Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
June 30
What does the principle of least action actually imply?
The principle of least action must be a fundamental law of physics, as it appears in a diverse range of physical theories from quantum mechanics to general relativity to string theory. But what does the principle of least action actually imply about nature?
You would think you would find the answer in the articles principle of least action or action (physics) but you can't. Why can't you just fudge the action to create any equations of motion? Why then do you need a principle of least action at all? Does the principle of least action actually place any restrictions on the equations of motion? It must do, because (for example) Liouville's theorem is a statement about mechanical systems that obey the principle of least action (as opposed to arbitrary dynamical systems).
For that matter, what is an action, really? As far as I can tell, it is just a function of the space of candidate solutions which is stationary at the true solution. Now, it is true that, in classical mechanics, the action takes a certain form as an integral of a Lagrangian which depends only on certain properties (position and velocity) of points on the trajectory, and this may place restrictions on the possible actions. On the other hand, the Einstein-Hilbert action is not of this form (actually it probably is, but disguised). What makes the Einstein-Hilbert action an action?
I would like to know what the principle of least action actually says about nature. PeterPresent (talk) 14:24, 30 June 2018 (UTC)
- In my opinion the PLA is an observation and a hypothesis and an axiom, in various fields, but it is not quite a law. It's one of those things where it is quite obvious the observable universe obeys it, yet I doubt anybody would be massively surprised to find an exception. Greglocock (talk) 20:11, 30 June 2018 (UTC)
At least in classical mechanics, the PLA (like in Lagrangian dynamics) is a theorem you can derive from Newton's equations of motion (F=ma etc). See calculus of variations for some discussion. In more generality, conservation laws arise from Noether's theorem. 173.228.123.166 (talk) 23:55, 30 June 2018 (UTC)
- And you can derive the Einstein-Hilbert action from the Einstein field equations. But what does the Einstein-Hilbert action have in common with the classical action? What is an action, fundamentally? What is a Lagrangian in abstract mathematical terms? --PeterPresent (talk) 01:09, 1 July 2018 (UTC)
- Was the article action (physics) of any help? This stuff is too advanced for me but I'm imagining an action as something like a path length on a surface, that is minimized on a geodesic resulting from some conservation law. This Mathoverflow thread might be interesting. It might be ok to ask on MO if there is a more formal notion. 173.228.123.166 (talk) 02:32, 1 July 2018 (UTC)
I'm no physicist and I don't understand any of the mathematics, so I don't know if this helps, but according to Andrew Thomas in Hidden in plain sight 2
- The lagrangian is the difference between the kinetic energy of a system minus its potential energy.
- We can calculate the lagrangian of a moving system at any point in time to get a series of values.
- The sum of all these values is the action.
- If we consider the value of a moving system over a period of time the value of the action will always be the lowest posible value as if nature moves objects so as to minimise the action - this gives us the principle of least action, so, for intance, if you throw a ball it will follow a smooth curve as that results in the smallest action.
- We don't yet know why this value is always as small as possible but theorists are convinced that action must be incredibly important.
All this is in the introduction - which is a far as I've got up to now - but he goes on to say he will discuss later in the book more about the balance (and imbalance) of energies which is the key factor in determining the motion of objects. The Hidden in plain sight set of books are incredibly cheap as as ebooks from Amazon but they are very good. Richerman (talk) 00:01, 2 July 2018 (UTC)
- I wonder if there is a reason they make physics students study the equipartition theorem of thermal and statistical physics right before they introduce the Lagrangian formulation of classical dynamic systems. Maybe, maybe, the formal mathematical study of different applications in physics provides insight into generalities of the universe that can only be appreciated after working hundreds of equations over the span of many months of intense study; and that these difficult insights cannot be more rapidly or succinctly conveyed - not even in the best writings by our greatest proponents of popular science. Nimur (talk) 12:38, 2 July 2018 (UTC)
- I merely offered this in case it was of any help to the original poster. There's no need to be so dismissive. Richerman (talk) 17:23, 2 July 2018 (UTC)
- Richerman, I think your post was unhelpful because it was pretty obvious that the OP was already familiar with the classical Lagrangian and its general relativity equivalent (and probably the quantum version using the Feynman path integral). So I think they were asking for a more abstract description of an action, the way Noether's theorem abstractly describes conservations laws. I took a stab at it and didn't reach an actual answer, but I think that was in the direction of what OP wanted. Nimur, at my school the more serious version of the intro physics course did mechanics, e&m, and thermodynamics in that order, and the Lagrangian was introduced in the mechanics part, fwiw. 173.228.123.166 (talk) 22:25, 3 July 2018 (UTC)
- I merely offered this in case it was of any help to the original poster. There's no need to be so dismissive. Richerman (talk) 17:23, 2 July 2018 (UTC)
July 1
Lexell's Comet
I just read the Lexell's Comet article that's currently on the Main Page, and I was confused by the conclusion. In a 2018 paper (DOI: 10.3847/1538-3881/aab1f6), a team examined four asteroids and evaluated whether they could be the remains of the comet; one had a 99.2% chance of being the comet, one had a 74% chance, and the other two had less than 1%. Mathematically, how does this work? Isn't this saying that there's a 173% chance of one of the first two being the comet? I can't imagine such a basic mistake getting past peer-reviewers, so I'm guessing I've misunderstood something. Maybe they said that it broke in pieces, and therefore both of those asteroids could be parts of it? Nyttend (talk) 12:06, 1 July 2018 (UTC)
- See Probability#Not_mutually_exclusive_events. You have to subtract the probability that both are remains of the comet, which comes to 0.992×0.74 = 0.734 (assuming the probabilities are independent). Hence the probability that one or the other (or both) are remains is 99.8%. --Wrongfilter (talk) 12:15, 1 July 2018 (UTC)
July 2
Are the chromosomes in human nucleus with different genes or the same?
Always I thought that DNA is found in the nucleus as one long chain of genes. But yesterday I red the book "the stuff of life - genetics" that if I understand correctly it explains that it's not one long chain of DNA but the 23 chromosomes (in humans) are 23 parts of the whole one DNA. Is it correct?--31.210.182.248 (talk) 00:29, 2 July 2018 (UTC)
- Yes. --Jayron32 04:47, 2 July 2018 (UTC)
- See Human genome. It's a bit more complicated than that. It's 23 pairs of chromosomes for the nuclear DNA, plus separate mitochondrial DNA. Each of the 23 pairs is different from the others. To grossly oversimplify, within each pair, each gene is represented on both pairs, but for each gene the pairs may have different variants of the gene. -Arch dude (talk) 05:35, 2 July 2018 (UTC)
- Eukaryotes like "eu" and me have one or more linear chromosomes. The number of chromosomes varies between species. As stated above, humans are diploid, and have 23 pairs of chromosomes, and therefore 46 total, in all the normal, nucleate somatic cells. (Human red blood cells lose their DNA while maturing.) Human gametes are haploid, having only a single set of chromosomes; two gametes fuse to make the diploid organism. Prokaryotes most commonly have a single circular chromosome, but there is variation. Many have plasmids floating around as well. As also mentioned, our mitochondria have their own DNA. Moreover, they have it in the form of…a single circular chromosome! This is a huge clue to their origins as bacteria that moved in to early eukaryote cells and struck up a partnership. The plastids found in plants similarly have their own DNA, because they originated the same way. There's all kinds of fascinating stuff you can learn from DNA. Another neat one is that humans have two fewer chromosomes than chimpanzees, our closest living relatives. What happened to the other two? In the lineage that lead to humans, they fused together to form human chromosome 2; we can tell because that chromosome has inside it remnants of structures from the pre-fusion chromosomes. --47.146.63.87 (talk) 07:11, 2 July 2018 (UTC)
Psychology of introverts who have given up with close friends and relationships
I come across many people in the world who seem to be introverts (like their solitary time and prefer doing things on their own because it’s easier). Often they also seem to not want close friends or relationships and instead opt to get their social needs nets in organised ways such as language classes or hobby groups. They are not interested in meeting new people. Isn’t this contradictory of an introvert who I thought prefers close friends? 82.132.186.29 (talk) 07:22, 2 July 2018 (UTC)
- A language group or hobby group is quiet and orderly compared to a party. Dmcq (talk) 08:30, 2 July 2018 (UTC)
- The concept of introversion (originally formulated by Carl Jung) has changed somewhat over time. Modern psychologists tend to use the term as it was conceptualized by Hans Eysenck. Quoting from out article on extraversion and introversion: Hans Eysenck described extraversion-introversion as the degree to which a person is outgoing and interactive with other people. ... Extraverts seek excitement and social activity in an effort to heighten their arousal level, whereas introverts tend to avoid social situations in an effort to keep such arousal to a minimum. Looie496 (talk) 13:12, 2 July 2018 (UTC)
Ideal Gas Problem
I have been trying to do this problem from my physical chemistry textbook. I think I understand what the apparatus looks like that they're describing. If I understand it correctly, then with the first pivot configuration, when 423.22 torr of CHF3 is surrounding the bulb, it becomes buoyant enough to reach the balance point. This would be when the density of the CHF3 is the same as the average density of the bulb. For the same pivot setting, we would expect that the unknown fluorocarbon would also balance at the same density. We can get an expression for the density of the CHF3 at the given pressure using the relationship and rearranging to . Now we don't actually know the value of T as it's not given. We could make a reasonable assumption that it's about 300K, or we can instead just set the expression for . Now we can just multiply both sides by RT to get rid of what we don't know. Now, we know punknown, pCHF3 and MCHF3, we we can just solve for MUnknown. I get ~102g/mol. Now if we go through the same procedure with the numbers given for the other pivot setting, we should be able to confirm the answer, but instead I get a much lower result for MUnknown of 90.5g/mol. There's few issues here. One is that it's really weird to have a chemical formula in the form CxHyFz where it has a half Dalton for a molecular weight. Fluorine, carbon and hydrogen are all fairly close to round numbers, so it seems unlikely that that answer is possible. Another is that the pressure values given for the second setting are a bit weird. The pressure needed to bring the unknown into balance was much lower than in the first experiment, but the pressure needed to bring the CHF3 into balance was slightly higher. All things being equal, that points to a higher density of the CHF3 and a lower density of the unknown. If all things are not equal, and the temperature changed drastically between experiments, then might account for the diversion in the pressures of the two gasses required, but it seems like a pretty large deviation. I've been going over this problem for some time and haven't managed to make any breakthroughs. I'm starting to wonder if I'm either tackling it completely wrong, or if the problem itself has an issue. 202.155.85.18 (talk) 10:03, 2 July 2018 (UTC) Edit: In case anyone else has been wreaking their brain trying to solve this, I found the answer in the solutions manual. It's essentially what DMacks said below. 202.155.85.18 (talk) 01:22, 3 July 2018 (UTC)
- I think this is from a fairly advanced textbook (physical chemistry—mid/upper-level undergraduate?), so even if you have correctly solved it, you may have to think about "real-world" issues. I agree with your math for each of the two cases. So consider that the ideal gas laws are only approximate and these values are actual experimental data. I have no idea how "ideal" your gases are, but are the gas laws more reliable at higher or lower pressures? Given one has a "physically impossible/unsolveable" decimal-value, does that cast doubt on the reliability of that measurement and you should exclude that result? Or if you know one gives a suspicious result, does that mean the other is also suspicious (albeit not by direct appearance) and you should somehow average them? DMacks (talk) 10:33, 2 July 2018 (UTC)
- You could be right that additional, practical issues need to be taken into account, but my feel from doing dozens of other problems in this text is that they directly ask for you to comment on practical issues when presenting these questions. Certainly, the point you make about whether the first result is only a plausible value by pure chance, and that it ought to be regarded with the same scepticism as the second is something I'd been mulling over too. As far as the significance of the figures is concerned, I should be able to calculate precise values for the molecular mass to the second or third decimal place, but whether or not it's reasonable to lend such weight to those decimal places in an experiment of this type is just guesswork. This particular problem is from the section on ideal gases (actually, the text insists on the terminology "perfect gas" because the author draws some technical distinction between a perfect gas and a merely ideal gas), and this section is followed by the section on real gases. For that reason, I'd assume that this problem is intended to be solvable with only the concepts taught in the ideal/perfect gas section. Then again, maybe the problem is trying to demonstrate the limits of the ideal gas law to whet our appetites for the real gas section. Who knows? To answer your question, the ideal gas law is valid in the limit of so the lower pressure results should be closer to ideal behaviour. Anyhow, thanks for your answer and your confirmation of my math and strategy for solving the problem. Whether or not I've actually solved the problem, it's made me think long and hard about the issues behind it and at the end of the day that's what the problems are meant to do. 202.155.85.18 (talk) 00:57, 3 July 2018 (UTC)
- There seems to be an issue there. As you mention, you need to increase pressure of the known calibration gas and decrease that of the unknown gas to retrieve equilibrium in the second setting compared to the first one, so it cannot be that gas densities are equal at equilibrium (even outside the ideal gas law, at a given temperature and composition, density always increases with pressure). I suspect the problem is wrong (though I cannot find any plausible typo to fix the values), or that there is more to the experiment's description that you or I understood (after all, why give you two settings of the beam, since one can already obtain a molar mass measurement?). TigraanClick here to contact me 17:36, 2 July 2018 (UTC)
- I guess the typo could be something as simple as them mixing up the pressure values? I'm doing the same amount of head scratching over why they overspecified the problem by giving the results of two experiments, unless it is just to illustrate the level of imprecision. 202.155.85.18 (talk) 00:57, 3 July 2018 (UTC)
- Pressure values are given to 5 significant figures, so that's a big pedagogy mistake from a chemistry textbook, if the measurement imprecision is about 20%. I did think of a typo in the pressure values, but could not find any permutation of values or digits that yields something similar in both cases from a cursory search. TigraanClick here to contact me 07:57, 3 July 2018 (UTC)
- I guess the typo could be something as simple as them mixing up the pressure values? I'm doing the same amount of head scratching over why they overspecified the problem by giving the results of two experiments, unless it is just to illustrate the level of imprecision. 202.155.85.18 (talk) 00:57, 3 July 2018 (UTC)
Astronomy: How does an object get in a Lagrange point?
For instance, if an asteroid crosses the path of a LP, what are (very roughly) the sort of conditions needed for it to get trapped? And if we drop an object at some place a LP will be at later, can it get picked up? (Maybe the article could have a short section about it?) 193.253.244.40 (talk) 17:58, 2 July 2018 (UTC)
- Only L4 and L5 points are stable and therefore objects can only be trapped in these two points. Trapping generally occurs when the velocity of an object becomes sufficiently low with respect to one of those points. This usually happens due to perturbations by other orbiting bodies. However this condition is reversible - if other bodies can push an object into a Lagrangian point, they can also push it out of that point. Ruslik_Zero 18:39, 2 July 2018 (UTC)
- Is ths a variation of Newton's First Law of Motion: A system of bodies either remains gravitationaly bound or continues to be unbound, unless acted upon by a gravitational force? The gravitational force is generated by a change in the collective mass of the system. Thus, the smallest number of bodies required to form or disrupt the smallest system of two bodies, is three. During formation or disruption, the third body is ejected, and can either be pre-existing or generated from the breakup of a single body. IMO. Plasmic Physics (talk) 06:31, 4 July 2018 (UTC)
- No. Newton's First Law says only that an object either remains at rest or continues to move at a constant velocity, unless acted upon by a force. It speaks only qualitatively, not quantitatively, about the force; it is a law of inertia that does not encompass his quantitative Law of Gravity nor by itself give solutions to 2- or 3-body Orbit stability problems. These may be solved by applying small perturbations, see Orbit#Orbital_perturbations.DroneB (talk) 09:46, 4 July 2018 (UTC)
- I'm just saying that a body approaching a LP won't slow down of its own accord, it will need to exchange momentum at just the right moment and vector, with a another body besides the host. Plasmic Physics (talk) 19:51, 4 July 2018 (UTC)
- A small slowly moving body approaching the L4 or L5 point will enter into an orbit around the point. This is the trapping mentioned by Ruslik_Zero. There is no sudden stopping exactly at the LP. The LPs exist in the combined gravitational field of two hosts. DroneB (talk) 10:08, 6 July 2018 (UTC)
- I'm just saying that a body approaching a LP won't slow down of its own accord, it will need to exchange momentum at just the right moment and vector, with a another body besides the host. Plasmic Physics (talk) 19:51, 4 July 2018 (UTC)
- No. Newton's First Law says only that an object either remains at rest or continues to move at a constant velocity, unless acted upon by a force. It speaks only qualitatively, not quantitatively, about the force; it is a law of inertia that does not encompass his quantitative Law of Gravity nor by itself give solutions to 2- or 3-body Orbit stability problems. These may be solved by applying small perturbations, see Orbit#Orbital_perturbations.DroneB (talk) 09:46, 4 July 2018 (UTC)
- Is ths a variation of Newton's First Law of Motion: A system of bodies either remains gravitationaly bound or continues to be unbound, unless acted upon by a gravitational force? The gravitational force is generated by a change in the collective mass of the system. Thus, the smallest number of bodies required to form or disrupt the smallest system of two bodies, is three. During formation or disruption, the third body is ejected, and can either be pre-existing or generated from the breakup of a single body. IMO. Plasmic Physics (talk) 06:31, 4 July 2018 (UTC)
- The Lagrangian points are weird in that they simulate a two-body problem, i.e. a "gravity well" of sorts, albeit one surrounded by tadpole orbits. Even so, they aren't really a two-body problem; you have the asteroid or probe, the moon, and the planet - hypothetically, an object might be captured or released by putting energy into or out of the planet-moon system. I don't know if this actually allows for some very clever orbit to infiltrate or exfiltrate objects from the point without acceleration, though that is never the way the topic is presented. There is some data that might be gotten from this, but I haven't read it, or possibly understood from here, though its emphasis is different. Note that an object wandering outward will eventually escape from a tadpole orbit to a horseshoe orbit, i.e. go from orbiting L4 to orbiting both L4 and L5. Most bodies do make such transitions over time, but I don't know if these necessarily rely on outside forces from other planets (which are unusually relevant, since the object can be nearly on the far side of the sun from the planet and moon it is "orbiting"!) . Wnt (talk) 11:48, 5 July 2018 (UTC)
DNA analysis of siblings
Siblings have (approximately) half of their DNA in common. With the analysis that shows what countries your ancestors came from, do siblings have the same breakdown? Bubba73 You talkin' to me? 20:13, 2 July 2018 (UTC)
- There can be a meaningful amount of variation, as a result of genetic recombination. Consider, as an arbitrary example, chromosome 1. Each child gets two copies of chromosome 1, one from the father and the other from the mother. The copy from the father is actually an amalgamation of the father's two versions of chromosome 1, obtained by splitting the two versions at a more-or-less arbitrary point and rejoining with the versions crossed. (If you don't understand this description, try reading the article.) The upshot is that, although one copy of chromosome 1 is guaranteed to come entirely from your father, the parts of it that come from your father's father and your father's mother are highly variable. The same thing basically applies to every other chromosome except the X and Y sex chromosomes, where the story is more complicated. Looie496 (talk) 21:18, 2 July 2018 (UTC)
- Short answer, no. They will usually be similar, but there will be differences since half their DNA is different and those different segments could have different ancestries. Dragons flight (talk) 22:17, 2 July 2018 (UTC)
- Thank you for the replies. Bubba73 You talkin' to me? 22:26, 2 July 2018 (UTC)
- Bubba73, my father and his siblings had analysis done on them at the end of last year, and they all came out differently. Yes, Dragonsflight's "similar" is correct, but they weren't identical. Nyttend (talk) 22:36, 2 July 2018 (UTC)
- Thank you for the replies. Bubba73 You talkin' to me? 22:26, 2 July 2018 (UTC)
- I had mine done and I've compared to my daughter and a first cousin on both sides (different cousins!). There are naturally commonalities and differences. Bubba73 You talkin' to me? 23:12, 2 July 2018 (UTC)
- This is all going to depend very heavily on the precise analysis done by a company. Suppose they used three genes with alleles that came from Africa or Asia. Then 1/8 of the children of a purebred African-Asian marriage would be African and 1/8 would be Asian. Hence ... they use more markers. But only so many are available, and they aren't really so easily localized to one continent, but just have differences in frequency between continents. To decide how much each means you're making a model of what the historical genetic makeup of the continent was, and then of countries within it, which means defining when you are talking about (100% of New Jersey residents have New Jersey ancestry!). There's even some necessary revisionism involved (do the Irish have British ancestry, because their country was occupied by Britain, or (I think) once considered part of the Britannia Province of Rome?) The more I think about it the more the wheels come off this wagon, and there's a reason - because you don't inherit nations, you inherit genes. Wnt (talk) 11:59, 5 July 2018 (UTC)
- The Romans didn't bother with Ireland, they called it Hibernia (winter land) which makes most land at that latitude laugh as to them the Irish winter is weak and mild like a little lamb. At least one Roman travelled to the west shore of Ireland (the Cliffs of Moher I think) and thought it was the edge of the World. Sagittarian Milky Way (talk) 17:48, 5 July 2018 (UTC)
July 3
Outstretched Fingers Form Circular Pattern
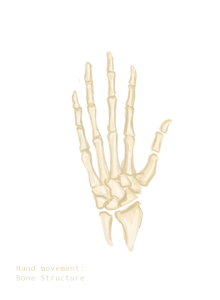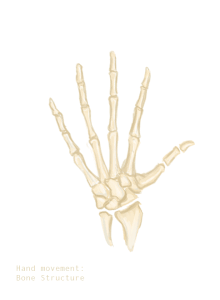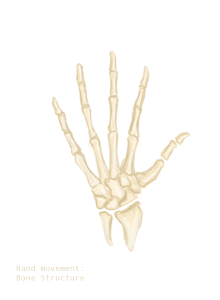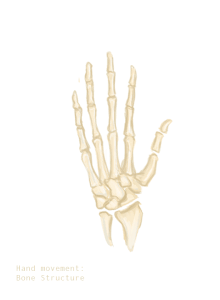
When outstretched, the fingers of the human hand seem to form a circular pattern. Is it true ? Is it known ? If so, what would be the biological explanation ? — 79.118.184.15 (talk) 03:45, 3 July 2018 (UTC)
- I'm not seeing anything obvious in Google, but here's something you could try: Position your hand in that way on a piece of paper, and put a mark for each fingertip. Then see if you can fit a circle to it. ←Baseball Bugs What's up, Doc? carrots→ 04:36, 3 July 2018 (UTC)
- The center of the circle seems to coincide with the center of the palm. Furthermore, the lengths of the pinky and the thumb appear equal, as do those of the index and ring finger. — 81.196.204.42 (talk) 16:41, 3 July 2018 (UTC)
- The biological explanation is that the fingers radiate from the carpals, as shown in this animation.--Shantavira|feed me 06:23, 3 July 2018 (UTC)
- It looks oval rather than circular. ←Baseball Bugs What's up, Doc? carrots→ 12:00, 3 July 2018 (UTC)
The subject at hand (!) could have been a better wing, flipper or purr-fect scratchy-grippy tool but instead you have something that does better than any of them on any of these. DroneB (talk) 14:03, 3 July 2018 (UTC)
- I'm not quite sure what the OP is referring to, but digit ratio seems to be the appropriate article; there are apparently lots of things that influence finger length ratios (and hence the overall shape of the outstretched hand). Matt Deres (talk) 15:29, 3 July 2018 (UTC)
- The immediate developmental reason is that the limb bud forms a rough circle, which is carved up by apoptosis into individual fingers. Of course, this doesn't have to lead on to a final circular pattern -- see File:Pterosaur Wing Anatomy.png about pterosaurs. It is not straightforward to decide whether fingers are the same length because this development is just "easier" or whether there is an adaptive reason (easier to clutch a fistful of silver ... probably not) Wnt (talk) 10:24, 4 July 2018 (UTC)
Diesel engines
I was asked to put this in a separate question. If a diesel engine burns pure cetane it's probably a standardized cetane number testing engine burning it for a short time maybe for calibration or something and that for the purposes of comparability might have its design frozen since the invention of cetane numbers and thus not have to deal with the many thousands of injector psis or hundreds of thousands of miles of modern engines. So I'm wondering if 100% n-hexadecane is just overkill or if it'd actually be a poor fuel for other reasons like lubricity even in like Nauru (all time low temperature: 20C, melting point: 18) Sagittarian Milky Way (talk) 19:52, 3 July 2018 (UTC)
July 4
Please ID the flower
[1] Thank you. 104.162.197.70 (talk) 02:17, 4 July 2018 (UTC)
- Looks like Scadoxus multiflorus (Blood lily) although I've never seen them in blue... Abecedare (talk) 02:32, 4 July 2018 (UTC)
- I'd just remark in passing that it's not entirely clear which flower the OP is asking us to identify. There appear to be at least two, and possibly as many as five, different species of flower in the picture. --Trovatore (talk) 03:58, 4 July 2018 (UTC)
SpaceX
Hi guys,
This graph should be updated to "12" for 2018. Source. Thank you, designers ;) Ericdec85 (talk) 03:15, 4 July 2018 (UTC)
- You may wish to consider raising the issue with Kjerish or one of the editors who has previously updated the graph. I see no indication of which software was utilized to create the infographic on the file page and while no particular software is required, others on the articles where the graph is employed will probably prefer continuity. Snow let's rap 07:35, 4 July 2018 (UTC)
- @Ericdec85: @Snow Rise: Done — Kjerish (talk) 09:57, 4 July 2018 (UTC)
- Thank you Kjerish, that was very accommodating of you. :) Snow let's rap 10:01, 4 July 2018 (UTC)
Is there or was there a diseases so called "hadroken" or "hadrokan"?
In the Talmud there is a word for a disease called "הדרוקן" (there are many options how to read it: Hadroken, Hadrokan, Hydroken, Hydrokan, hedroken, hadrokan). I tried to google them but I didn't find answer. Also it makes sense that it has to do something with water or liquid (hydro prefix), I'm not sure if it's not something more specific (congestion, edema? or maybe something else which should be solved when such word will be found in the ancient times).--2A02:ED0:6D6D:F300:14FF:7A4C:A0F5:D524 (talk) 13:19, 4 July 2018 (UTC)
- This was also posted at the language desk. ←Baseball Bugs What's up, Doc? carrots→ 13:24, 4 July 2018 (UTC)
- Please continue at the Language Desk. Alansplodge (talk) 16:49, 4 July 2018 (UTC)
Quantum spin pairing in atomic and molecular species
I can't seem to find a relevant article to answer this question. I know that spin pairing can occur over degenerate energy levels to turn a triplet into a singlet state, but what about over non-degenerate levels of an excited state? Plasmic Physics (talk) 13:21, 4 July 2018 (UTC)
- You can look at Helium_atom - there are both singlet and triplet states. Ruslik_Zero 20:22, 4 July 2018 (UTC)
- So, yes? If this is true please have a look at [2]. Why is the Ath state higher in energy than the bth state if the Ath and ath states have the same configuration but multiplicities? The only way that this makes sense, is if the pi orbitals are infact higher in energy than the px+px sigma orbital. Does that mean that most of the MO diagrams on Google are wrong? i.e - is the electronic configuration: 1σ2
g1σ2
u2σ2
g2σ2
u3σ2
g1π2
u? Plasmic Physics (talk) 01:52, 5 July 2018 (UTC)- A and a states have the same projection of the orbital momentum on the molecular axis and the same parity but different spin. However this does not mean that their electronic configuration are similar - they can be very different. Ruslik_Zero 20:11, 5 July 2018 (UTC)
- I see what the problem is. Where can I find a demonstration of how to correctly determine a term symbol from an MO diagram. I thought I have figured it out, but it seems I'm wrong. I need to understand how to determine the angular momentum projections (Λ and Ω). Plasmic Physics (talk) 08:42, 6 July 2018 (UTC)
- A and a states have the same projection of the orbital momentum on the molecular axis and the same parity but different spin. However this does not mean that their electronic configuration are similar - they can be very different. Ruslik_Zero 20:11, 5 July 2018 (UTC)
- So, yes? If this is true please have a look at [2]. Why is the Ath state higher in energy than the bth state if the Ath and ath states have the same configuration but multiplicities? The only way that this makes sense, is if the pi orbitals are infact higher in energy than the px+px sigma orbital. Does that mean that most of the MO diagrams on Google are wrong? i.e - is the electronic configuration: 1σ2
Is there a scientific basis for the claim that carbonated water expand the stomach?
I heard that carbonated water expand the stomach because of the gases and therefore it can cause to obesity (the bigger stomach the more food). Is there a scientific basis for the claim that carbonated water expand the stomach?--2A02:ED0:6D6D:F300:14FF:7A4C:A0F5:D524 (talk) 21:56, 4 July 2018 (UTC)
- That sounds Not even wrong. Bloating and obesity are two different things. Drinking carbonated water could cause bloating. Obesity is ultimately caused by consuming more Food energy than one uses (though it can be influenced by other factors). Sodas have carbonated water but usually also have a lot of sugar. Ian.thomson (talk) 22:00, 4 July 2018 (UTC)
- I really don't understand your answer, obesity can also caused indirectly by stomach expansion that cause to more consumption of fats or carbs. Compare with Sleeve gastrectomy that analogically works based on this principle. --2A02:ED0:6D6D:F300:7869:435F:5ED7:13CC (talk) 00:40, 6 July 2018 (UTC)
- Competitive eaters who want to stretch their stomachs to make them larger don't use carbonation. It just makes them burp. See Competitive eating#Training and preparation and [3][4][5][6] for how they do it. --Guy Macon (talk) 22:40, 4 July 2018 (UTC)
July 5
Scientifically, is it possible to improve IQ score?
Scientifically, is it possible to improve IQ score? Or what human have it's constant for him forever, no matter what he does to improve it? --2A02:ED0:6D6D:F300:7869:435F:5ED7:13CC (talk) 13:09, 5 July 2018 (UTC)
- See Flynn effect. IQs are on the rise for society as a whole. Section 3 of the article gives several possible explanations for this increase. --Doroletho (talk) 14:44, 5 July 2018 (UTC)
- If you look further you'll see there is some evidence the effect is ending or has ended and IQs are now decreasing if anything. Dmcq (talk) 11:21, 6 July 2018 (UTC)
- My mother was a schoolteacher and administered several tests. She would also (in between crosswords and knitting) solve the papers for herself. So on an IQ test (or at least, the Cattell tests she was familiar with) she could regularly nail a 180+ score. Now she was smart, but not that smart. Andy Dingley (talk) 17:12, 5 July 2018 (UTC)
- The article here on wiki is not clear in the light of these answers. --2A02:ED0:6D6D:F300:7869:435F:5ED7:13CC (talk) 00:42, 6 July 2018 (UTC)
- Why not?? My mother did one of those societies tests and got 165 and one of my sons 183 so it certainly isn't unreasonable. Dmcq (talk) 11:30, 6 July 2018 (UTC)
Measles
This news article about a measles case in Portland says, "A person is considered immune to measles if they were born before 1957, had the measles previously, or have been fully vaccinated for measles." How does being born before 1957 afford immunity? Is this just a poorly worded sentence based on the assumption that most people born before 1957 are immune because they are likely to have gotten measles in their childhood? (In this case, the sentence would be more accurate if "were born before 1957" were simply removed from the sentence.) ANDREVV (talk) 13:20, 5 July 2018 (UTC)
- It's because they will have lived through several epidemics and so are likely to be immune see [7] Richerman (talk) 14:28, 5 July 2018 (UTC)
- The year is included because it is the date at which the (U.S.) Centers for Disease Control and Prevention advise that people do not need the measles vaccine booster - Nunh-huh 16:11, 5 July 2018 (UTC)
When 20 is not the double of 10 in a scale
How do you call a scale like Celsius, in which 20 degrees is not the double of 10? Or SPF, where SPF 30 does not protect 50% more than SPF 20? --Doroletho (talk) 14:36, 5 July 2018 (UTC)
- Celsius is a relative temperature scale with arbitrary reference points, conventionally 0 °C and 100 °C for the freezing and boiling temperatures of water. One may however convert a Celsius reading to Kelvin using [K] = [°C] + 273.15. The Kelvin scale is absolute so "twice the Kelvin" means "twice as hot".
- SPF (Sun Protection Factor) is a reciprocal expression of the fraction of sunburn-producing UV rays that a Sunscreen allows to reach the skin. For example, "SPF 15" means that 1⁄15 of the radiation reaches the skin, or conversely that one may sunbathe 15 times longer with the same risk of burn as without the sunscreen. SPF 30 should allow 50% longer sunbathing than SPF 20. DroneB (talk) 16:55, 5 July 2018 (UTC)
- Our article Level of measurement explains the relevant concepts. It distinguishes four types of scale: nominal, ordinal, interval, and ratio. Celsius is an interval scale. SPF is a ratio scale. Looie496 (talk) 17:15, 5 July 2018 (UTC)
- So in the terminology of that article, the answer to the question is "it's not a ratio scale". However, note that SPF is a ratio scale: SPF 30 does protect 50% more than SPF 20, if everything else is equal.
- However, while I don't imagine that the authors of the article made it up, I do not think this "ratio scale" terminology is widely used. I read quite a bit about units of measurement and I don't remember ever hearing of it before. --76.69.47.228 (talk) 02:09, 6 July 2018 (UTC)
- IP is correct. See Level of measurement. The terms you are likely looking for are nominal, ordinal, interval, and ratio scales. Nominal scales are things like gender, religious affiliation, or race. Ordinal scales are ones that can be ordered from most to least or largest to smallest, like likert scales, but the distance between each value is unknown. Interval scales are rather uncommon but examples are Fahrenheit and Celsius and dress sizes. They are like ordinal scales butt the distance between each value is known to be equal. However, they do not have a true zero value. Ratio scales have all the attributes of interval scales but have a true zero value. An example would be the Kelvin scale. EvergreenFir (talk) 02:15, 6 July 2018 (UTC)
- That's my take too; even in the Kelvin scale you can't say "twice as hot". There'd have to be a scale based on the average velocities of the particles. Abductive (reasoning) 23:07, 6 July 2018 (UTC)
- As an aside, I've been following this thread and find it very intriguing/educational. Thanks to all who've participated, even though I'm not the OP! Killiondude (talk) 23:21, 6 July 2018 (UTC)
- I've heard of million, billion, trillion, etc. How many is a killion? ←Baseball Bugs What's up, Doc? carrots→ 01:52, 7 July 2018 (UTC)
- IP is correct. See Level of measurement. The terms you are likely looking for are nominal, ordinal, interval, and ratio scales. Nominal scales are things like gender, religious affiliation, or race. Ordinal scales are ones that can be ordered from most to least or largest to smallest, like likert scales, but the distance between each value is unknown. Interval scales are rather uncommon but examples are Fahrenheit and Celsius and dress sizes. They are like ordinal scales butt the distance between each value is known to be equal. However, they do not have a true zero value. Ratio scales have all the attributes of interval scales but have a true zero value. An example would be the Kelvin scale. EvergreenFir (talk) 02:15, 6 July 2018 (UTC)
- Unless I'm misunderstanding the question, any logarithmic scale fits the description. Most commonly used ones are base-10 logarithmic, meaning each level on the scale represents a power of ten. A pH of 5 is ten times more acidic than a pH of 6, and so forth. --47.146.63.87 (talk) 06:41, 7 July 2018 (UTC)
Children of homosexual couples.
I recently came across an entry for Linda Perry. In her personal section it says her wife gave birth to their child. This seems to imply that biologically something happened. I do realize that this is a sensitive topic, however, for accuracy wouldn't it be more appropriate to specify surrogacy (or another method?) There may come a time where science allows for a biological child, but for now it is technically incorrect to state or imply that this child belongs biologically to both partners
https://en.wikipedia.org/wiki/Linda_Perry
https://en.wikipedia.org/wiki/Sara_Gilbert — Preceding unsigned comment added by 24.144.1.3 (talk) 17:31, 5 July 2018 (UTC)
- This comment belongs on the article’s talk page, not here where the purpose is to request references. Loraof (talk) 19:27, 5 July 2018 (UTC)
- This is addressed at Talk:Sara Gilbert#Parentage. -- ToE 07:53, 6 July 2018 (UTC)
July 6
Why soda (Sodium bicarbonate) isn't considered as anti acidic drug?
There are many antacids drugs, but my question Why soda (Sodium bicarbonate) which anybody consumes it as a carbonated water form isn't considered as anti acidic drug? According to my logic, soda is base and base is antacid. Isn't it? --2A02:ED0:6D6D:F300:7869:435F:5ED7:13CC (talk) 00:46, 6 July 2018 (UTC)
- The drink "soda" is likely to have no sodium bicarbonate in it. The carbon dioxide will be partly in the form of carbonic acid which then might make a tiny amount of bicarbonate as it ionises. But this is balanced by the hydrogen ions, so it is not classified as a base, but more as an acid. I won't comment on drug classification as to antacid use. Graeme Bartlett (talk) 02:03, 6 July 2018 (UTC)
- Not true. Many of them do have sodium bicarbonate: See here for example (club soda ingredients of schweppes). 188.120.129.140 (talk) 15:09, 6 July 2018 (UTC)
- And here is a listing of pH values for a number of popular non-alcoholic, non-dairy drinks. You'll note that almost all of them have pH<7.0, i.e. are acidic. Abecedare (talk) 02:17, 6 July 2018 (UTC)
- Some soda water has potassium carbonate added, whihc would be in the form of potassium bicarbonate once carbonated. Also there are mineral waters with calcium bicarbonate or magnesium bicarbonate in solution. Graeme Bartlett (talk) 03:55, 6 July 2018 (UTC)
- Magnesium salts cause diarrhea don't they? And another one maybe aluminum causes constipation. So if the OP wants a cheap antacid that should be taken into consideration (and that the cheapest chemicals would have impurities that might make them unfit for human consumption. I'm not sure if reagent grade is pure enough to be sure it's food grade if the chemical you're getting 99.99x% of is even safe to eat in antacid quantities in the first place. Also some bases are too strong to eat without great dilution. i.e. Sodium hydroxide is a base and that article has a photo showing that it eats organic tissue without a lot of dilution. This is also why wet cement itches (alkalinity) cement is not a good antacid obviously) Sagittarian Milky Way (talk) 06:02, 6 July 2018 (UTC)
- Some soda water has potassium carbonate added, whihc would be in the form of potassium bicarbonate once carbonated. Also there are mineral waters with calcium bicarbonate or magnesium bicarbonate in solution. Graeme Bartlett (talk) 03:55, 6 July 2018 (UTC)
- And here is a listing of pH values for a number of popular non-alcoholic, non-dairy drinks. You'll note that almost all of them have pH<7.0, i.e. are acidic. Abecedare (talk) 02:17, 6 July 2018 (UTC)
- Almost no one in richer countries is short on sodium and lots of people eat too much. That's probably why. Sagittarian Milky Way (talk) 02:37, 6 July 2018 (UTC)
- And sodium bicarbonate isn't generally a major ingredient in soda drinks for enjoyment because too much would add saltiness. Perhaps someone is selling some sodium bicarbonate water somewhere to drink as a "keep healthy" or dubious remedy for something but it probably wouldn't taste good or sate your thirst if it has enough sodium bicarbonate to use as an antacid. Sagittarian Milky Way (talk) 02:45, 6 July 2018 (UTC)
- Sodium bicarbonate is baking soda. It can make non-carbonated acidic drinks like juice bear some resemblance to soda with the carbon dioxide bubbles and can remove all of its acidity but probably also makes it taste too salty. Sagittarian Milky Way (talk) 02:55, 6 July 2018 (UTC)
- In addition to the points above, sodium bicarbonate (actual sodium bicarbonate, not soda water) is often used an an antacid. Our article you linked to specifically mentions it although as it also mentions, alternatives are frequently preferred for various reasons. While our article primarily mentions drug like formulations which for many given simplicity, dosage etc are preferred; some people do just take regular baking soda [8], generally mixed with water. Nil Einne (talk) 03:06, 6 July 2018 (UTC)
July 7
Does hypoalbuminemia causes decrease in oncotic pressure or osmotic pressure?
Does hypoalbuminemia causes decrease in oncotic pressure or osmotic pressure? I have red two different sources which everyone says something different, one says it decreases oncotic pressure and one says it decreases osmotic pressure. Who's more correct? --2A02:ED0:6D6D:F300:E469:6A54:1B75:3955 (talk) 01:26, 7 July 2018 (UTC)
- If you re-read the article Kwashiorkor that you linked in your next post, you'll see that it says in parts:
- "Proteins, mainly albumin, are responsible for creating the colloid osmotic pressure (COP) observed in the blood and tissue fluids. The difference in the COP of the blood and tissue is called the oncotic pressure."
- and:
- "The typical swollen abdomen is due to two causes: ascites because of hypoalbuminemia (low oncotic pressure), and enlarged fatty liver."
- So, to answer your first query; hypoalbuminemia doesn't cause a decrease in oncotic pressure, it is a low (decreased) oncotic pressure. To address your second query; both are correct – the oncotic pressure is the difference between the two osmotic pressures, so if you alter one of those osmotic pressures, you will by definition alter the oncotic pressure. Hope this helps. {The poster formerly known as 87.81.230.195} 90.220.213.151 (talk) 06:54, 7 July 2018 (UTC)
Why in starvation there is mainly ascites rather than edema?
After reading the article Kwashiorkor I have noticed that in photos of people (especially children) in starvation always have bloated abdomen as if they are fatty but according to the mentioned articale (as well as more articles) this is due to hypoalbuminemia. Now my question is if hypoalbuminemia causes to leak of fluid into the abdominal cavity (ascites) due to changes in osmotic / oncotic pressure, then it should be anywhere in body (edema) rather than ascites only. Isn't it? But always in photos of starvation we see only bloated abdomen so it says that there is something special in the blood vessels in the abdomen (which causes to this phenomenon in the abdomen only) that in other vessels there is no, and I don't understand what it is. 2A02:ED0:6D6D:F300:E469:6A54:1B75:3955 (talk) 01:38, 7 July 2018 (UTC)
Hæmatophyte (plant blood parasites)
I found this page through Wiktionary's list of dictionary-only words. It contains citations about unicellular plants (described in one entry as either fungi or algae) that infect, or live in, the bloodstream. What is this actually referring to? Are there actually algae and fungi living in the bloodstream, or do the citations refer to something else that was incorrectly classified as such? 169.228.156.220 (talk) 04:10, 7 July 2018 (UTC)




