months. I don't know what the figure is for hydroelectric plants. Again, the point is that any renewable source producing transportation fuel will make it carbon neutral. Using the same CO2 feedstock processes for plastic lumber represents an essentially permanent economical means of carbon sequestration which has the advantage of reducing deforestation. 71.212.237.20 (talk) 19:03, 22 April 2012 (UTC)
- There's a hole in the bucket, dear Liza, dear Liza, there's a hole....http://www.youtube.com/watch?v=ElLpKewnxp4&feature=related Ratbone58.170.172.202 (talk) 09:57, 22 April 2012 (UTC)
- Manufacturing solar cells is very energy intensive due to the zone refining that is required. I wonder if you could set up a zone refining solar cell factory in the desert where you have plenty of sand for raw materials and use an array of mirrors to heat the ingot... 203.27.72.5 (talk) 07:21, 22 April 2012 (UTC)
- Well, until heavy government subsidies, here in Australia at any rate, only nutters and remote area folks who had no alternative, did buy solar panels. The energy required to make the panels is why they are so expensive. Wind power and hydro is totally different. Hydro has been mainstrean power in favourable areas since the elctricity industry started. Ratbone121.215.58.238 (talk) 03:42, 22 April 2012 (UTC)
- If solar panels (or wind turbines, or hydroelectric stations) did not produce more energy than it takes to manufacture and install them, nobody would purchase them. 71.212.237.20 (talk) 03:22, 22 April 2012 (UTC)
- If wind or hydro, yes, but the ouput is electrical, so just distribute it on the grid. Solar is NOT carbon nuetral if photovoltaic, nothing like it. To make the panels takes considerable energy. Solar power via heating a fluid that spins a turbine is theoretically better, but it seems that nobody has come up with a reliable maintenance free and efficent device yet. Ratbone121.215.58.238 (talk) 01:08, 22 April 2012 (UTC)
- The point is that if the energy comes from wind, hydropower, or solar, then it's carbon neutral. 71.212.237.20 (talk) 17:22, 21 April 2012 (UTC)
- It's not quite what you're asking, but essentially it is the US: corn ethanol. We in the UK don't have anything like that scale of a biofuel industry. SmartSE (talk) 12:30, 21 April 2012 (UTC)
- Fuel which raises the cost of food is bad news, and isn't really carbon neutral because of agricultural fuel use. 71.212.237.20 (talk) 17:22, 21 April 2012 (UTC)
- But it is carbon neutral if the fuel used for agriculture is sourced from the very agriculture it's performing. 203.27.72.5 (talk) 07:16, 22 April 2012 (UTC)
- For a couple of crops, maybe. Then you need fertiliser, which takes energy to produce. Biofuel is like solar panels - the energy consumed is so high it only works commercially if governments prop it up with laws and subsidies. Ratbone58.170.172.202 (talk) 09:57, 22 April 2012 (UTC)
- See compost, sir. Whoop whoop pull up Bitching Betty | Averted crashes 18:14, 22 April 2012 (UTC)
- Where does the material to make compost come from? The point is, if you expect to get energy out of an area of land for an indefinite period, without bringing in fertiliser from somewhere else, you are basically expecting to make just another form of perpetual motion machine. Ratbone60.230.215.57 (talk) 01:30, 23 April 2012 (UTC)
- See compost, sir. Whoop whoop pull up Bitching Betty | Averted crashes 18:14, 22 April 2012 (UTC)
- For a couple of crops, maybe. Then you need fertiliser, which takes energy to produce. Biofuel is like solar panels - the energy consumed is so high it only works commercially if governments prop it up with laws and subsidies. Ratbone58.170.172.202 (talk) 09:57, 22 April 2012 (UTC)
- But it is carbon neutral if the fuel used for agriculture is sourced from the very agriculture it's performing. 203.27.72.5 (talk) 07:16, 22 April 2012 (UTC)
- Fuel which raises the cost of food is bad news, and isn't really carbon neutral because of agricultural fuel use. 71.212.237.20 (talk) 17:22, 21 April 2012 (UTC)
- See Energy returned on energy invested and [3] (the source cited for the ERoEI graph). Corn ethanol has an ERoEI of, at best, 1.6 -- any inefficiency in production, and you'll be losing energy on every gallon of fuel you make. Biodiesel is even worse, at an ERoEI of 1.3. For comparison, photovoltaic energy has an ERoEI of 6.8 or so, wind is 18, and hydroelectric is somewhere upwards of 100. --Carnildo (talk) 02:16, 24 April 2012 (UTC)
ban all plastic bags ... and then?
Crude oils and natural gases around the world are very complex mixtures of organic materials. We get all kinds of fuels and raw materials from these non-renewable natural resources.
I can certainly reduce my plastic bag consumption. However, I still consume much gasoline. If I don't reduce my use of fuel oils (driving cars, use electricity directly and indirectly ...), then the oil industry still has to pump up so much crude oil to produce so much fuel for me and also so much organic byproducts that will be used to manufacture plastic bags, forks, cup lids, styrofoam peanuts, lousy key-ring toys, all these miserable disposable things that will never be disposed .......
You can kill a fat cow to get two hundred lbs of meat. In the mean time, you get bones, fats, horns, hoofs, hair, .... I think crude oils contain much less waste because many "cow ingredients" are more difficult to use. They may be useful to some people, but the market demand much less of these low-end "cow ingredients".
Without the plastic bag industry, how do we make good use of all these chemical soups that will become LDPE and many other low-end plastics? I think it may be a good idea to store billion tons of low-end plastic pellets somewhere for future generations. The costs can be added to fuel prices. However, these low-end plastics may still degrade in storage.
The best way is to reduce oil consumption so we don't need to pump up so much crude oil. But what will happen if we just ban plastic bags? -- Toytoy (talk) 05:49, 21 April 2012 (UTC)
- Amongst other things, we will have fewer discarded (because trivially cheap) plastic bags littering the streets and countryside, and being washed via drains and rivers into the sea where they contribute (along with other discarded plastic items) significantly to harming marine life (which ingests them or their fragments both involuntarily and through mistaking them as food items: rubber bands resemble marine worms, plastic bags jellyfish, etc). {The poster formerly known as 87.81.230.195} 90.197.66.64 (talk) 08:47, 21 April 2012 (UTC)
- The thing about the crude oil 'cow' is that you don't have to settle for what comes in from the field; if you need more 'meat' (or 'hoof', or 'eyeball', for that matter) there are catalytic refining processes that can get it for you. A modern oil refinery uses quite a few different techniques to separate the different components of crude oil, and then to manipulate the different fractions to produce the mix of products required at market. Taking your specific question about polyethylene, steam cracking is a high-temperature process used to break longer-chain, saturated hydrocarbons into the smaller, unsaturated compounds (like ethylene) used to make plastics. Ethylene isn't a naturally-occurring component of crude oil; it's deliberately manufactured in order to feed downstream industrial users. If there were a reduced demand for ethylene, then the hydrocarbons used to make it would be used to generate a different end product instead. TenOfAllTrades(talk) 13:39, 21 April 2012 (UTC)
- Both cow slaughter and oil rerfining are highly efficient. There is little waste in either process. You may not eat those low value parts (or may not realize what exactly is in your hot dog or gelatin dessert) but your dog or garden plants may eat them. Some end up as industrial feedstocks too. Rmhermen (talk) 14:06, 21 April 2012 (UTC)
- It's not even just those parts. Some people find those parts of the animal to be very desirable, e.g. Menudo, headcheese, black pudding, chitterlings, lengua, etc. etc. --Jayron32 23:55, 21 April 2012 (UTC)
- Both cow slaughter and oil rerfining are highly efficient. There is little waste in either process. You may not eat those low value parts (or may not realize what exactly is in your hot dog or gelatin dessert) but your dog or garden plants may eat them. Some end up as industrial feedstocks too. Rmhermen (talk) 14:06, 21 April 2012 (UTC)
- If we develop advanced virtual reality worlds that jack directly into our minds then limited physical resources become an irrelevant concern. All society would need then is the power to operate the simulated reality and a little extra nutrition to keep our bodies ticking along until we replace our minds with digital simulations. I don't think we'll need plastic bags at that point. SkyMachine (++) 21:56, 21 April 2012 (UTC)
- Touché. 203.27.72.5 (talk) 01:49, 22 April 2012 (UTC)
Insect ID


Can anyone identify either of these two pretty critters, both from southern Vietnam? The moth was somewhere in the order of 15cm across; the other (weevil?) perhaps 6-8. I'd particularly like to know if the hook shapes on the weevil's antennae serve a specific purpose. Thanks, HenryFlower 10:19, 21 April 2012 (UTC)
- The black and yellow chap looks like the longhorn beetle, Pachyteria dimidiata [4] Sean.hoyland - talk 10:38, 21 April 2012 (UTC)
- Thanks--one down! The moth resembles the Emperor Gum Moth (Opodiphthera_eucalypti); not quite the same though (that one's Australian and lacks the transparent 'windows' in the eyes). HenryFlower 12:19, 21 April 2012 (UTC)
- I have no particular knowledge of this species (being UK/European based), but I believe some lepidoptera can lose patches of specialised (scent-bearing?) scales from their wings as they get older, so yours might simply be an older specimen; alternatively, it might be a sexual dimorphism. On balance, however, I think it's a different but closely related species. {The poster formerly known as 87.81.230.195} 90.197.66.219 (talk) 02:17, 22 April 2012 (UTC)
- (ec)It appears to be a Tussah moth, Genus Antheraea perhaps it is the Antheraea pernyi since it is similar in appearance to those pictured here. --Modocc (talk) 02:22, 22 April 2012 (UTC)
- I have no particular knowledge of this species (being UK/European based), but I believe some lepidoptera can lose patches of specialised (scent-bearing?) scales from their wings as they get older, so yours might simply be an older specimen; alternatively, it might be a sexual dimorphism. On balance, however, I think it's a different but closely related species. {The poster formerly known as 87.81.230.195} 90.197.66.219 (talk) 02:17, 22 April 2012 (UTC)
- Thanks--one down! The moth resembles the Emperor Gum Moth (Opodiphthera_eucalypti); not quite the same though (that one's Australian and lacks the transparent 'windows' in the eyes). HenryFlower 12:19, 21 April 2012 (UTC)
~ Health-Related Questions: For Only Knowledge & Experienced People of Excellence Only ~
- what is the median cost of birth on state, and usa level. possibly include UK, AUS, etc. if you know.
- what is the most reputable rankings for Maternity Hospitals?
- what are the 1-2 most reputable ranking for each section on mastersinhospitaladministration.com/2011/hospital-rankings-in-the-usa-the-ultimate-list/ -- include one sentence as to why
- what is the average hip size, or average range of hip sizes?
- will ask on ehealthforum.com or SOMEWHERE if nobody knows
Thingstofollow (talk) 10:57, 21 April 2012 (UTC)
Odd tree growth
Can anybody give me any information on this phenomenon?

The dense portion within the tree really seems to branches and twigs of the tree, not a parasite or epiphyte. There are at least three trees with this appearance at Nostell Priory, but I've never noticed the phenomenon anywhere else. Is it natural to this particular species, or the result of disease? Or has it been pollarded in some odd way?
I haven't managed to identify the species, or even the genus of the tree: only a few leaf-buds are open yet, so I can't tell the size or final shape of the leaves, but from the young leaves I've seen I think it might be a lime, or perhaps an elm. --ColinFine (talk) 11:34, 21 April 2012 (UTC)
- It's called a witch's broom (for obvious reasons). It is a symptom of many different diseases and can occur on many different species. EB also have an article which is a bit different to ours, and there are many scientific publications about it to be found. SmartSE (talk) 12:27, 21 April 2012 (UTC)
- From the branches that looks like a lime tree, and I've seen this phenomenon in a number of places in southern UK usually, on lime trees. I would say it is unusual but not rare. Richard Avery (talk) 13:27, 21 April 2012 (UTC)
- These growths are associated with burrs (or "burls" if you're American). The Common Lime Tilia × europaea is noted for them and they distinguish it from the many other lime species and cultivars. The Field Guide to the Trees of Britain and Northern Europe by Alan Mitchell says; "Bark... burred and densely sprout-infested especially at the base". The poet Robert Browning noted the same feature in the English Elm Ulmus procera, in Home Thoughts from Abroad;
- And whoever wakes in England
- Sees, some morning, unaware,
- That the lowest boughs and the brushwood sheaf
- Round the elm-tree bole are in tiny leaf
- While the chaffinch sings on the orchard bough
- In England—now! Alansplodge (talk) 17:04, 21 April 2012 (UTC)
- Are we entirely uncertain this isn't mistletoe? See File:Mistletoe Abundance Wye Valley.jpg. --Jayron32 19:31, 21 April 2012 (UTC)
- Well, I'm entirely certain it isn't mistletoe. Or ivy. I went and looked. --ColinFine (talk) 17:36, 24 April 2012 (UTC)
- It's possible, but every part of mistletoe is light green. We seem to be seeing a mass of dark twigs in this instance. Alansplodge (talk) 21:03, 21 April 2012 (UTC)
- Mistletoe has an easily recognizable geometry, too. It doesn't have straight, parallel branches like the twigs on this tree. And it has leaves on it all year round. When I first say the picture, I thought it was ivy. But then I noticed that the bottom portion of the trunk is bare. Looking closely, I have to agree that it is some type of hyper-proliferation of the tree itself rather than an epiphyte. Dominus Vobisdu (talk) 23:19, 21 April 2012 (UTC)
- Let's not let mistletoe off so easily. It does indeed steal resources from its host, making it parasite. ;) 96.233.247.233 (talk) 01:43, 22 April 2012 (UTC)
- Mistletoe has an easily recognizable geometry, too. It doesn't have straight, parallel branches like the twigs on this tree. And it has leaves on it all year round. When I first say the picture, I thought it was ivy. But then I noticed that the bottom portion of the trunk is bare. Looking closely, I have to agree that it is some type of hyper-proliferation of the tree itself rather than an epiphyte. Dominus Vobisdu (talk) 23:19, 21 April 2012 (UTC)
Thanks, Smartse and Alansplodge. --ColinFine (talk) 17:38, 24 April 2012 (UTC)
alternative explanation
I heard about the book fingerprints of the gods which offered a different explanation for some archaeological discoveries, i understand that this is farfetch explanation but the idea is fascinating, can you guys give me a list of this kinds of things?
explanations of phenomenons that is not particularly accepted by mainstream science but is interesting to know. thanks 203.112.82.129 (talk) 16:30, 21 April 2012 (UTC)
- I'm not kidding or being sarcastic when I say try using Youtube for this sort of thing. Start with your topic and browse the related links on the right.. eg http://www.youtube.com/watch?v=o52zJEGqkhI etc.Oranjblud (talk) 18:25, 21 April 2012 (UTC)
- The idea is interesting, but resolving it is like nailing Jello to a tree. The hypothetical First Race Of Man was at once so thoroughly careful about covering up its tracks that we find no highways or monuments or garbage dumps or plastic baby dolls from their era. Yet they were sorry enough for their poor sorry brethren that they let slip a few key ideas in mathematics or architecture or something. Because no evidence can exist for the idea it is not falsifiable, which means it's not really science, except maybe as hypothesis. Of course, we still have no real idea whether our planet is just an ornament in the highway median of some ultimate galactic civilization, protected from the truth by a few low-grade holograms. And so forth - it leads smoothly to solipsism and related philosophical black holes.
- That said, of course, there is doubtless much that our ancestors were capable of that we don't know. Shorelines from the Ice Ages are under water and only beginning to be explored. But the key here is, you don't need to be able to build spaceships to know how to do calculus. The ancients could have developed lots of clever math and lost it without leaving much of a trace.
- I fear that too often such arguments are meant, consciously or otherwise, to denigrate the achievements of certain races. When people say that the native Central Americans couldn't have built pyramids without contact from Egyptians, or aliens, or gods, or predecessors, what they're saying is that they are what? Too stupid to pile rocks together? It reflects a lack of awareness of how extensively the New World was populated before smallpox and other diseases decimated their civilization. The reason why such races in such early times could do things like modern man can, is that despite economic and educational disadvantages they were our equals in intellect and character. Wnt (talk) 19:31, 21 April 2012 (UTC)
- (edit conflict)I am also not kidding when I say that Youtube is a haven for nutjobs and conspiracy theorists. You are more likely to get a good scientific treatment by asking random people on the street.
- The problem with theories like this is that they seem plausible if you look at them in isolation, and only take the evidence presented. This theory will conveniently omit all the evidence that their theory is physically impossible. The idea that a pole shift could have made Antarctica habitable as recently as 10000 years ago is absurd and scientifically impossible (I suggest you read the articles True polar wander and geomagnetic reversal, because many people don't actually understand what a pole shift really is). Additionally, the Antarctic ice caps are demonstrably millions of years old. If you're interested in a balanced treatment of this theory, I'd read the articles I linked (especially true polar wander). If you're interested in wild speculation or downright foolishness, I'd try Youtube. Don't think I'm being condescending: the people in the linked interview make their prejudices apparent right off the bat. They feel oppressed because the scientific community takes their theories for what they are: wildly speculative, based on thread-bare evidence, and based on near-impossible to impossible assumptions. All this when their "evidence" is easily explained away by coincidence and human nature. "Suppression of knowledge" is a phrase only used by people who don't care about scientific proof. -RunningOnBrains(talk) 19:41, 21 April 2012 (UTC)
- See also Crackpot index; Graham Hancock's books (of which I have actually read several) all rank fairly high. What makes Hancock so engaging is that he saves his crazy for the last fifth of the book or so. He does a good case of gathering a bunch of seemingly innocuous evidence without letting you know what he's gonna do with it. Once you've bought into his evidence, he throws in the "The aliens did it" bit at the end (or whatever crackpot theory he's proposing). Since the first part of the book is usually so artfully crafted and well written, it makes it easy to get trapped in it, and then fall for his wacko conclusions. Of course, Hancock's Fingerprints of the Gods is basically a rehash of Erik von Daniken's Chariots of the Gods, which pretty much established the genre of this type of work. --Jayron32 20:13, 21 April 2012 (UTC)
- I can tell you're not kidding because youtube is where i heard about the book, in fact its from Joe Rogan, and thats what amaze me. I just asked this on ref desk because i already know about ancient aliens and other similar stuff, i just know that some, if not most of you guys can tell me what other interesting things to look for. — Preceding unsigned comment added by 203.112.82.128 (talk) 21:12, 21 April 2012 (UTC)
- One alternate theory was proposed in response to a contest to come up with valid theories in violation of Occams's Razor. In it, the Earth is inside-out (a hollowed out sphere), with us living on the inside, and as we approach the center of the sphere (our sky), atoms become steadily smaller, until infinitely small. The theory didn't explain why this happens. However, the effect apparently would cause a visual distortion making it appear the Earth is how we think of it. StuRat (talk) 14:08, 22 April 2012 (UTC)
See my userpage. Count Iblis (talk) 01:36, 23 April 2012 (UTC)
Vactrain effects
What effects, if any, would a Vactrain have on the human body? And how exactly would passengers be let off the train? 64.229.204.143 (talk) 19:57, 21 April 2012 (UTC)
- As long as the train was kept pressurised, it would have no effect on the body. When you fly in a modern airliner, the air pressure around the plane is very low indeed, but because planes are reasonably airtight, and air pressure is maintained by air pumps on board, all most passengers experience is ear popping. Indeed, the Vactrain should be safer than a plane in this respect, since in an emergency you can simply flood the tunnels with air. The way the passengers get off is that once the pipes operate on an airlock type system - when the train is in the station (or on the approach to it, since there's no need for vacuum tunnels on the low-speed tracks in stations) the ends of the tunnel simply open to let the train out, and close behind it. There are also more advanced ideas for how to seal the end of the tunnel - the StarTram concept for instance uses what's called a "plasma window" - electrically charged gas controlled with a magnetic field to form a "curtain" that seals the tunnel to air but lets the train can pass out. Smurrayinchester 21:10, 21 April 2012 (UTC)
- I can see a few problems:
- 1) Air exchange with the environment is normally used in a train to keep the indoor air fresh. This can't be done while in a vacuum. They may not even want to vent air from the train because that would steadily lessen the vacuum in the tunnel. So, they would then need to recycle the air in some way. Since this is rather expensive, I bet they would do the minimum required, resulting in stale air (low oxygen, high in carbon dioxide and pollutants).
- 2) Getting out in an emergency would have to be slower. Even if they were willing to flood the tunnel with air, this would take a long time, if not done with explosive force. So, if there's an emergency requiring immediate evacuation, like a fire, many could die. The alternative of supplying space suits for all would be prohibitively expensive. Perhaps they could evacuate everyone to one car, and disconnect from the burning car, though.
- 3) If there's a hole blown in the train, say by a terrorist, this could lead to explosive decompression.
- 4) A slow leak could result in low air pressure, causing people to pass out and eventually die. This occasionally happens on planes.
- 5) The higher speeds allowed by such a train pose their own increased risks. A collision, for example, would become more deadly.
- So, such a train would be more dangerous than a train in normal air. StuRat (talk) 13:56, 22 April 2012 (UTC)
- You wouldn't need to disconnect the burning car. Once it is evacuated and sealed off, you can just vent the air, which would put the fire out instantly. --Tango (talk) 17:25, 22 April 2012 (UTC)
- That would fill the tunnel with smoke and deposit soot everywhere, but I suppose that's OK. StuRat (talk) 01:54, 23 April 2012 (UTC)
- Also, if you're flooding the tunnel with exterior air, it could be done with explosive force—you're doing it very infrequently, so that would become a possibility. Whoop whoop pull up Bitching Betty | Averted crashes 17:57, 22 April 2012 (UTC)
- When the shock wave hit the cars it would blow them apart, or at very least bounce the passengers around inside. A 10 PSI difference hitting the end of the car, let's say 10 feet by 10 feet, or 14400 square inches, would exert an instantaneous 144,000 pound force on the end of the car. StuRat (talk) 01:54, 23 April 2012 (UTC)
- Just 10 PSI? That is easy to withstand! Whoop whoop pull up Bitching Betty | Averted crashes 18:38, 23 April 2012 (UTC)
Biomorph-producing fractal formulas
I need some biomorph producing fractal formulas... does any one know any? except the mandelbrot, and I don't want it to be just interesting, but really look like biomorphs....--Irrational number (talk) 20:25, 21 April 2012 (UTC)
- This is a very vague question. Who judges how biomorphic a certain image is ?! Nevertheless, you may be interested in L-systems, which can broadly generate life-like patterns. Also note that "fractal" formulae are not necessary for the generation of "biomorph" patterns. See e.g. reaction-diffusion systems. In particular, If you are interested in some of the mathematical properties of biological pattern formation, I highly recommend "The Chemical Basis of Morphogenesis", (Turing, 1952) [5]. SemanticMantis (talk) 01:52, 22 April 2012 (UTC)
- Turing's patterns are not fractal (they are spots and things like that), but the L-system reference is a good one. Mandelbrot's book contains a bunch of examples. Our article on fractal broccoli might also have a helpful pointer or two. Looie496 (talk) 02:01, 22 April 2012 (UTC)
- Indeed, Turing patterns are not fractals. That is why I said ""fractal" formulae are not necessary for the generation of "biomorph" patterns." before I suggested the other links. I suppose I should have made the distinction more clear. SemanticMantis (talk) 03:20, 22 April 2012 (UTC)
- I didn't say that fractals necessarily make biomorphs, I'm looking for formulas that do make such things. I've been doing some "experiments" in "ultra fractal" program seeing different formulas and looking for those that resemble biomorphs, which, although interesting, was not very successful. I also found an article in the references of the pickover stalk article, which seemed to have some biomorph producing Iteration formulas, but I tried them and they were all blank.So, I wanted to try finding some here...--Irrational number (talk) 10:10, 22 April 2012 (UTC)
- Turing's patterns are not fractal (they are spots and things like that), but the L-system reference is a good one. Mandelbrot's book contains a bunch of examples. Our article on fractal broccoli might also have a helpful pointer or two. Looie496 (talk) 02:01, 22 April 2012 (UTC)
Any thoughts?--81.31.188.28 (talk) 10:41, 23 April 2012 (UTC)
April 22
Caffeine in Nesquik
How much caffeine is there in a 16 oz bottle of Nestle Nesquick fat free chocolate milk? The label simply says "99% caffeine free" without giving a mg amount. Magog the Ogre (talk) 00:32, 22 April 2012 (UTC)
- I don't know the answer, but I'd like to take this moment to laugh out loud at the phrasing. Most of the drinks on this list have less than 1% caffeine by weight. One fluid ounce of water-based drink weighs approximately 30 grams, so anything listed there with less than 300 mg/fluid ounce will technically fit this description.-RunningOnBrains(talk) 02:25, 22 April 2012 (UTC)
- Ha! Good point! I wonder, does that phrase even have a legal meaning e.g. in the USA? Definitely <1% caffeine by weight is a low bar to cross :) SemanticMantis (talk) 03:25, 22 April 2012 (UTC)
- Any amount higher than that should be fatal. Maybe the marketing department of Nestle can run with that and promote Nesquick with the slogan "It won't kill you." SkyMachine (++) 08:17, 22 April 2012 (UTC)
- "...at least not immediately." StuRat (talk) 13:47, 22 April 2012 (UTC)
- This article appears from a google snippet to have the answer (it mentions chocolate Nesquik and has a table of caffeine content) but I don't have full access. By the way, the Nesquik company site makes the same claim for both the powder and the bottled drink. I am guessing that the "99% free" claim was merely transferred from the powdered product without any actual though being put into its meaning. One could deduce from that that the powder contains about 1% caffeine which will be diluted depending on how much milk it is mixed with. SpinningSpark 10:38, 22 April 2012 (UTC)
Flying car
Your proposed design has four fans each of radius 50 cm. Each fan sucks in air from above the car and blows it downward. The downward flow of air can be approximated as a column of radius equal to the radius of the fan traveling at uniform speed downwards.
A) How fast must the air be blown downwards if the car plus passangers weigh 1000 kg?
B) If the car is 100% efficient, how much power would it use while hovering?
The densidad of air is
Is this the correct calculation for the upward force: , and I think so . Overall I got for part A and for part B. Widener (talk) 08:25, 22 April 2012 (UTC)
- Do your own homework. Whoop whoop pull up Bitching Betty | Averted crashes 17:50, 22 April 2012 (UTC)
- The poster has done their homework. They have already shown an attempt at the question and are asking if they have made any errors. SpinningSpark 18:12, 22 April 2012 (UTC)
- Your answers look correct. If this is for a calculus-based physics class, they're fairly certainly going to want you to work this out in terms of derivatives, i.e., start with F=dp/dt instead of F=p/t, but your equations work OK as long as you're careful about the definitions and assumptions being used. Red Act (talk) 01:19, 23 April 2012 (UTC)
- For a general audience such as Wikipedia editors, please define each variable you use rather than just throwing out random Greek or Latin letters. Some readers took physics decades ago and the letters used might have been different. Few are likely enrolled in your particular class with access to your lectures, homework assignments and textbook.F=force?, t=time?, s=seconds?, mv=millivolts? What is nu?, What is rho?? Is h=Planck's constant? Meet us halfway. Edison (talk) 03:14, 23 April 2012 (UTC)
- I wouldn't sweat this so much—the people who are likely to be able to assess this type of equation are probably fluent in the usual meanings of these symbols, and it's only important to define unusual or non-standard symbols. For basic physics formulas, F is always a force, p is always momentum, t is always time, m is always mass, v is always velocity, and ρ is always a density. h is generally either a height or Planck's constant; the correct choice should be obvious from context in nearly all circumstances (as it is here). There is no nu in the above equations; I'm guessing that you're (mis)reading the stylized v used in Wikipedia formulas.
- There's no need to define standard SI units. ms-1 is potentially ambiguous, as it could represent meters per second (m/s) or inverse milliseconds: (ms)^(-1); it may be best to write it as either m·s-1 or m/s to emphasize that the m isn't a prefix. Not that it particularly matters in this situation, as the context clearly demands a velocity. W, finally, is always a watt.
- If there are editors who aren't familiar with the notation used in these types of problems, I encourage them to ask—but there's no need to browbeat the OP. This usage would be familiar to any first-year undergraduate physics student. TenOfAllTrades(talk) 03:42, 23 April 2012 (UTC)
High buoyancy and low drag
If you build a very large capsule (hence with enormous buoyancy) but with a narrow pointed shap (hence very low drag), could you get this capsule to rise so fast it could actually rise up a waterfall?
According to my calculations, I see no reason why this could not be done. I calculate the terminal velocity as . ( is the density of the water and is the density of the capsule). If you make sufficiently large then you can make as large as you want. If you can release it at any depth, then it can come as close to this terminal velocity as you want. Does anyone object to this? Widener (talk) 17:25, 22 April 2012 (UTC)
- For a capsule in water, you cannot ignore parasitic drag, which increases linearly (IIRC) with total wet area. So your very aqua-dynamic capsule will still experience significant drag that is proportional to its volume. So in practical terms, I doubt it's possible. --Stephan Schulz (talk) 17:38, 22 April 2012 (UTC)
- Oh I see, of course at high velocities the drag equation underestimates the true drag. Widener (talk) 17:47, 22 April 2012 (UTC)
- I'm not much of a scientist, but I imagine that there aren't many waterfalls which are a column of solid water without any voids in them. As soon as your capsule finds some air, it's going to stop rising and fall out of the side of the waterfall. While I was typing that, I remembered that water with a lot of air bubbles in it (ie "whitewater") is a lot less bouyant than water without, so your caclations are going to go awry before you start. Good luck with your experiment. Alansplodge (talk) 01:14, 23 April 2012 (UTC)
- Oh I see, of course at high velocities the drag equation underestimates the true drag. Widener (talk) 17:47, 22 April 2012 (UTC)
- You use hydrostatic pressure to calculate the buoyancy. It does not apply very well to falling water, whether it has air in it or not. Any vertical pressure gradient in the water would act against gravity and slow down the falling, but as far as I know waterfalls are close to free fall. --145.94.77.43 (talk) 05:06, 23 April 2012 (UTC) Though on a second reading I take you are planning to "jump" from a depth rather than starting at the base of the fall? --145.94.77.43 (talk) 05:12, 23 April 2012 (UTC)
- There's a much more fundamental problem here than those discussed so far: bouyancy is driven entirely by the pressure gradient in a standing fluid. (It just happens that it can be stated simply in terms of density in that case.) In free fall, there is no such pressure variation (as the fluid is free to move around to suppress it) and bouyancy just doesn't happen. --Tardis (talk) 13:00, 23 April 2012 (UTC)
- On rereading I see that this is fundamentally what 145.* already said. I should point out that an idealized cylinder of water falling through air will of course eventually reach a sort of terminal velocity where the shear from the air balances the acceleration due to gravity. There's no reason you can't (in principle) float up somewhat past this point before you reach too good an approximation to free fall to continue. --Tardis (talk) 13:11, 23 April 2012 (UTC)
Some Science questions
1)When the electric charge is quantized, how the quarks having fraction electric charge exists?
2)Why can't we use D.C. current everywhere? I mean, what if all the devices are also made to use dc. and a rectifier is added at each house.
3)Here is a practical problem.I have ammeter , 2 volt battery and a 2 volt dc rectifier. When i connect both voltage sources to ammeter why it shows different readings?V and R of ammeter is constant, so I must be constant according to Ohm's law.
- For 1: Charge is still quantized, just maybe not in the size that was originally thought (i.e. quarks always have a charge that is a multiple of 1/3).
- For 2: War of Currents. The problems are largely historical. There are some places with High-voltage direct current distribution. You could use DC current everywhere, but we don't, because it used to be unsuitable for long-distance distribution. Buddy431 (talk) 21:31, 22 April 2012 (UTC)
- For number 3: How different? What are the actual readings for the battery and the rectifier? If we knew that, it would help answer the question. --Jayron32 22:01, 22 April 2012 (UTC)
- This entirely depends on what the rectifier is being powered from. If it is the battery, a diode voltage drop would be expected at the output. Two volts is an unusual value for both batteries and rectifiers by the way. Also, you appear to be connecting the ammeter directly across the voltage sources. If this is so, there will be a significant volt drop at the source output and in general this will not be the same for both sources. SpinningSpark 22:10, 22 April 2012 (UTC)
- For number 3: How different? What are the actual readings for the battery and the rectifier? If we knew that, it would help answer the question. --Jayron32 22:01, 22 April 2012 (UTC)
- There's something weird about the fractional electric charge. Quarks can't exist in combinations that have non-fractional charge, and can't transition in ways that would work out to an odd fraction. They go, say from 2/3 to -1/3, not 2/3 to 1/3, emitting W particles. This is sort of the way that electron spin can be +1/2 or -1/2, but the photons have to carry an angular momentum of just plain 1 (Planck's constant, that is). Not sure why things are that way at the bottom - is there a way to understand that intuitively? Wnt (talk) 00:50, 23 April 2012 (UTC)
- For question 2: It is really simple to have an electric generation and distribution to serve a large area wherein the electricity is generated, then stepped up by simple transformers to a very high voltage AC at the generating station and sent in all directions, then stepped down to say 12 KV at substations every few miles, and distributed to a neighborhood, then stepped down to say 120 or 240 volts (depending on the country) by a transformer every block or so, to be sent into houses. With DC, the generating stations would have to be every 2 miles or so apart, producing 120 (or 240) volts, because of "copper losses." The same power could be provided by DC, but much, much more copper would be required. Copper currently costs close to 4$ (US) per pound. Edison (talk) 03:09, 23 April 2012 (UTC)
- It makes me kind of uneasy that AC power is being advocated here by Edison, of all people. —Akrabbimtalk 12:40, 23 April 2012 (UTC)
- So far as I know the War of Currents stuff is sort of out of date. As I recall HVDC is now a serious contender for very long range distribution networks, like proposed transmission from solar or wind stations in the Sahara to Europe. (Of course, as you can tell from the sound of an idea like that, it may be a little starry eyed) In former times, changing DC voltage from one value to another was difficult, making it easier to use high-voltage lines (less loss) with AC, but as I understand it this is no longer such an obstacle. Wnt (talk) 14:09, 23 April 2012 (UTC)
- What can be done now that could not be done decades ago is the use of switchmode power convertors to change the voltage. Until the development of high power Field Effect Transistors, efficient low cost switch mode convertors (aka DC to DC convertors) could not be made. Until 30 years ago all power operated consumer electronics used transformers to bring the 120V or 240V house power down to whatever voltage was required internally - now virtually all consumer electronics uses switchmode. However, don't expect switchmode to be used in electricity substations and street distribution any time soon, as the reliability, while good enough for consumer products, is no where near that of a transformer, and not good enough for power companies. Transformers are also very tolerant of short term (<1 second or so) overload, which tends to be frequent in power distribution. Switchmode on even small overload must shut down to protect itself, whereas transformers even on 300% or more overload just keep on delivering close to the normal voltage, allowing fuses & circuit breakers to operate where necessary, with discrimination. Discrimination is the feature provided by a hierachial arrangment of fuses or circuit breakers that result in only the minimum of consumers suffer blackouts due to faults. Keit120.145.185.73 (talk) 14:34, 23 April 2012 (UTC)
- Thanks for a really informative response. I put the link above into standard Wikilink format (hope you don't mind). Wnt (talk) 23:29, 23 April 2012 (UTC)
- What can be done now that could not be done decades ago is the use of switchmode power convertors to change the voltage. Until the development of high power Field Effect Transistors, efficient low cost switch mode convertors (aka DC to DC convertors) could not be made. Until 30 years ago all power operated consumer electronics used transformers to bring the 120V or 240V house power down to whatever voltage was required internally - now virtually all consumer electronics uses switchmode. However, don't expect switchmode to be used in electricity substations and street distribution any time soon, as the reliability, while good enough for consumer products, is no where near that of a transformer, and not good enough for power companies. Transformers are also very tolerant of short term (<1 second or so) overload, which tends to be frequent in power distribution. Switchmode on even small overload must shut down to protect itself, whereas transformers even on 300% or more overload just keep on delivering close to the normal voltage, allowing fuses & circuit breakers to operate where necessary, with discrimination. Discrimination is the feature provided by a hierachial arrangment of fuses or circuit breakers that result in only the minimum of consumers suffer blackouts due to faults. Keit120.145.185.73 (talk) 14:34, 23 April 2012 (UTC)
- For question 2, some devices, such as AC motors, require AC. Of the devices that use DC internally, such as radios, televisions, and computers, they need different values of DC, so it has been easier to have one standard AC voltage and build transformer-rectifier units to step down to the DC voltage needed by an individual device. The evolution of cheap and efficient DC to DC converters is much more recent than most of the electrical infrastructure. Jc3s5h (talk) 14:22, 23 April 2012 (UTC)
Cholera rates in Kenya
Hi Reference Desk,
I'm look for the prevalence of cholera in Kenya. I can find [rates], but nothing on cholera. Please help me. Wiki or external links that directly inform me of this information would be great! Thank you. 208.22.79.249 (talk) 23:15, 22 April 2012 (UTC)
- There's some information at WHO: [6]. It lists the number of cases of Cholera for many countries, including Kenya. RudolfRed (talk) 00:32, 23 April 2012 (UTC)
- The WHO also has a short article here. Someguy1221 (talk) 00:36, 23 April 2012 (UTC)
- Thank you. If anyone else has anything, please help. -original poster 208.22.79.249 (talk) 04:11, 23 April 2012 (UTC)
April 23
Chemistry/Amylose and amylopectin Determination.
How can i determined amylose and amlopectine in starch sample. — Preceding unsigned comment added by 78.138.24.1 (talk) 10:19, 23 April 2012 (UTC)
- Do your own homework. Whoop whoop pull up Bitching Betty | Averted crashes 10:23, 23 April 2012 (UTC)
- The basic schoolchild test for amylose starch is given in the Wikipedia article. A test specific to amylopectin is trickier, and I haven't studied Chemistry for more years than I care to remember, so I'll leave it for someone else to comment of the appropriateness of formamide. Are you wanting to determine the relative proportions of the starches? Dbfirs 07:16, 24 April 2012 (UTC)
Kills 99 per cent of germs...
That's the claim of household bleaches and surface cleansers etc - but are they doing the same thing that antibiotics are doing? What of the one per cent of germs that survive? Are we breeding supergerms? Bleach-resistant e-coli? Chlorine-tolerant salmonella bacteria?
Ta
Adambrowne666 (talk) 11:34, 23 April 2012 (UTC) There is a major difference between antibiotics and bleach: the 1st is a bacteria-only poison, working by a very specific damage it deals to the bacteria protein making process. Without protein making on the run no living cell can, well, stay a living cell. It dies and eventually decompose or is eaten by a still-living cells. Bleach is a different thing altogether: it is a highly reactive compound. It reacts with the cell membrane, dissolving it. It reacts with many, many molecules inside the shattered cell, destroying anything and everything. It consumes the cell and it contents. From this it is clear that, unlike antibiotics, bleach is poison for every living cell, not just bacteria. Since bacteria does not live in a protective layer of dead skin as we do, contact with fairly low concentrations of bleach for several dozen minutes kills it. 109.65.9.22 (talk) 13:10, 23 April 2012 (UTC)
- Bleach works in part by a specific mechanism, which is opposed by Hsp33 and EF-Tu. [7][8][9][10] Now it's loss of Hsp33 that makes a strain of cholera bacteria extra sensitive to bleach. I have not seen anything in NCBI about evolution of more bleach resistant bacteria, and it is possible that this is much harder because toxicity affects many different things, all of which would have to simultaneously evolve to overcome the problem. Nonetheless, until proven otherwise, my guess is that such bacteria can, will, and probably have evolved to develop greater bleach resistance, and that this adversely affects people when their own cells' attacks with oxidizing agents are better rebuffed. Wnt (talk) 13:59, 23 April 2012 (UTC)
- I suspect the 99% business is a truth-in-advertising CYA to avoid charges of unreasonable claims, but bleach kills pretty much 100% of germs unless they're somehow shielded from contact with the bleach. Scratches in a surface, for instance, coverage by some other substance - grease, for instance, etc. would be a shield - and which could be seen to negate a 100% claim. The building contaminated by Reston virus was decontaminated (initially, anyway) by bleach heated in electric skillets. Acroterion (talk) 20:29, 23 April 2012 (UTC)
Quantum entanglement and kinetic energy
Let's say you have two particles that are entangled. If you impart kinetic energy to one particle, will kinetic energy be imparted to the other particle? ScienceApe (talk) 16:55, 23 April 2012 (UTC)
- No. When you change the particle by adding energy (using some unspecified mechanism), its quantum mechanical energy state is no longer identical to its previous state, and therefore no longer identical to its pair partner. Rather, its state is "not necessarily identical," because even if you specify how you modify the energy, we don't know what you did to the quantum mechanical energy state until you measure it again, ad infinitum. Quantum mechanical entanglement sure becomes a lot less interesting, (insofar as it has no useful practical consequences), when it is treated correctly! Nimur (talk) 17:44, 23 April 2012 (UTC)
Female scientist question
Looking for the name of a Female scientist , who was a pioneer nd gave and lost life to science. She was homeless in early childhood. She grew up in a European city.
I thought Marie curie but looks like some other fits the description. Any help will be appreciated. I searched google/wikipedia with no luck. — Preceding unsigned comment added by 183.83.244.183 (talk) 17:08, 23 April 2012 (UTC)
- I doubt that you meant Hypatia but I will mention her because she deserves more publicity. What field was this scientist a pioneer in? SpinningSpark 19:25, 23 April 2012 (UTC)
- You might have fun with List of female scientists before the 21st century. {The poster formerly known as 87.81.230.195} 90.197.66.239 (talk) 00:07, 24 April 2012 (UTC)
Population growth vs accelerating returns in technology
From Accelerating change:
- For example, it can be claimed that inventions are generally created by a fixed population of human inventors at a constant rate, regardless of their current technological prowess, and therefore technological "progress" is actually a function of population growth, not past inventions.
Have any studies attempted to assess the accuracy of this claim, i.e. to determine whether historical statistics still support the Law of Accelerating Returns once adjusted for the (possibly superlinear, and possibly time-lagged) effect of an increased population? NeonMerlin 17:21, 23 April 2012 (UTC)
- My reading of the article is that that is not a claim, but a dialectical argument against accelerating change. Personally, I very much doubt that there is evidence to back it. It just does not stack up when comparing, say, the Golden Age of Greece against the stagnation of medieval Europe. Population size will clearly come into this equation, but equally, if not more, important are good communications, a social system allowing freedom to express ideas, and a society wealthy enough to allow individuals to pursue those ideas. SpinningSpark 19:16, 23 April 2012 (UTC)
- The problem with anything like this is deciding on a metric to measure the rate of invention with. I'm sure you could find plausible metrics to both prove and disprove that claim. --Tango (talk) 21:54, 23 April 2012 (UTC)
- That is exactly the point being made in the quotation taken from the article. It is not a claim in itself. SpinningSpark 23:27, 23 April 2012 (UTC)
Carmine microbial production
"The carminic acid used to produce the pigment can also be extracted from various microbes engineered for the purpose." This is unreferenced- can I get some further information on microbial production of this compound? 71.223.9.1 (talk) 18:46, 23 April 2012 (UTC)
Some Biology questions
1)Till what age an average human gets taller?I mean, after what age the human height generally stops to increase?
2)Is the death is certain for every human being?.If it is provided all the necessities and is cured from all diseases then how the death would come? Max Viwe | Wanna chat with me? 19:52, 23 April 2012 (UTC)
- 2) Yes, death is certain. Some reasons:
- 2a) Parts of our body wear out. Some are never replaced, like nerve cells.
- 2b) Other parts can be replaced, but our bodies slow down the rate of replacement as we age. This seems to be evolution at work, using mechanisms like telomeres to ensure that we grow old and die, to make room for new individuals with new genes, so the species is better able to adapt to changing conditions. (Note that there may be a way to fight telomere shortening, as with telomerase reverse transcriptase, but there are probably other mechanisms at work, too.)
- 2c) We also have an accumulation of damage to our bodies, such as scars and genetic damage to DNA from UV light and other radiation.
- 2d) If nothing else killed us, we would eventually die in an accident or violence. There are some plants and simpler organisms with no fixed lifespan, and this is what kills them all off, eventually.
- 2e) As for artificial immortality: One day, if we can duplicate out brain in a computer, we might be able to live forever as a robot. With sufficient backups located far away (Pluto ?), this would make us reasonably safe from accidents, too. StuRat (talk) 20:40, 23 April 2012 (UTC)
- Human height has a chart (for the US). Looking at it, it appears the average female stops at around 19, the average male at about 20. Taxes are certain; as for death, check out Immortality#Prospects for human biological immortality. Clarityfiend (talk) 21:09, 23 April 2012 (UTC)
- 2b the main function of telomere shortening IIRC is to prevent rogue cells from becoming cancerous and killing you early in life. Only that fraction of cancer cells that manage to switch on their telomerase genes become really dangerous. That allows you to escape cancer for quite a while at a young age (when it really matters to evolution). The breakdown of the human body is also because of antagonistic pleiotropy - some genes that let you have a good early life at the cost of long term damage. An example is the choice between a strong immune response and the body eventually slipping up and attacking itself (diabetes and arthritis).Staticd (talk) 05:13, 24 April 2012 (UTC)
- Death is by no means certain - like most biological phenomena, it sort of dissolves when you try to think about it. If the two halves of your brain were split apart from each other, and put into similar heads and sewn together with halves from some other person, would you still be alive? What if you took half of each those halves and repeated? And so on, until just one neuron from your brain was in each other head? Would you be alive then? Well, what if every neuron you have in your head is more or less identical to one neuron which is in some other person's head, somewhere in the world, right now? Would you be alive then, in those heads, even if the original head were no more? Wnt (talk) 23:24, 23 April 2012 (UTC)
- There is a difference between continuum of biology and continuum of self-aware personhood. Cell lines can be made to become immortal. Swapping hemispheres of the brain out would result (if it could be made to work in humans) in a completely new person. From a human perspective it is continuum of personhood that matters in the quest for immortality. Maybe we can achieve for people something similar to these lobsters. SkyMachine (++) 23:59, 23 April 2012 (UTC)
- 1) According to Introduction to Child Development by John Dworetzky, boys reach full height between 18 and 20 years, while girls between the ages of 15 and 17. Source: Roche & Devila 1972. Using the same source, Bodyspace: Anthropometry, Ergonomics, and the Design of Work by Stephen Pheasant gives a median age for reaching adult stature as 21.2 years for boys and 17.3 for girls, although 10% of boys were still growing after 23.5 and 10% of girls after 21.1 years. Alansplodge (talk) 01:00, 24 April 2012 (UTC)
- For #2: I hope so. I can't take much more than 50-60 years of this shit... --Jayron32 04:05, 24 April 2012 (UTC)
what is the year old of the donkey when it begin to bray?
which is year old of the donkey when it start to bray? it's bray from the start when it's born? thank you. 95.35.155.88 (talk) 21:16, 23 April 2012 (UTC)
- Are you asking about a Chinese zodiac year? There is a year of the horse, but no donkey. If this is not what you are asking about, perhaps you can clarify your question by providing context. -- ToE 03:17, 24 April 2012 (UTC)
- My guess is that the question is "at what age does a donkey start to bray?". (I don't know the answer.) --Trovatore (talk) 03:18, 24 April 2012 (UTC)
- Oh, of course! Thanks. The answer might be found in some YouTube videos of newborn donkeys. I don't have the bandwidth here to receive video, but I'll check later if I reception improves. -- ToE 03:28, 24 April 2012 (UTC)
- yea, I meant about the age of donkey start to bray. I have found the answer in You tube. Thank all of you95.35.240.97 (talk) 13:11, 24 April 2012 (UTC)
- Oh, of course! Thanks. The answer might be found in some YouTube videos of newborn donkeys. I don't have the bandwidth here to receive video, but I'll check later if I reception improves. -- ToE 03:28, 24 April 2012 (UTC)
- My guess is that the question is "at what age does a donkey start to bray?". (I don't know the answer.) --Trovatore (talk) 03:18, 24 April 2012 (UTC)
April 24
Curlews?
In February and March I have seen flocks of water birds wading in the flooded rice fields between Sacramento and Yuba City California. Their silhouettes and size are best described as Curlews but to me they appeared very dark or black in plumage. My Birds of North America field guide shows no illustrations or descriptions that fit the color of the many birds I have seen. I am curious about these birds. Can you help me with a well-educated guess? — Preceding unsigned comment added by 75.19.157.83 (talk) 00:59, 24 April 2012 (UTC)
- Did they have the curlews' distinctive "long, slender, downcurved bills"?--Shantavira|feed me 07:35, 24 April 2012 (UTC)
M notation in chemical reactions described for rate data
In looking at chemical reaction rate data, I notice that common notation for describing the reactions often includes the symbol "M", which I initially thought stood for "any molecule" - a molecule that provides an opportunity for collision but is not changed by the reaction, implying that the rate is substantually independent of M. For example: CH4 + M → CH3 + H + M. However it seems that the rate coefficients (Arrhenius parameters) are very strongly dependent on just what molecule the M actually is. So why is the "M" notation used? Why not give the reaction in a form stating what the colliding molecule actually is, as in CH4 + Ar → CH3 + H + Ar? Is it some sort of historical reason? I've looked at many reaction rate papers and chemistry books - they all just use the notation without explaining why. Ratbone124.182.165.65 (talk) 02:56, 24 April 2012 (UTC)
- Usually 'M' indicates a metal atom. Plasmic Physics (talk) 03:07, 24 April 2012 (UTC)
- No, that can't be it. Refeences listed on the NIST database, e.g., Baulch, Cobos, etc, (J Phys. Chem. Ref Data, Vol 21 page 411 onward) use M to signify Ar, N2, O2, CHn, you name it, most of them are not metals, and most are not atoms. Keit121.215.138.9 (talk) 03:36, 24 April 2012 (UTC)
- M does mean "a molecule". The idea is that you're looking at a specific reaction here, which appears to be the homolytic (radical) cleavage of a hydrogen atom from methane to make a methyl radical and a hydrogen atom. If you want to do a series of studies as to the effect of various collision targets for such a reaction, you first define the general reaction, using "M" for the target, then you would have a table or chart in the article which would define rate law as a function of the identity of "M". That's, at least, how I would read this. M is a variable, which will later in the article be defined as a specific set of molecules. That would make the most sense to me, if I am reading your question correctly. --Jayron32 04:03, 24 April 2012 (UTC)
- To put it another way, chemistry has a few algebra-like "variables". M = some molecule, just as H-X typically means some halide, or CH3-R means something bound to some side chain. The point is to allow the writer to say M, X, or R = this, that, or the other thing. Wnt (talk) 05:49, 24 April 2012 (UTC)
- M does mean "a molecule". The idea is that you're looking at a specific reaction here, which appears to be the homolytic (radical) cleavage of a hydrogen atom from methane to make a methyl radical and a hydrogen atom. If you want to do a series of studies as to the effect of various collision targets for such a reaction, you first define the general reaction, using "M" for the target, then you would have a table or chart in the article which would define rate law as a function of the identity of "M". That's, at least, how I would read this. M is a variable, which will later in the article be defined as a specific set of molecules. That would make the most sense to me, if I am reading your question correctly. --Jayron32 04:03, 24 April 2012 (UTC)
- No, that can't be it. Refeences listed on the NIST database, e.g., Baulch, Cobos, etc, (J Phys. Chem. Ref Data, Vol 21 page 411 onward) use M to signify Ar, N2, O2, CHn, you name it, most of them are not metals, and most are not atoms. Keit121.215.138.9 (talk) 03:36, 24 April 2012 (UTC)
I've seen this M notation before in the context of gas phase kinetics like this. My recollection is that M stands for any atom or molecule whose purpose in the reaction is to give or receive energy by collision. M doesn't chemically react, it's just there to bump into. If, for example, a molecule of CH4 collides with a molecule of M, CH4 might end up with more energy than it had before the collision. You often see such "excited" species highlighted with an asterisk, e.g. CH4*. The process would be expressed in an equation:
- CH4 + M → CH4* + M
This isn't a chemical reaction in the traditional sense, it's redistribution of translational, rotational and vibrational energy. The important point is CH4 becomes vibrationally excited as a result of the collision with M. Vibrational excitation ("chemical activation") is often necessary for a chemical reaction, such as the homolytic fission of a C–H bond:
- CH4* → CH3 + H
The extra vibrational energy CH4 obtained from collision with M has been spent on breaking the bond. This could not happen without activation, since bond breaking requires energy (is endergonic or endothermic?).
The two processes of (i) vibrational excitation by collision with M and (ii) bond dissociation often occur simultaneously, so are expressed together:
- CH4 + M → CH3 + H + M
I've often heard M referred to as the "bath gas", meaning something like argon that you might use as an inert gas to dilute a reactive gas like methane for kinetic studies. I believe the general placeholder M rather than a specific symbol like Ar is used because M could be either an atom of Ar or another CH4 molecule, it doesn't matter. M signifies the role of the molecule in the reaction, rather than its chemical identity. See Lindemann mechanism for an example of the use of M. --Ben (talk) 09:54, 24 April 2012 (UTC)
Diamminehydridoboron(0) [BH(NH3)2]
Is it possible to generate this compound, or will it fly appart like an overfilled closet? Or perhaps rearrange itself and spit out anything that doesn't quite fit, like a box full of mattress springs? Plasmic Physics (talk) 04:31, 24 April 2012 (UTC)
Apparently, diamminedihydridoboron(1+) [BH2(NH3)2]+ does exist. Plasmic Physics (talk) 04:34, 24 April 2012 (UTC)
- I'm seeing several references to diaminoboranes here, some over 30 years old: [11]. Assuming you mean HB(NH2)2. If, instead, you really mean ammineboron complexes, then this search shows similar complexes, such as dihydridodiammineboron(III) ions. That may give you some leads. --Jayron32 04:46, 24 April 2012 (UTC)
Simplest being to show affection
Which simplest (taxonomically lowest) living organism is capable to show affection and/or love?--176.241.247.17 (talk) 09:46, 24 April 2012 (UTC)
- With all due respect, your question is very problematic. Affection and love are not concepts amenable to biological discussion. We can talk about animal behaviors, but we have very little way of knowing their internal states (which is what love and affection are in humans). You might be interested in Beetle#Parental_care, or Frog#Parental_care, but understand that we cannot say a beetle loves its children because it takes care of them. (sadly parental care is completely human-centric, we need a parental care_(biology)...) SemanticMantis (talk) 12:49, 24 April 2012 (UTC)
Hermaphroditism
Does this NSFW image depict a true hermaphrodite, or just a person with both genetalia? Crisco 1492 (talk) 10:20, 24 April 2012 (UTC)
- Well, the museum page shows the person was a hermaphrodite. --SupernovaExplosion Talk 11:55, 24 April 2012 (UTC)
- According to this paper (p.3), "As early as 1860 the French photographer ... Nadar, took a series of nine photographs – which I take as the first examples of medical pictures of intersex patients ... that depicted a young intersex patient. They show the genitals of a hermaphrodite...". --SupernovaExplosion Talk 12:00, 24 April 2012 (UTC)
- There is a journal article titled Early Photo-Illustration of a Hermaphrodite by the French Photographer and Artist Nadar in 1860. Hope this clears all the doubt. --SupernovaExplosion Talk 12:01, 24 April 2012 (UTC)
- In that case these photographs could have their own article... Imagine the DYK... But if I'm not mistaken, intersex and hermaphrodite are different. Crisco 1492 (talk) 12:51, 24 April 2012 (UTC)











