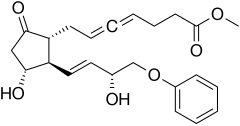
Enprostil
Appearance
This article needs more links to other articles to help integrate it into the encyclopedia. (March 2016) |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C23H28O6 |
| Molar mass | 400.46 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Enprostil is a synthetic prostaglandin designed to resemble dinoprostone. Enprostil was found to be a highly potent inhibitor of gastric HCl secretion.[1]
References
- ^ Roszkowski, AP; Garay, GL; Baker, S; Schuler, M; Carter, H (1986). "Gastric antisecretory and antiulcer properties of enprostil, (+/−)-11 alpha, 15 alpha-dihydroxy-16-phenoxy-17,18,19,20-tetranor-9-oxoprosta-4,5,13(t)-trienoic acid methyl ester". The Journal of Pharmacology and Experimental Therapeutics. 239 (2): 382–9. PMID 3095537.
External links
- Toshina, K.; Hirata, I.; Maemura, K.; Sasaki, S.; Murano, M.; Nitta, M.; Yamauchi, H.; Nishikawa, T.; et al. (2000). "Enprostil, a Prostaglandin-E2 Analogue, Inhibits Interleukin-8 Production of Human Colonic Epithelial Cell Lines". Scandinavian Journal of Immunology. 52 (6): 570–5. doi:10.1046/j.1365-3083.2000.00815.x. PMID 11119262.
- Tari, Akira; Hamada, Masanori; Kamiyasu, Toshiki; Sumii, Koji; Haruma, Ken; Inoue, Masaki; Kishimoto, Shinya; Kajiyama, Goro; Walsh, John H. (1997). "Effect of enprostil on omeprazole-induced hypergastrinemia and inhibition of gastric acid secretion in peptic ulcer patients". Digestive Diseases and Sciences. 42 (8): 1741–6. doi:10.1023/A:1018825902055. PMID 9286243.
- Ching, C. K.; Lam, S. K. (1995). "A comparison of two prostaglandin analogues (enprostil vs misoprostol) in the treatment of acute duodenal ulcer disease". Journal of Gastroenterology. 30 (5): 607–14. doi:10.1007/BF02367786. PMID 8574332.
