Prednisolone: Difference between revisions
Pharming16 (talk | contribs) image added |
Pharming16 (talk | contribs) Mechanism of Action |
||
| Line 114: | Line 114: | ||
==Mechanism of action== |
==Mechanism of action== |
||
Prednisone is the active drug metabolite of prednisone.<ref>{{Cite journal|url = http://download.springer.com/static/pdf/955/art%253A10.1007%252Fs40262-012-0007-8.pdf?originUrl=http%3A%2F%2Flink.springer.com%2Farticle%2F10.1007%2Fs40262-012-0007-8&token2=exp=1446589366~acl=%2Fstatic%2Fpdf%2F955%2Fart%25253A10.1007%25252Fs40262-012-0007-8.pdf%3ForiginUrl%3Dhttp%253A%252F%252Flink.springer.com%252Farticle%252F10.1007%252Fs40262-012-0007-8*~hmac=1fac44cb5e3d8fefd855e0bd9221b87040c3d70c8420947cf4f88559a9c62960|title = Clinical Pharmacokinetics and Pharmacodynamics of Prednisolone and Prednisone in Solid Organ Transplantation|last = Bergmann|first = Troels|date = 7 September 2012|journal = Clinical Pharmacokinetics|doi = |pmid = |access-date = }}</ref> As a synthetic glucocorticoid (GC), its lipophilic structure allows for easy passage through the cell membrane where it then binds to its respective glucocorticoid receptor (GCR) located in the cytoplasm. Upon binding, formation of the GC/GCR complex causes dissociation of chaperone proteins from the glucocorticoid recepetor enabling the GC/GCR complex to translocate inside the nucleus. This process occurs within 20 minutes of binding. Once inside the nucleus, the homodimer GC/GCR complex binds to specific DNA binding-sites known as glucocorticoid response elements (GREs) resulting in gene expression or inhibition. Complex binding to positive GREs leads to synthesis of anti-inflammatory proteins while binding to negative GREs block the transcription of inflammatory genes.<ref>{{Cite journal|url = http://ac.els-cdn.com/S0303720707002110/1-s2.0-S0303720707002110-main.pdf?_tid=3ad5455a-827d-11e5-b74d-00000aab0f26&acdnat=1446591187_d76fcee3317cd77021dada53514f4310|title = Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists|last = Stahn|first = Cindy|date = 17 May 2007|journal = Molecular and Cellular Endocrinology|doi = |pmid = |access-date = }}</ref> |
|||
Prednisolone irreversibly binds with [[glucocorticoid receptor]]s (GR) alpha and beta for which they have a high affinity. AlphaGR and BetaGR are found in virtually all tissues with variable numbers between 3000 and 10000 per cell, depending on the tissue involved. Prednisolone can activate and influence biochemical behaviour of most cells. The steroid/receptor complexes dimerise and interact with cellular DNA in the nucleus, binding to steroid-response elements and modifying gene transcription. They induce synthesis of some proteins, and inhibit synthesis of others.<ref>Prednisolone. Australian Medicines Handbook 2010. Adelaide: Australian Medicines handbook Pty Ltd.; 2010. 497, 598.</ref><ref>Rang HP, Dale MM, Ritter JM, Moore PK. The pituitary and the adrenal Cortex. Hunter l, editor. Pharmacology. 5th ed. London: Churchill Livingstone; 2003. 413, 415.</ref> |
|||
Not all metabolic actions on genes are known. Most mediator proteins are enzymes, e.g., cAMP-dependent kinase |
|||
Anti-inflammatory and immunosuppressive actions: |
|||
*Inhibition of gene transcription for [[Cyclooxygenase-2|COX-2]], cytokines, cell adhesion molecules, and [[inducible NO synthase]] |
|||
*Blockage of Vitamin D3-mediated induction of osteocalcin gene in [[osteoblasts]] |
|||
*Modification of collegenase gene transcription |
|||
*Increase synthesis [[Annexin A1|annexin-1]], important in negative feedback on [[hypothalamus]] and [[anterior pituitary gland]] |
|||
Regulation of gene suppression leads to systemic suppression of inflammation and immune response. This is of clinical usefulness but ultimately leads to [[gluconeogenesis]], proteolysis and lipolysis. Gene transcription returns to normal after cessation, but sudden stoppage can cause Addison's disease. Osteoporosis is permanent. |
|||
==Banned status in athletics== |
==Banned status in athletics== |
||
Revision as of 22:51, 3 November 2015
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 11,17-dihydroxy-17- (2-hydroxyacetyl)- 10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17- dodecahydrocyclopenta [a]phenanthren-3-one |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682794 |
| License data |
|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 2-3 hours |
| Excretion | urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.020 |
| Chemical and physical data | |
| Formula | C21H28O5 |
| Molar mass | 360.444 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
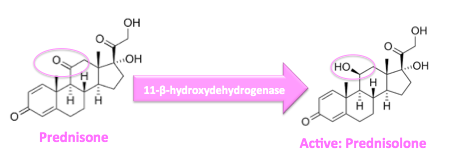
Prednisolone is a synthetic glucocorticoid, a derivative of cortisol, which is used to treat a variety of inflammatory and auto-immune conditions. It is the active metabolite of the drug prednisone[1] and is used especially in patients with liver failure, as these individuals are unable to metabolize prednisone into active prednisolone; it is primarily metabolized via the liver enzyme, 11-β-hydroxydehydrogenase.[2]
Medical uses
Prednisolone is a corticosteroid drug with predominant glucocorticoid and low mineralocorticoid activity, making it useful for the treatment of a wide range of inflammatory and auto-immune conditions[3] such as asthma,[4] uveitis, pyoderma gangrenosum, rheumatoid arthritis, ulcerative colitis, pericarditis, temporal arteritis and Crohn's disease, Bell's palsy, multiple sclerosis,[5] cluster headaches, vasculitis, acute lymphoblastic leukemia and autoimmune hepatitis,[6] systemic lupus erythematosus, Kawasaki disease, [7] dermatomyositis[8], and sarcoidosis.[9]
Prednisolone acetate ophthalmic suspension (eye drops) is an adrenocortical steroid product, prepared as a sterile ophthalmic suspension and used to reduce swelling, redness, itching, and allergic reactions affecting the eye.[10][11]
Prednisolone can also be used as an immunosuppressive drug for organ transplants[12][13] and in cases of primary adrenal insufficiency (Addison's disease)[14][15].
Corticosteroids inhibit the inflammatory response to a variety of inciting agents and, it is presumed, delay or slow healing.[16] They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation with inflammation.[17]
Adverse effects
There are several adverse reaction from the use of prednisolone:
- Increase appetite, weight gain, nausea, and malaise
- Cardiovascular events children
- Dermatological effects including reddening of face, bruising/ skin discoloration, impaired wound healing, thinning of skin, skin rash and fluid build up abnormal hair growth
- Patients with diabetes may need increase insulin or diabetic therapies
- Menstrual abnormalities
- Less response to hormones especially during stressful instances such as surgery or illness.
- Change in electrolytes: rise in blood pressure and increase sodium, low potassium leading to alkalosis, potassium loss
- GI system effects: Swelling of stomach lining, reversible increase liver enzymes and stomach ulcers
- Muscular and skeletal abnormalities such as muscle weakness and loss, osteoporosis, long bone fractures, tendon rupture and back fractures.
- Neurological effects include involuntary movements (convulsions), headaches, and vertigo [18]
Loteprednol is an analog drug that has reduced adverse ocular effects.
Prednisolone may cause serious mental health problems, these affect around 5% who take such steroids.[19] Symptoms include:
- depression, including suicidal thoughts
- feeling high (mania) or mood swings
- anxiety
- insomnia
- difficulty in thinking / confusion
- memory loss
- visual, auditory or tactile hallucinations
- having strange and frightening thoughts, changing behaviour or having feelings of being alone
Nasal septum perforation and bowel perforation are also notable adverse effects that restrict steroids' use in some pathologic conditions.[20][21]
Withdrawal from prednisolone can be problematic after taking large doses or over more than two weeks.[22] This is caused by prednisolone inhibiting the natural production of corticosteroids in the "Hypothalamic-Pituitary-Adrenal Axis" (HPAA)[22]
Use in Pregnancy and Breastfeeding
Although there are no major human studies of prednisolone use in pregnant women, studies in several animals show that it cause birth defects including increase cleft palate. Prednisolone should be used in pregnant women when benefits outweigh the risks and children born from mothers using prednisolone during pregnancy should be monitored for impaired adrenal function.
Prednisolone is found in breast milk of mothers taking prednisolone. [23]
Mechanism of action
Prednisone is the active drug metabolite of prednisone.[24] As a synthetic glucocorticoid (GC), its lipophilic structure allows for easy passage through the cell membrane where it then binds to its respective glucocorticoid receptor (GCR) located in the cytoplasm. Upon binding, formation of the GC/GCR complex causes dissociation of chaperone proteins from the glucocorticoid recepetor enabling the GC/GCR complex to translocate inside the nucleus. This process occurs within 20 minutes of binding. Once inside the nucleus, the homodimer GC/GCR complex binds to specific DNA binding-sites known as glucocorticoid response elements (GREs) resulting in gene expression or inhibition. Complex binding to positive GREs leads to synthesis of anti-inflammatory proteins while binding to negative GREs block the transcription of inflammatory genes.[25]
Banned status in athletics
As a glucocorticosteroid, unauthorized or ad-hoc use of prednisolone during competition via oral, intravenous, intramuscular or rectal routes is banned under WADA anti-doping rules.[26] The drug may be used in competition with a TUE (Therapeutic Use Exemption), in compliance with WADA regulations. Local or topical use of prednisolone during competition as well as any use out of competition is not regulated.
See also
References
- ^ Davis M, Williams R, Chakraborty J, et al. (June 1978). "Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability". British Journal of Clinical Pharmacology. 5 (6): 501–5. doi:10.1111/j.1365-2125.1978.tb01664.x. PMC 1429358. PMID 656293.
- ^ Frey, Brigitte (March 1985). "Pharmacokinetics of 3 prednisolone prodrugs. Evidence of therapeutic inequivalence in renal transplant patients with rejection". Transplantation. doi:10.1160/TH11-09-0672. PMID 3883592.
{{cite journal}}:|access-date=requires|url=(help) - ^ Czock D, Keller F, Rasche FM, Häussler U (2005). "Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids". Clin Pharmacokinet. 44 (1): 61–98. PMID 15634032.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fiel SB, Vincken W (Jun 2006). "Systemic corticosteroid therapy for acute asthma exacerbations". J Asthma. 43 (5): 321–31. doi:10.1080/02770900600567163. PMID 16801135.
- ^ Thrower BW (Jan 2009). "Relapse management in multiple sclerosis". Neurologist. 15 (1): 1–5. doi:10.1097/NRL.0b013e31817acf1a. PMID 19131851.
- ^ Lambrou GI, Vlahopoulos S, Papathanasiou C, Papanikolaou M, Karpusas M, Zoumakis E, Tzortzatou-Stathopoulou F (2009). "Prednisolone exerts late mitogenic and biphasic effects on resistant acute lymphoblastic leukemia cells: Relation to early gene expression". Leuk Res. 33 (12): 1684–95. doi:10.1016/j.leukres.2009.04.018. PMID 19450877.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Miura M, Tamame T, Naganuma T, Chinen S, Matsuoka M, Ohki H (2011). "Steroid pulse therapy for Kawasaki disease unresponsive to additional immunoglobulin therapy". Paediatrics & Child Health. 16 (8): 479–84. PMC 3202387. PMID 23024586.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Product Information: ORAPRED ODT(TM) orally disintegrating tablets, prednisolone sodium phosphate orally disintegrating tablets. Cima Labs Inc, Alpharetta, GA, 2006.
- ^ "Acute alveolar sarcoidosis presenting with hypoxaemic respiratory failure". BMJ Case Rep. April 30th, 2014. doi:10.1136/bcr-2013-202247. PMID 24789154. Retrieved 11/03/15.
{{cite journal}}: Check date values in:|access-date=and|date=(help) - ^ "Pred Forte Package Insert" (PDF).
- ^ Product Information: OMNIPRED(TM) ophthalmic suspension, prednisolone acetate ophthalmic suspension. Alcon Laboratories Inc, Fort Worth, TX, 2005
- ^ Vethe, Nils; Midtvedt, Karsten; Åsberg, Anders; Amundsen, Rune; Bergan, Stein. "Legemiddelinteraksjoner og immunsuppresjon hos organtransplanterte". Tidsskrift for Den norske legeforening. 131 (20). https://plus.google.com/114358066502844682758: 2000–2003. doi:10.4045/tidsskr.11.0138.
{{cite journal}}: External link in|publisher= - ^ Product Information: ORAPRED ODT(TM) orally disintegrating tablets, prednisolone sodium phosphate orally disintegrating tablets. Cima Labs Inc, Alpharetta, GA, 2006.
- ^ Husebye, E. S.; Allolio, B.; Arlt, W.; Badenhoop, K.; Bensing, S.; Betterle, C.; Falorni, A.; Gan, E. H.; Hulting, A.-L. (2014-02-01). "Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency". Journal of Internal Medicine. 275 (2): 104–115. doi:10.1111/joim.12162. ISSN 1365-2796.
- ^ AMA Department of Drugs: AMA Drug Evaluations, 6th. American Medical Association, Chicago, IL, 1986.
- ^ "Glucocorticosteroid inhibition of cytokine production: relevance to antiallergic actions". J Allergy Clin Immunol. January, 1996. PMID 8568145. Retrieved 11/03/15.
{{cite journal}}: Check date values in:|access-date=and|date=(help) - ^ "Mechanisms of Glucocorticoid Action in Chronic Rhinosinusitis". Allergy Asthma Immunol Res. November, 2015. doi:10.4168/aair.2015.7.6.534. PMID 26333699. Retrieved 11/03/15.
{{cite journal}}: Check date values in:|access-date=and|date=(help) - ^ "Draft Package Insert for Orapred ODT" (PDF).
- ^ Drugs Information. "Prednisolone Tablets 1mg, 5mg (Actavis UK Ltd)". Retrieved 13 December 2012.
- ^ "Bowel Perforation in Steroid-Treated Patients"
- ^ "Intranasal steroids and septum perforation--an overlooked complication?"
- ^ a b Kaminstein, David. "Steroid Withdrawal". medicinenet. Retrieved 23 December 2012.
- ^ "PEDIAPRED" (PDF).
- ^ Bergmann, Troels (7 September 2012). "Clinical Pharmacokinetics and Pharmacodynamics of Prednisolone and Prednisone in Solid Organ Transplantation" (PDF). Clinical Pharmacokinetics.
- ^ Stahn, Cindy (17 May 2007). "Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists" (PDF). Molecular and Cellular Endocrinology.
- ^ "The 2011 Prohibited List".
