From Wikipedia, the free encyclopedia
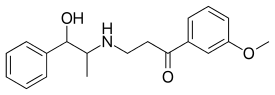
Oxyfedrine is a vasodilator and a β adrenoreceptor agonist . It was found to depress the tonicity of coronary vessels, improve myocardial metabolism (so that heart can sustain hypoxia better) and also exert a positive chronotropic and inotropic effects, thereby not precipitating angina pectoris . The latter property (positive chronotropic and inotropic effects) is particularly important, because other vasodilators used in angina may be counter productive causing coronary steal phenomenon.
Synergistic effects with antibiotics have been suggested.[ 1]
References
α1
Agonists Antagonists
Abanoquil Ajmalicine Alfuzosin Anisodamine Anisodine Atiprosin Atypical antipsychotics (e.g., brexpiprazole , clozapine , olanzapine , quetiapine , risperidone )Benoxathian Beta blockers (e.g., adimolol , amosulalol , arotinolol , carvedilol , eugenodilol , labetalol )Buflomedil Bunazosin Corynanthine Dapiprazole Domesticine Doxazosin Ergolines (e.g., acetergamine , ergotamine , dihydroergotamine , lisuride , nicergoline , terguride )Etoperidone Fenspiride Hydroxyzine Indoramin Ketanserin L-765,314 mCPP Mepiprazole Metazosin Monatepil Moxisylyte Naftopidil Nantenine Neldazosin Niaprazine Niguldipine Pardoprunox Pelanserin Perlapine Phendioxan Phenoxybenzamine Phentolamine Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Piperoxan Prazosin Quinazosin Quinidine Silodosin Spegatrine Spiperone Talipexole Tamsulosin Terazosin Tiodazosin Tolazoline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , imipramine , trimipramine )Trimazosin Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Urapidil WB-4101 Zolertine
α2
Agonists Antagonists
1-PP Adimolol Amesergide Aptazapine Atipamezole Atypical antipsychotics (e.g., asenapine , brexpiprazole , clozapine , lurasidone , olanzapine , paliperidone , quetiapine , risperidone , zotepine )Azapirones (e.g., buspirone , gepirone , ipsapirone , tandospirone )BRL-44408 Buflomedil Cirazoline Efaroxan Esmirtazapine Fenmetozole Fluparoxan Idazoxan Ketanserin Lisuride mCPP Mianserin Mirtazapine NAN-190 Pardoprunox Phentolamine Phenoxybenzamine Piperoxan Piribedil Rauwolscine Rotigotine Setiptiline Spegatrine Spiroxatrine Sunepitron Terguride Tolazoline Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Yohimbine
β