Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
November 25
Monstrous shape-shifting owl
I've just been sent a link to this YouTube video, which I found really freaky and disturbing, for some reason that I can't quite place my finger on.
http://www.youtube.com/watch?v=Es52WQKLumI
Does anyone know if this owl is actually a real species and can really do this? I'm thinking that while the first display might be real, the second (demonic looking) one is CGI.--84.69.185.215 (talk) 00:38, 25 November 2009 (UTC)
That was really cool. I don't know if it is real or not. I like it. Dauto (talk) 01:02, 25 November 2009 (UTC)
- This has come up on the Reference Desk before, see Wikipedia:Reference desk/Archives/Science/2009 July 26#Cat bird owl?. Looie496 (talk) 01:11, 25 November 2009 (UTC)
- Northern White-faced Owl also references that video. SteveBaker (talk) 03:57, 25 November 2009 (UTC)
- Remarkable as it is it is quite small beer when compared to some of the courting postures of some Birds of Paradise of Asia.This is just one example. [1] Not much of a song but what a super blue smiley face. Richard Avery (talk) 08:46, 25 November 2009 (UTC)
- Some more about the species here:[2] Here's pics of it in action:[3][4] Other "scops-owls" adopt a similar thin upright posture with ear tufts.[5][6][7] Fences&Windows 18:33, 25 November 2009 (UTC)
- The owl reminds me (facially) somewhat of a Ring-tailed Lemur during the first transformation. It's probably not intentional - but I just thought that I'd make that observation. I felt slightly unsettled myself when I saw the owl's 'demon' form (anyone have any thoughts as to what it's trying to imitate with that?) - which may indeed be *exactly* what the owl wants you to feel. It's something about the way that its eyes appear to change shape that doesn't rest easy with me. --Kurt Shaped Box (talk) 01:34, 26 November 2009 (UTC)
- The resemblance to a cat is striking, and a photographer described them as giving a "rarely heard cat-like cry",[8] Some kind of Batesian mimicry might be going on as an adaptation of the defence posture, but that's speculation. The general significance of ear tufts in owls has been discussed on a science blog here, and mimicry of mammalian predators is one suggestion. But other owls do adopt a similar posture, e.g. the Northern Pygmy-owl, in which it is described as a 'concealing' posture,[9] so the appearance to humans of the Northern White-faced Owl could just be a coincidence. Fences&Windows 12:57, 26 November 2009 (UTC)
- The owl reminds me (facially) somewhat of a Ring-tailed Lemur during the first transformation. It's probably not intentional - but I just thought that I'd make that observation. I felt slightly unsettled myself when I saw the owl's 'demon' form (anyone have any thoughts as to what it's trying to imitate with that?) - which may indeed be *exactly* what the owl wants you to feel. It's something about the way that its eyes appear to change shape that doesn't rest easy with me. --Kurt Shaped Box (talk) 01:34, 26 November 2009 (UTC)
- The advantages of making oneself looking bigger is obvious. But what can be the advantage of making oneself looking smaller and weaker, as in the second posture? --131.188.3.20 (talk) 14:59, 28 November 2009 (UTC)
- Concealment. Fences&Windows 18:55, 28 November 2009 (UTC)
Early Hominina finding in Americas
What is the oldest known Hominina remains found in Americas before the Homo sapiens arrived here? 174.114.236.41 (talk) 03:12, 25 November 2009 (UTC)
- I find this: Models of migration to the New World. Bus stop (talk) 03:16, 25 November 2009 (UTC)
- That article is about Homo sapiens, the OP is explicitly asking about pre-Homo sapiens. --Tango (talk) 03:23, 25 November 2009 (UTC)
- There were no hominids in the Americas before Homo sapiens. The only primates in the Americas before the arival of Homo sapiens were the New World monkeys, and they are among the most distantly related primates to Homo sapiens around (far more distantly related than any other apes or monkeys). The first Hominids to arrive in the Americas were fully modern Homo sapiens. See Models of migration to the New World. There is some controversy as to the actual date the first people arrived; or from which direction, or whether they arrived in a single migration or in multiple waves, but even the oldest possible date for humans arriving is about 50,000 years ago, and that date is considered pretty old by most mainstream theories, which hold a date of about 17,000 years ago as the first arrival of humans. For comparison, the last non-Homo sapiens hominid, homo erectus, died out some 1,000,000 years ago. There is simply zero fossil evidence for any pre-homo sapiens hominids in the Americas; indeed as noted above, the nearest relatives are actually quite distant, those New World monkeys branched off of the "human family tree" well before any Apes or Old World monkeys did, some hundreds of millions of years ago. --Jayron32 03:26, 25 November 2009 (UTC)
- There is some dispute about which was the last non-Homo sapiens hominid - it depends on whether you consider Neanderthals to be a subspecies of Homo sapiens or not (the experts can't agree). If you consider them a separate species then that moves to date from which Homo sapiens have been alone to, possibly, 30,000 years ago (although probably more). However, they were in Europe, not the Americas. --Tango (talk) 03:30, 25 November 2009 (UTC)
- There is also Homo floresiensis, aka. "The hobbit", which may or may not have been a subspecies of Homo sapiens or a distinct species. Given that there are no actual extant hominids besides homo sapiens, it is hard to say whether any of these examples are "subspecies" or "species" - level relatives, it probably has a lot more to do with politics than real science as to which side of the debate one falls. Still, no fossil evidence of any of these hominids, or indeed of any other, before the arrival of modern Homo sapiens. --Jayron32 03:33, 25 November 2009 (UTC)
- (Just today - and reported right there on the Wikipedia front page - is news that some rather convincing new studies show that florensiensis was indeed a separate species.) SteveBaker (talk) 04:31, 25 November 2009 (UTC)
- Well, there ya go. But floresiensis wasn't in the Americas either. --Jayron32 04:36, 25 November 2009 (UTC)
- Indeed not. SteveBaker (talk) 04:57, 25 November 2009 (UTC)
- Well, there ya go. But floresiensis wasn't in the Americas either. --Jayron32 04:36, 25 November 2009 (UTC)
- (Just today - and reported right there on the Wikipedia front page - is news that some rather convincing new studies show that florensiensis was indeed a separate species.) SteveBaker (talk) 04:31, 25 November 2009 (UTC)
- There is also Homo floresiensis, aka. "The hobbit", which may or may not have been a subspecies of Homo sapiens or a distinct species. Given that there are no actual extant hominids besides homo sapiens, it is hard to say whether any of these examples are "subspecies" or "species" - level relatives, it probably has a lot more to do with politics than real science as to which side of the debate one falls. Still, no fossil evidence of any of these hominids, or indeed of any other, before the arrival of modern Homo sapiens. --Jayron32 03:33, 25 November 2009 (UTC)
- There is some dispute about which was the last non-Homo sapiens hominid - it depends on whether you consider Neanderthals to be a subspecies of Homo sapiens or not (the experts can't agree). If you consider them a separate species then that moves to date from which Homo sapiens have been alone to, possibly, 30,000 years ago (although probably more). However, they were in Europe, not the Americas. --Tango (talk) 03:30, 25 November 2009 (UTC)
Hood Canal "tide computer"

I came across this interesting snippet in the 'forum' posts at the end of this:
- "...until very recent times the Ptolemaic theory was sufficient to calculate tide tables and it was a whole lot easier to use it rather than to puzzle out multi-body solutions with Newton's formula. The ancient Ptolemaic tables were good enough. At the Hood Canal locks here in Seattle there used to be a giant analog computer consisting of a heavy chain about fifteen feet long. The chain was fixed on one end and stretched horizontally with a series of pulleys representing the celestial bodies each able to indent the stretched chain and make it shorter or longer. The free end culminated in a pointer that moved up and down a scale indicating the expected tide."
I can't find anything about this on the web - does anyone have any more information about it? Hood Canal has nothing about it. Tide-predicting machine has a lot of discussion of these kinds of gizmo but nothing there sounds like it would have fifteen feet of "heavy chain" - it seems to discuss relatively delicate-looking gadgets.
SteveBaker (talk) 03:48, 25 November 2009 (UTC)
- Um...since the Hood Canal isn't really a canal, but rather a fjord, why would it have locks? --jpgordon::==( o ) 05:32, 25 November 2009 (UTC)
- Wow. I did find the thing. "Old Brass Brains", they called it; it was used until 1965! Don't know the Hood Canal connection. This is from Technical World magazine in 1921; it's on page 205. It's a big download! There's also a modern writeup about it and others here. So cool! (--jpgordon::==( o ) 06:21, 25 November 2009 (UTC)
- Of course, I could have just looked more closely at tide-predicting machine. But it was fun research. Anyway, "Old Brass Brains" was big! It weighed 2500 pounds and was 11.5 feet tall. So "heavy chains" might be accurate. --jpgordon::==( o ) 06:33, 25 November 2009 (UTC)
- I had looked at the "Old Brass Brains" thing before I posted the question - but I didn't see the Hood Canal connection - and big though it is - it didn't seem like it would have 15 feet of "heavy chain" - and what chain it has isn't "stretched horizontally", it seems to zig-zag through the mechanism. It's clear that the person who I quoted (above) didn't understand the machine. Unless the one he's talking about is very different from the "normal" mechanical tide predictors, it would be summing an empirically derived Fourier series to make the predictions - not employing some kind of ancient Ptolomaic theory of how sun, moon and planets move as implied by "pulleys representing the celestial bodies". Mathematically and mechanically, the results might be kinda similar - you're adding circular motions - but "Old Brass Brains" certainly isn't using Ptolomaic principles. That's why I wanted to find out more about this supposed Hood Canal machine in Seattle. SteveBaker (talk) 14:30, 25 November 2009 (UTC)
- Yeah. But as I said -- there don't appear to be any locks in the Hood Not-really-a-canal. --jpgordon::==( o ) 15:38, 25 November 2009 (UTC)
- Why would anyone make such a large tidal calculator as Old Brass Brains and not scale it down to a portable device like the Antikythera mechanism? How many of the Fourier series coefficients (up to 24 on one machine) correspond to identifiable astronomic influences? Cuddlyable3 (talk) 18:37, 25 November 2009 (UTC)
- Seattle has Chittenden Locks - I don't think I'd describe them as being on the Hood Canal - but they are close enough that maybe someone could assume that they were. Still no sign of a tide predictor there though. SteveBaker (talk) 23:38, 25 November 2009 (UTC)
- Why would anyone make such a large tidal calculator as Old Brass Brains and not scale it down to a portable device like the Antikythera mechanism? How many of the Fourier series coefficients (up to 24 on one machine) correspond to identifiable astronomic influences? Cuddlyable3 (talk) 18:37, 25 November 2009 (UTC)
- Yeah. But as I said -- there don't appear to be any locks in the Hood Not-really-a-canal. --jpgordon::==( o ) 15:38, 25 November 2009 (UTC)
- I had looked at the "Old Brass Brains" thing before I posted the question - but I didn't see the Hood Canal connection - and big though it is - it didn't seem like it would have 15 feet of "heavy chain" - and what chain it has isn't "stretched horizontally", it seems to zig-zag through the mechanism. It's clear that the person who I quoted (above) didn't understand the machine. Unless the one he's talking about is very different from the "normal" mechanical tide predictors, it would be summing an empirically derived Fourier series to make the predictions - not employing some kind of ancient Ptolomaic theory of how sun, moon and planets move as implied by "pulleys representing the celestial bodies". Mathematically and mechanically, the results might be kinda similar - you're adding circular motions - but "Old Brass Brains" certainly isn't using Ptolomaic principles. That's why I wanted to find out more about this supposed Hood Canal machine in Seattle. SteveBaker (talk) 14:30, 25 November 2009 (UTC)
- Of course, I could have just looked more closely at tide-predicting machine. But it was fun research. Anyway, "Old Brass Brains" was big! It weighed 2500 pounds and was 11.5 feet tall. So "heavy chains" might be accurate. --jpgordon::==( o ) 06:33, 25 November 2009 (UTC)
- Wow. I did find the thing. "Old Brass Brains", they called it; it was used until 1965! Don't know the Hood Canal connection. This is from Technical World magazine in 1921; it's on page 205. It's a big download! There's also a modern writeup about it and others here. So cool! (--jpgordon::==( o ) 06:21, 25 November 2009 (UTC)
Type of truss bridge



Is the upper drawing a Warren truss bridge? I would guess so from attempting to read the description (I don't know French), but it differs from the lower drawing, which we currently use to illustrate the Warren section of truss bridge — the upper drawing has vertical supports, which are entirely missing from the lower drawing. If it's not a Warren, what is it? Nyttend (talk) 04:54, 25 November 2009 (UTC)
- By the way, the reason that I'm asking is that I'm trying to ascertain the type of bridge shown in the photo. Nyttend (talk) 05:14, 25 November 2009 (UTC)
- (ec) List of truss types identifies the lower image as a Warren (non-polar) truss - which agrees with the main article. The upper image does not appear in any of those types because the diagonal elements alternate in direction - unlike a Howe truss - and has verticals, unlike a Warran (non-polar) truss. The (french) caption says "Warren avec montants" - "avec" means "with" - so this is a Warren truss with "montants". My wife (who is French) says that "montants" are the vertical elements in window frames (the horizontal bits are "traverses")...and Wiktionary says that a montant is any vertical piece of carpentry in a framework. So evidently, your bottom image is a bona-fide Warren truss - and your top image is a modified version of a Warren - with those vertical bits (the montants) added in for whatever reason. SteveBaker (talk) 05:17, 25 November 2009 (UTC)
- Well, now you've confused things - the photo you just added has only every second "montant" present! So that's yet another variation! I'm guessing though that this is still a Warren truss. SteveBaker (talk) 05:19, 25 November 2009 (UTC)
- Briefly skimming an old engineering book I happen to have here at home - the critical thing about a Warren truss is how the weight is distributed between compressive and tensile forces in the diagonal elements. The Howe truss has all of the diagonals in the left-hand half of the span going from bottom-left to top-right and all the ones on the other side going the other way. A Pratt truss does the complete opposite - and which one you use depends on the building materials you have (are they better in tension or compression?) and whether you expect the truss to be fairly evenly loaded along it's entire length - or have a small, heavy load at just one point along the truss. It seems that the Warren design (by alternating the diagonals) is some kind of compromise between the two. The vertical elements...I have no clue...they seem to play little part in the discussion of the relative merits of the three designs - but so many real bridges seem to have them that they must be important in some manner. SteveBaker (talk) 05:32, 25 November 2009 (UTC)
- Thanks for the details; I've never quite understood the different types of trusses, so I wasn't at all sure that the montants weren't significant for the type of truss. If you're an engineer, you may find this location quite interesting: it's the Deep Cut of the Miami and Erie Canal. Nyttend (talk) 05:47, 25 November 2009 (UTC)
- By the way - I doubt that "montant" is the right word for the upright beams in bridge structures. It's evidently the right word in French - but probably not in English. I was merely using the word as a shorthand for whatever the correct term is! SteveBaker (talk) 06:01, 25 November 2009 (UTC)
- Thanks for the details; I've never quite understood the different types of trusses, so I wasn't at all sure that the montants weren't significant for the type of truss. If you're an engineer, you may find this location quite interesting: it's the Deep Cut of the Miami and Erie Canal. Nyttend (talk) 05:47, 25 November 2009 (UTC)
- Briefly skimming an old engineering book I happen to have here at home - the critical thing about a Warren truss is how the weight is distributed between compressive and tensile forces in the diagonal elements. The Howe truss has all of the diagonals in the left-hand half of the span going from bottom-left to top-right and all the ones on the other side going the other way. A Pratt truss does the complete opposite - and which one you use depends on the building materials you have (are they better in tension or compression?) and whether you expect the truss to be fairly evenly loaded along it's entire length - or have a small, heavy load at just one point along the truss. It seems that the Warren design (by alternating the diagonals) is some kind of compromise between the two. The vertical elements...I have no clue...they seem to play little part in the discussion of the relative merits of the three designs - but so many real bridges seem to have them that they must be important in some manner. SteveBaker (talk) 05:32, 25 November 2009 (UTC)
- According to this [10], it is a "varying depth Warren truss". --Cookatoo.ergo.ZooM (talk) 09:11, 25 November 2009 (UTC)
- The varying depth refers to the polygonal arrangement of the chords, not the upright posts. We are still looking for a name for a Warren truss with posts. SpinningSpark 12:59, 25 November 2009 (UTC)
- This page and this page call it a "subdivided Warren truss". If you want a fancy architectural term for the vertical members, you could call them mullions. Gandalf61 (talk) 13:25, 25 November 2009 (UTC)
- Yeah, doesn't seem to have an actual name, just Warren truss with verticals seems the most usual description. The vertical members, according to this book, are called posts and hangers depending on whether they are taking a compressive or tensile load. SpinningSpark 13:31, 25 November 2009 (UTC)
- Mullion is a term used in connection with windows and doors and other openings. The side of a bridge is not intended as an opening (either for people of light) so I think it would be unlikely to be used. SpinningSpark 13:41, 25 November 2009 (UTC)
- This page and this page call it a "subdivided Warren truss". If you want a fancy architectural term for the vertical members, you could call them mullions. Gandalf61 (talk) 13:25, 25 November 2009 (UTC)
Dehumidifiers and climate change control
Given water vapour's role in the greenhouse effect, could large-scale dehumidifiers be used to offset emissions of other greenhouse gases and control climate change? NeonMerlin 05:25, 25 November 2009 (UTC)
- Well, yes and no. Water vapor is itself a greenhouse gas - so I suppose a dehumidification of the upper atmosphere would help to alleviate the greenhouse effect. However, water droplets in the form of clouds and fog banks are very bright and reflect sunlight back out into space - keeping the earth cooler. So in that regard, getting rid of the clouds would make matters worse. (This was noted in the days after the 9/11 terrorist attacks when all North American airliners were grounded. The lack of contrails - which are mostly white water vapor - caused a noticable increase in temperatures throughout the USA.)
- But either way, it's crazy to imagine that we could build dehumidifiers on a large enough scale to make any impact without burning so much energy to drive them that our net carbon footprint would go through the roof!
- So "No" for a couple of reasons. SteveBaker (talk) 05:40, 25 November 2009 (UTC)
- If you could then you could water the entire Sahara with the water taken out of the atmosphere...--BozMo talk 07:10, 25 November 2009 (UTC)
- Also, don't dehumidifiers generate a lot of heat in their operation? I suspect the heat generated would be more than the heat allowed to escape into space by the reduced water vapour in the air, even without factoring in the albedo effects. --Tango (talk) 23:32, 25 November 2009 (UTC)
- Unlike other greenhouse gasses, water vapor is fairly self-regulating: on average, the atmosphere is fairly close to saturation, so any increase in evaporation will be countered by an increase in rainfall. --Carnildo (talk) 02:02, 26 November 2009 (UTC)
- The Thermohaline circulation means that there is naturally, on average, more water vapour in some places than in others. So taking vapour out of the atmosphere could result in more or less heat in some areas, resulting in storms, displaced quasi-stationary highs and lows, wind patterns...and ultimately the Thermohaline itself. But this is mostly speculation. ~AH1(TCU) 02:17, 2 December 2009 (UTC)
do blue tulips occur in nature?

do blue tulips occur in nature?
- Define "natural". Tulips are a highly cultivated genus of plants, see tulip. Like maize and other highly cultivated plants, it is doubtful if you have ever seen a truly "wild" tulip before (you may have seen feral tulips, but that is different than a truly wild species). There is apparently a variety called "Tulipa sylvestris" which is known as the "wild" tulip; however we have no article on this, so I have no reference for what "natural" tulips look like. --Jayron32 05:34, 25 November 2009 (UTC)
- Type "Tulipa sylvestris" into Google Images and you'll be greeted by a ton of bright yellow flowers with petals that are much more open than the classical idea of what a Tulip should be. So, no - no blue tulips in the wild...or red ones, or orange ones...just yellow. SteveBaker (talk) 05:55, 25 November 2009 (UTC)
- Photoshop trumps nature. Cuddlyable3 (talk) 08:20, 25 November 2009 (UTC)
- Eeew!! Are you certain about that? ;-)) Richard Avery (talk) 08:33, 25 November 2009 (UTC)
- Photoshop trumps nature. Cuddlyable3 (talk) 08:20, 25 November 2009 (UTC)
- There are a few cultivars that approach blue: for example 'Blue Parrot', seen here [11]. As for naturally occuring species (about 150 [12] ), I don't think there are any blue species, although there are certainly purplish species. --Eriastrum (talk) 00:08, 26 November 2009 (UTC)
North Sea gas
In The Death of Regginald Perrin by David Nobbs, Jimmy attempts suicide by putting his head in the oven, and Reggie laughs at his ineptitude: "North Sea gas isn't poisonous." I thought this was either a bizarre blunder by the author, or exceedingly obscure humor. But now I'm reading a story by P. D. James in which a murder attempt fails: "The conversion, that's why. We're on natural gas from this evening. That North Sea stuff. It isn't poisonous. The two men from the Gas Board came just after nine o'clock."
What the heck do they pump out of the North Sea? Hydrogen? —Tamfang (talk) 05:28, 25 November 2009 (UTC)
- North Sea Gas (aka "Natural" gas) is mostly Methane - which isn't poisonous. If the air gets too full of the stuff - it would eventually displace all of the oxygen and you'd suffocate - but that's not really likely from a gas oven. The biggest concern would be explosions. The old "Coal Gas" or "Town Gas" that was used in the UK before the North Sea oil boom was a mixture of hydrogen, methane and carbon monoxide. The carbon monoxide would kill you pretty quickly. I'm pretty sure the UK was in that transition period right when Reggie Perrin was imagining his mother in law as a hippo - so it's perhaps understandable that someone would still attempt suicide that way if they didn't know any better. Maybe the advertising of the benefits of the new gas was highly prevalent at the time the series was made - so perhaps the implication was that the guy who was attempting this was an idiot for not knowing about that. SteveBaker (talk) 05:50, 25 November 2009 (UTC)
- For the record, natural gas is not a term that applies just to "North Sea Gas" — reading through the article, "natural gas" appears to be a super-generalization, describing many variants. Comet Tuttle (talk) 19:02, 25 November 2009 (UTC)
- I was an accident investigator for Shell when Tokyo switched. Funnily the pattern of failed suicides (booze, oven, wake up with headache) was sometimes followed by lighting a cigarette and starting a non fatal gas explosion. When I left they were looking at smart meters to contact the police for suspected suicide. It is similar to using car exhaust to kill yourself when you have a Cat Converter on the car. --BozMo talk 07:03, 25 November 2009 (UTC)
- Oh, there goes that plan. Thanks, all, for the clarification. —Tamfang (talk) 07:07, 25 November 2009 (UTC)
- At the time of the changeover there was a lot of public debate over North Sea gas being odourless. I believe something is added to it to give it a human detectable odour but it is very mild compared to the strongly sulfurous smell of the old coal gas and this led to some public concern. It was a stock response from industry and government spokesmen that there was no need to do anything about this because the gas is not poisonous. The issue was therefore likely to be in people's minds at the time and they would have got the joke. SpinningSpark 12:23, 25 November 2009 (UTC)
- Seriously? "Not poisonous" is not much comfort when your house goes up like a bomb. Was there an uptick in explosions corresponding to the downtick in poisonings? --Trovatore (talk) 18:15, 25 November 2009 (UTC)
- I doubt that - if someone dies from carbon monoxide poisoning with their head in a gas oven - they are unlikely to turn it off afterwards! Hence, probably, the number of explosions would be pretty similar. But once people knew that this was not a viable suicide technique - the rate of explosions due to that ought to have dropped substantially. SteveBaker (talk) 23:01, 25 November 2009 (UTC)
- Seriously? "Not poisonous" is not much comfort when your house goes up like a bomb. Was there an uptick in explosions corresponding to the downtick in poisonings? --Trovatore (talk) 18:15, 25 November 2009 (UTC)
- Do they not use Butanethiol in the UK? --Jmeden2000 (talk) 16:04, 25 November 2009 (UTC)
- Yes, I think that's what is used, and I know it is a very nasty chemical in high concentrations. But it is used in tiny quantities and definitely does not smell anywhere near as repulsive as coal gas. One whiff of that had us immediately opening all the windows to dispel it. Natural gas simply does not cause that same reaction in people. At least, with the additives used in Britain, I could not speak for elsewhere. SpinningSpark 17:35, 25 November 2009 (UTC)
- [This] link claims the UK smell is due to a blend of mercaptans and sulphides. But a trace of any thiol is going to smell enough to detect. I would suspect a blend is used so that the gas detector vans can check the ratio of the odourants and confirm a gas leak - it might be that a nearby plant could be using one of the components, but the ratio in the detector would then be different. LPG uses ethanethiol to make it smell - I think the butanethiol is too high boiling here - it would tend to concentrate towards the end of the bottle - I know that the ethanthiol has a small tendency to do that - and I can tell (on the boat) when the bottle is starting to get low by the smell of the cooker. Ronhjones (Talk) 19:35, 25 November 2009 (UTC)
- Someone should really move that article to butyl mercaptan in accordance with WP:COMMONNAME. In my opinion the chemistry editors are overly respectful of IUPAC's silly nomenclature. But I'm not a chemist so I won't do it myself. --Trovatore (talk) 18:12, 25 November 2009 (UTC)
- At the time of the changeover there was a lot of public debate over North Sea gas being odourless. I believe something is added to it to give it a human detectable odour but it is very mild compared to the strongly sulfurous smell of the old coal gas and this led to some public concern. It was a stock response from industry and government spokesmen that there was no need to do anything about this because the gas is not poisonous. The issue was therefore likely to be in people's minds at the time and they would have got the joke. SpinningSpark 12:23, 25 November 2009 (UTC)
- See a full account in Kreitman, Norman (1976). "The coal gas story. United Kingdom suicide rates, 1960-71". Br J Prev Soc Med. 30 (2): 86–93.. Fences&Windows 18:02, 25 November 2009 (UTC)
overies
wat happens if the size of overies s larger??? —Preceding unsigned comment added by Shilpa.upadhya (talk • contribs) 07:37, 25 November 2009 (UTC)
- A common cause of swollen ovaries is a cyst or PCOS. We have an article about PCOS[13] and there is more information on the web.Cuddlyable3 (talk) 08:14, 25 November 2009 (UTC)
Balancing chemical equations
Is there any real, concrete method for balancing chemical equations? If not, can anyone offer any advice or tips? Thank you. —Preceding unsigned comment added by 161.165.196.84 (talk) 08:51, 25 November 2009 (UTC)
- There must be such methods, because automatic equation balancers exist, e.g. http://www.webqc.org/balance.php.
- A good tip is to start by balancing one particular element (i.e. making sure there are equal numbers of the chosen element on both sides of the equation) and then putting in enough of the other elements to make the required product.
- Chemical equations can be written as simultaneous equations requiring integer solutions. Written in that form, any linear equation solver like Gaussian elimination or linear programming, or a complicated optimization method, can be applied. Finally, for convenience, the result is usually reduced by dividing out any common integer factors, if the solution method doesn't already take care of this. Nimur (talk) 13:34, 25 November 2009 (UTC)
- The best tip I give my students is that, if done correctly, the last element always balances itself; so if there is some element which is complicating the solution because it appears in multiple compounds on each side of the equation (oxygen does this often), leave THAT element till last, and it will work itself out. Additionally, some equations may seem to never balance; my suggestion in these cases is to consider using fractional coefficients and then multiply out to get rid of the fractions. Just a few tips. --Jayron32 04:28, 26 November 2009 (UTC)
How does an increased heart-rate stimulate the body's metabolism?
Obviously, excersise raises one's metabolism (although scientifically speaking, I'm not sure why), but how does just having an increased heart-rate (perhaps by consuming caffiene)stimulate the metabolism? Thank you.161.165.196.84 (talk) 09:27, 25 November 2009 (UTC)
- (For the impact of exercise on the body, see Exercise physiology.) I don't know the actual biochemistry involved, but by definition, the energy used by the heart would increase if its output were to increase. Zain Ebrahim (talk) 14:09, 25 November 2009 (UTC)
- I think we need to start by asking whether the body's metabolism is stimulated by increased heart rate. There are many ways to increase the heart rate, and many of those could affect metabolism in ways that don't directly involve the heart. One way to separate the effects would be to use an implanted cardiac pacemaker to increase the heart rate directly. Speeding up the heart above normal rate using a pacemaker is called ventricular overdrive pacing redlink!, and is used diagnostically and therapeutically in tachycardia. I haven't found a reliable source for this (yet), but my own experience suggests that metabolism is not globally increased during this maneuver. It is clear that cardiac metabolism is increased (PMID 8261221), as Zain has pointed out. -- Scray (talk) 14:24, 25 November 2009 (UTC)
- exercising raises one's metabolism for that particular part of the body exercised, there is increase of heart rate and respiratory rate (by both neurological and hormonal activation), with corresponding increase in metabolic activity in terms of cardiac muscle as well as the respiratory muscles. while doing exercise, organic acids and products of anaerobic respiration such as lactic acid will be released into circulation, and these will be metabolised by the liver, and this would lead to increase in metabolic rate in liver as well. bubu~ (talk) 15:29, 25 November 2009 (UTC)
Freezing contents of deeply chilled liquid.
What happens to a deeply chilled, but unfrozen drink; that in the few seconds,usually a bottle container, is opened, the liquid freezes immediately? In the many discussions held about this phenomenon, none could come up with a convincing answer. Some tried to say that when the oxygen enters the bottle container, it makes the liquid to freeze. It was not convincing since the oxygen entering the top of the bottle is warmer than the inside of the bottle. Please help41.17.171.15 (talk) 12:00, 25 November 2009 (UTC)
- The liquid has been supercooled but freezing will not start unless there are small particles present that allow nucleation to begin. Opening the bottle releases bubbles which provoke the nucleation process. SpinningSpark 12:07, 25 November 2009 (UTC)
- It could be that, or it could be that the phase diagram of water indicates that there is an inverse relationship between pressure and phase for water; that is water under higher pressure tends to be more likely to be a liquid, and it actually freezes as the pressure decreases. Additionally, the released gas can cause adiabatic cooling in the liquid (open a can of soda, its temperature will drop slighlty but measurably as the bubbles are released). I think it is some combination of these three effects; though I think that Spinningsparks answer on supercooling is likely the dominant effect here, the other two effects may play some role as well. --Jayron32 04:24, 26 November 2009 (UTC)
Finding power factor
Suppose I find the time delay between the voltage and current waveforms and divide that by the time period of half a cycle. Then subtracting it from 1, won't that give me the power factor? If not, please suggest how to. I want to avoid finding out phasor angular difference. 218.248.80.112 (talk) 14:18, 25 November 2009 (UTC)
- The time delay to the crest of the current sine wrt the crest of the voltage sine is only part of the power factor calculation (namely the leading/lagging portion) you still need to know the total power drawn vs the peak of the current sine (apparent power) to figure out what the number should be. If the current sine is perfectly identical to the voltage sine this might be a simple process (mathematically) if not you will need to perform some advanced calculations. Hope this helps! --Jmeden2000 (talk) 15:56, 25 November 2009 (UTC)
- What Jmeden2000 is alluding to is that the power factor may vary if your current and voltage waveforms aren't perfect sinusoids. If they are not, then all bets are off, and simple power-factor formulae are useless - you will need to perform a full integral over the waveforms, .
- In any case, your approach will yield:
- This is approximately, but not exactly equal, to (the correct value of the power factor), for sufficiently small and perfect sinusoids. I think you're missing a factor of pi, too. You probably meant:
- ...which is sort of like a first-order taylor series approximation of a cosine. (But not a correct one).
- The degree of applicability of this engineering approximation will depend on your application's precision needs. I can't think of any engineering application today where this approximation would be useful, because calculating the true phase difference and computing its cosine is trivial on even the most reduced forms of computers, microcontrollers, or even analog signal conditioners. Nimur (talk) 17:41, 25 November 2009 (UTC)
- After re-reading your question, it sounds like you just want to avoid using phasors. That's fine - but your approach has already calculated the angular difference in the time-domain (without using phasors): . Is there any reason you don't want to use cosine? Nimur (talk) 18:01, 25 November 2009 (UTC)
- I had to skip all those integrations and all because I am implementing this on an 8051 microcontroller which does not have any floating point capability and only 8bit math. So that was also the reason of not producing the cosine.. I think I will extend this project to simple power factor improvement (like connecting a capacitor through relay for low power angle) and protection (cutting of the circuit for very low power angle). Any suggestions ? And btw thanks for the analysis.. 218.248.80.112 (talk) 09:50, 26 November 2009 (UTC)
- Fixed point math throws some kinks in to things, but you can still compute cosine with a lookup table. But, if your only actions are to throw a relay or two, you may as well just solve for the phase difference that you want to use as trigger points (instead of power factor). You might also want to be aware that simply throwing a relay to change the circuit may result in a phase oscillation - for example, you may be right on the edge of an acceptable phase error, throw the relay, and then fall just on the other side, un-trigger the relay... in other words, unless you analyze the closed loop circuit, including your software logic, you may have created an unstable circuit. The correct way to design such a power factor correction circuit is a little bit more complicated. For example, take a look at Fairchild Semiconductor's Application Note AN-42047 - Power Factor Correction Basics. They detail the theory and practice of PFC circuits, and pitch some of their products that do this for you. Nimur (talk) 15:12, 26 November 2009 (UTC)
- Certainly you could use a lookup table for cosine - and if you need higher precision and/or are too limited on memory to get the precision you desire then you can dramatically improve the results by doing a simple linear interpolation of two consecutive table entries. SteveBaker (talk) 17:33, 26 November 2009 (UTC)
- Fixed point math throws some kinks in to things, but you can still compute cosine with a lookup table. But, if your only actions are to throw a relay or two, you may as well just solve for the phase difference that you want to use as trigger points (instead of power factor). You might also want to be aware that simply throwing a relay to change the circuit may result in a phase oscillation - for example, you may be right on the edge of an acceptable phase error, throw the relay, and then fall just on the other side, un-trigger the relay... in other words, unless you analyze the closed loop circuit, including your software logic, you may have created an unstable circuit. The correct way to design such a power factor correction circuit is a little bit more complicated. For example, take a look at Fairchild Semiconductor's Application Note AN-42047 - Power Factor Correction Basics. They detail the theory and practice of PFC circuits, and pitch some of their products that do this for you. Nimur (talk) 15:12, 26 November 2009 (UTC)
Ionic food preserver
Does anyone have any idea how this "ionic preserver" works to prevent mould growth, banana pigmentation, and the various other food spoilages that they are claiming? I searched Google but couldn't find a thing. The only clue from the food preservation article is nitrites and sulphites, but surely the device would run out of them eventually and there is no mention of a plug-in pack or the like. --Mark PEA (talk) 17:02, 25 November 2009 (UTC)
- Ionising air will produce ozone. The Wikipedia article on ozone says it can be used as a disinfectant for killing bacteria on food and to kill mold spores. I'm not sure if this is the specific mechanism for the "ionic preserver", or if the preserver would produce a sufficient concentration of ozone to do anything.
- Here is a scientific paper saying that negative ions preserve lettuce; they seem to reduce water loss (which would cause drying). --Maltelauridsbrigge (talk) 17:26, 25 November 2009 (UTC)
- Air ioniser and Air_purifier#Air_ionizers_and_ozone will tell you more about this idea in general, but not this specific product. The idea has been around since at least the 1950s.[17] Negative ions and ozone may restrict the growth of microorganisms.[18] Here's a study of the use of an ionizer in a domestic fridge that says it is effective:[19] Fences&Windows 17:51, 25 November 2009 (UTC)
- Okay, so basically it is 3O2 -> 2O3. Aerobes don't proliferate, cheese doesn't dry out, etc. I do wonder about the safety of the product though, I'm guessing there aren't any longitudinal studies looking at respiratory diseases of those who have these in their fridge. --Mark PEA (talk) 18:35, 25 November 2009 (UTC)
- It's a UK advert - which is good, because it will have to tell the truth (or the ASA will jump on them like a ton of bricks) - it's main claim as I see it is "it reduces the damaging gases involved in food decay" - I would guess that it will keep down the concentration of ethylene, that would slow down any ripening process, and if the food doesn't over-ripen, it won't go bad so fast. Ronhjones (Talk) 00:45, 26 November 2009 (UTC)
- Okay, so basically it is 3O2 -> 2O3. Aerobes don't proliferate, cheese doesn't dry out, etc. I do wonder about the safety of the product though, I'm guessing there aren't any longitudinal studies looking at respiratory diseases of those who have these in their fridge. --Mark PEA (talk) 18:35, 25 November 2009 (UTC)
Ozone is a strong oxidiser and will cleave unsaturated compounds by ozonolysis. Ozone will react with ethylene (possibly into formaldehyde units) cuz of the unsaturated pi bond. The side effect of applying ozone for too long I think is rancidification. John Riemann Soong (talk) 13:03, 26 November 2009 (UTC)
- This just reminded me of an A-level chemistry lecture I had a couple of years ago. Paintings discolour overtime due to UV light homolysing O2 in the atmosphere, leading to O3 and subsequent ozonolysis of the unsaturated glycerides in the paint. --Mark PEA (talk) 17:40, 27 November 2009 (UTC)
November 26
age of atoms
Is there a difference between an atom of carbon when it was created billions of years ago and one now that is billions of years old? In other words are carbon atoms mortal and if so over what time scale do they show any change? 71.100.11.112 (talk) 04:44, 26 November 2009 (UTC)
- Well there are different types of carbon atoms, or of any atom, called isotopes. According to our article Isotopes of Carbon, two of these isotopes are stable, meaning that they will last forever under ordinary conditions. The rest of the isotopes are "mortal" or unstable, the most famous of which is probably Carbon-14 which has a half-life of about 5703 years. When it "dies" or decays, it becomes a Nitrogen-14 atom, which is stable. Jkasd 04:59, 26 November 2009 (UTC)
Interesting...and what decays to become stable Carbon-12, or are we stuck with however many of them we started with? DRosenbach (Talk | Contribs) 05:02, 26 November 2009 (UTC)
- I think that most of the Carbon-12 in the universe is due to the CNO cycle in stars. Jkasd 05:07, 26 November 2009 (UTC)
- I am certainly no expert, but the answer to your question is "it depends." If the carbon atom is an unstable isotope (radioactive), it will spontaneously decay at a time, which in my understanding is quite random. There is a way to measure this decay, but it is not dependent on an individual atom. The measurement assumes that you have a mass of multiple unstable atoms of the same type. When half of the atoms have decayed (changed form into another atom), that is called the half life. Carbon decay is specifically significant; look at carbon dating, where people attempt to calculate age by what percentage of the estimated original unstable carbon isotopes remain in an object. As for the time scale, it varies from almost immeasurably small amounts of time to hundreds of thousands of years, depending on the atom. As for mortality, only living beings can be mortal, at least in my definition of the word. An atom, so far as anybody knows, has absolutely zero self awareness or thought process, and as such is abiotic. An atom is therefore neither mortal (it will die someday) or immortal (it will live forever). Also, theoretically if one returned the appropriate particles to a decayed atom, it would return to its unstable form (we can do that with some nuclear reactions). Finally, if you have two carbon atoms of the same isotope, but one is three billion years older than the other, I don't believe that there is any difference. I hope this gives you a start, and since I am not an expert I'm counting on other editors to correct me if I am wrong about something ;-). Falconusp t c 05:11, 26 November 2009 (UTC)
- I think the talk of isotopes is a bit of a distraction. In either case, a billion year old atom is the same as a newly created atom. That is, a billion year old atom of C-12 is the same as a newly created one and a billion year old atom of C-14 (if you could find one) will be exactly the same as a newly created atom of C-14. So, no, they don't "age". The unstable ones do randomly decay, but not because they are old, just because of a random event happening. (Don't think of it like trees dying when they get old, but dying when they are randomly struck by lightning, regardless of age.) StuRat (talk) 05:30, 26 November 2009 (UTC)
- Ah... So then are we speaking of conservation of something... perhaps conservation of stability? 71.100.11.112 (talk) 06:03, 26 November 2009 (UTC)
- No, I don't think it is really a conservation law. It's about the indistinguishability of atoms of the same isotope. --Tango (talk) 09:28, 26 November 2009 (UTC)
- Ah... So then are we speaking of conservation of something... perhaps conservation of stability? 71.100.11.112 (talk) 06:03, 26 November 2009 (UTC)
- Radiocarbon dating determines the age of a sample or population of atoms, not the "age" of an individual atom. Individual atoms, as StuRat and Tango said, do not age (in the case of unstable isotopes this means that an atom/nucleus just before it decays is indistinguishable from one that has just been created). --Wrongfilter (talk) 09:49, 26 November 2009 (UTC)
However, StuRat is correct in stating that I am not referring to radioactive decay of unstable isotopes but to aging of stable isotopes or aging of any stable particle. If you insist on including unstable isotopes then the question applies only to an isotope atom between the time it is created and the time it transmutes such as an atom of carbon-14 before transmuting to nitrogen-14. It is during the time the atom has not transmuted which concerns me and that I reference rather than to the transmutation itself. However, I leave the question open so you can answer it any way you will - transmutation included or not. 71.100.11.112 (talk) 10:19, 26 November 2009 (UTC)
- In that case, no. There is absolutely no ageing of atoms. An atom of a particular isotope of a particular element is identical to any other, there are no changes over time. There are some changes in energy levels, but they are fluctuations, not any consistent change - they take place over nanoseconds (or smaller timeframes) and are insignificant over longer timescales. --Tango (talk) 10:34, 26 November 2009 (UTC)
- What else in the Universe including the Universe itself does not grow older or undergo changes we can attribute to aging? 71.100.11.112 (talk) 10:49, 26 November 2009 (UTC)
- Well, the universe itself certainly ages. It expands (ie. becomes less dense) and the Cosmic Microwave Background cools down (that is part of the expansion) and entropy increases. Atoms and sub-atomic particles don't age. Molecules don't really age, although some large ones change shape over time (although I don't know of any such changes that happen in a particular direction so could be considered ageing). Stars certainly age, as do geologically active planets and planets large enough not to have finished cooling and whole galaxies and clusters of galaxies. Small planets and asteroids pretty much stop ageing after a certain amount of time (a billion years, say). They may pick up a few extra impact craters, but that's about it, although I suppose the isotope ratios change over time as the time since the matter that built up the asteroid was created in a supernova increases. So, in conclusion, if you are willing to look very closely pretty much anything larger than an individual molecule probably ages in some way. To an extent, this is a matter of definitions, though - everything changes, but for some things we consider any change to make it a new thing. If we considered an atom that undergoes radioactive decay to remain the same atom, just in a different form, then even atoms age - as they get older, they move towards more stable forms. This is a very statistical form of ageing, there is no way of measuring the age of a single atom, but I suppose it would be a form of ageing. We usually consider it a new atom, though, so that is irrelevant. I apologise for the stream of consciousness - this was going to be a coherent answer when I started it! I hope it is still useful. --Tango (talk) 11:13, 26 November 2009 (UTC)
- No need to apologize. The question begs the answer and I can imagine a stable particle aging completely within the entire duration of its creation especially if that time span is instantaneous. 71.100.11.112 (talk) 12:05, 26 November 2009 (UTC)
- Well, the universe itself certainly ages. It expands (ie. becomes less dense) and the Cosmic Microwave Background cools down (that is part of the expansion) and entropy increases. Atoms and sub-atomic particles don't age. Molecules don't really age, although some large ones change shape over time (although I don't know of any such changes that happen in a particular direction so could be considered ageing). Stars certainly age, as do geologically active planets and planets large enough not to have finished cooling and whole galaxies and clusters of galaxies. Small planets and asteroids pretty much stop ageing after a certain amount of time (a billion years, say). They may pick up a few extra impact craters, but that's about it, although I suppose the isotope ratios change over time as the time since the matter that built up the asteroid was created in a supernova increases. So, in conclusion, if you are willing to look very closely pretty much anything larger than an individual molecule probably ages in some way. To an extent, this is a matter of definitions, though - everything changes, but for some things we consider any change to make it a new thing. If we considered an atom that undergoes radioactive decay to remain the same atom, just in a different form, then even atoms age - as they get older, they move towards more stable forms. This is a very statistical form of ageing, there is no way of measuring the age of a single atom, but I suppose it would be a form of ageing. We usually consider it a new atom, though, so that is irrelevant. I apologise for the stream of consciousness - this was going to be a coherent answer when I started it! I hope it is still useful. --Tango (talk) 11:13, 26 November 2009 (UTC)
- What else in the Universe including the Universe itself does not grow older or undergo changes we can attribute to aging? 71.100.11.112 (talk) 10:49, 26 November 2009 (UTC)
- There are particles that are 'immune' to time: massless particles like photons that travel at the speed of light and therefore experience no time. Fences&Windows 12:16, 26 November 2009 (UTC)
- Massless particles seem to be problematic: c = (1/M)*(M*E)^(1/2) (excuse the plain text form) 71.100.11.112 (talk) 13:06, 26 November 2009 (UTC)
- I'm not sure where that formula came from, E=mc^2 rearranges to c=(E/m)^(1/2). While that looks problematic, that formula is for rest energy and massless particles can never be at rest, so the problem never comes up. If you interpret m as relativistic mass then that formula does give you the energy of the photon (but only because the relativistic mass is calculated using that formula!). --Tango (talk) 13:33, 26 November 2009 (UTC)
- Massless particles seem to be problematic: c = (1/M)*(M*E)^(1/2) (excuse the plain text form) 71.100.11.112 (talk) 13:06, 26 November 2009 (UTC)
- The article you need to read, I think, is indistinguishability, at least up to the point before it launches into all the wave mechanics notation. When discussing particles at the quantum level, the concept of individual particles with individual identities starts to lose its meaning. We cannot tell, even in principle, which particles are the oldest because separated particles are a misconception. It is a bit like asking which is the oldest wave in the sea. SpinningSpark 12:54, 26 November 2009 (UTC)
Blackholes don't age. Dauto (talk) 13:12, 26 November 2009 (UTC)
- They decay - see Hawking radiation. They also absorb background radiation (which any star sized or larger black holes will do more of at the moment than they radiate). --Tango (talk) 13:33, 26 November 2009 (UTC)
- Yes, they decay, but as pointed out above by others, decaying is not the same thing as ageing. In other words: an old blackhole is indistuinguishable from an young one, as long as they have the same mass, charge, and angular momentum. see the no hair theorem. Dauto (talk) 14:26, 26 November 2009 (UTC)
- True. Given one black hole there is no way to determine its age since you don't know the starting size. However, given the same black hole at two different times if is possible to determine which is the older, which can be interpreted as a kind of aging. --Tango (talk) 15:32, 26 November 2009 (UTC)
- Yes, they decay, but as pointed out above by others, decaying is not the same thing as ageing. In other words: an old blackhole is indistuinguishable from an young one, as long as they have the same mass, charge, and angular momentum. see the no hair theorem. Dauto (talk) 14:26, 26 November 2009 (UTC)
- Having recently sat through a NASA lecture about interplanetary petrology, I may have some insights. The definition of "age" of a "thing" is always a little ambiguous when dealing with cosmogenic timescales. Technically speaking, (actually, not technically speaking at all), according to our best understanding of the universe, everything always existed and always will exist (although even this one gets debated by the really nitpicky cosmologists. So when you want to know the "age of a thing", what you really are asking is "how long since ____ happened to these atoms?" The blank can be filled in with a lot of complicated particle physics, geology, etc. The actual atoms are the same ones that came out of stars (usually); but even if they are not, the subatomic particles they are made of are the same ones that came out of stars; and so on. So, Instead of asking the "age of an atom," it is really better to ask "how long has this rock contained these molecules", "how long has this molecule contained these atoms", "how long since this atom mixed with these other atoms that are in the same molecule," or "how long since the protons of this atom all stuck together?" These are more precise questions and they're much easier to get exact-ish years.
- But, moving past some moot details, in the beginning there was a great infinitely dense mixture of undifferentiated energy and matter that obeyed the laws of General Relativity. All this muck swirled around in a sort of mind-bending cosmic singularity, but suddenly it started to break symmetry, and various types of subnuclear particles manifested themselves and obeyed different force laws, and it became possible to identify those which were matter-like and those which were particle-like carriers of forces that had just started to be identifiable. So, by now, all the matter and energy in the universe "existed" - and strictly speaking, everything is this old (13 billion years, often quoted with more precision in case you want to calibrate your calendar). At this early stage, nothing was an atom yet, let alone an identifiable hunk of space-debris or rock or self-locomotive, talking, carbonaceous life. Nonetheless, everything is "this old", insofar as its primordial cosmogenic ancestors came from this material.
- Anyway, flashing forward a few hundred million boring years, past the formation of the proto-nuclei and arriving at the plasma-like single protons swirling around in space, we get to stellar formation of the first round, in which hydrogen squishes together and stellar nucleosynthesis produces the standard spectrum of nuclei (atoms) that are found in almost everything we observe in space. So from some standpoint, all the atoms are really this old (neglecting, of course, a tiny but relevant fraction of nuclei which were synthesized in Big Bang's first few moments before the expansion brought the density below critical levels). Many, but not all, of the atomic nuclei are this old.
- A key thing happens here: probably, this star extincts itself, blows out all these atoms somewhere else, and they accrete in a new cloud or disc, the solar nebula or solar disc. These atoms are all floating around, but they begin to gravitationally self-attract, and finally compress in to start the formation of Sol. This is another unique age for all the rocks we find in space, because at this point, differentiation becomes relevant (e.g. the melting of rock by gravitational force). Gravity-based melting distills out the heavy and light nuclei, and so a benchmark of separated-nuclei concentrations is present. This is our "baseline" particle-ratio for heavy-nucleus radioisotope dating, because after this point, fractionation causes different rocks to have different concentrations of things. And once rocks sink into a convective melting process inside a planet, complete with metamorphic rock formation, all bets are off as far as where an individual atom will go.
- Finally, once a planetary rock makes its way to the surface, the rock begins to weather and erode, and oxidize (depending on the planet). So we can see an exchange of material again taking place; and we can date the age of this interaction.
- All told, it's really the same material getting mixed up over and over again; but at different timescales, different amounts of mixing happen. Over the lifespan of the universe, a single atom has progressed from an undifferentiated Planck soup into a Hydrogen atom into a complex nucleus into a fully-grown atom with its own electrons; and finally, it found other atoms and formed a molecule with those; and those molecules might have changed around a lot (and even the atomic nuclei might have shattered once or twice); and the large chunks of molecules probably convected a lot inside of a planet, and then probably eroded a whole lot at the surface; so the "age" is whatever you want to define it to be: the time since ____ last happened. Nimur (talk) 15:48, 26 November 2009 (UTC)
- Are you saying that while the bricks don't age the mortar and the building does? 71.100.11.112 (talk) 20:26, 26 November 2009 (UTC)
- I'm saying that the age of the building or the wall is not the same as the age of the brick. You could spin that into your summary, as long as you recognize the limits of the metaphor. Nimur (talk) 20:51, 26 November 2009 (UTC)
- Are you saying that while the bricks don't age the mortar and the building does? 71.100.11.112 (talk) 20:26, 26 November 2009 (UTC)
Both analog and digital possible?
Are there any FTA receivers which can receive both analog and digital satellite television? --84.61.167.221 (talk) 14:07, 26 November 2009 (UTC)
- I think that they exist, but there is so little analog on satellite that it is probably not worthwhile. Graeme Bartlett (talk) 09:19, 27 November 2009 (UTC)
Sun looks different in size
Is there a scientific explanation for the reason we seen the Sun bigger in size during sunrise/sunset than in the mid-noon. I might be wrong but many have been asking this.--Email4mobile (talk) 14:53, 26 November 2009 (UTC)
- Thanks a lot, Gandalf61.--Email4mobile (talk) 15:09, 26 November 2009 (UTC)
- It's a very powerful illusion. I work in computer graphics and one task I've been handling recently is drawing the sun and moon realistically in computer games. When I calculate the mathematically correct sizes to draw them, they look ridiculously small because that moon illusion works in reverse on a computer screen. I had to draw them both at four times the realistic size in order to please the majority of the 30 or so co-workers I polled for their judgement of the right size! I find this deeply disturbing to my sense of "doing it right" - so I eventually compromised at three times the correct size. SteveBaker (talk) 17:26, 26 November 2009 (UTC)
- I've read the moon illusion, but couldn't get a final explanation for that phenomenon. Can you give me a conclusion for that?--Email4mobile (talk) 17:36, 26 November 2009 (UTC)
- It's a surprisingly powerful illusion - nearly everyone overestimates the size of the moon - but you can use the "Can you cover the moon with the tip of your pinky finger at arms-length?" test (yes, you can) to convince yourself that sun & moon are indeed the same size no matter where they are in the sky. Basically - when the moon (or sun or whatever) is far above the horizon, you have no context for judging it's size - when it's close to the horizon, you have other objects to compare it against - since it's "behind" everything else, your brain registers that it's a long way away - since it's obviously larger than things like houses and trees - it looks gigantic (which is fair because it IS gigantic). SteveBaker (talk) 18:33, 26 November 2009 (UTC)
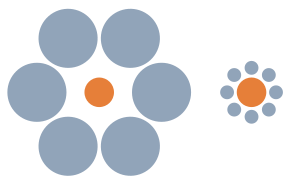
- So it is a state of relativistic views rather than light intensity effect. Before, I asked and before reading about it, I was thinking it was due to different light intensity during the different times. Thanks SteveBaker.--Email4mobile (talk) 21:33, 26 November 2009 (UTC)
- I wouldn't call it relativistic, I would call it relative - just to avoid ambiguity about the actual effect. Nimur (talk) 22:38, 26 November 2009 (UTC)
- Right - the image at right really explains it well. It's called "The Ebbinghaus illusion". Imagine that the orange dot is the moon - but with the largeness of the sky it looks smaller than with the small details of the horizon. SteveBaker (talk) 23:32, 26 November 2009 (UTC)
- Doesn't the atmosphere have a sort of magnifying effect as well? Granted it would be fairly small, but perhaps noticeable. Googlemeister (talk) 15:32, 30 November 2009 (UTC)
- Right - the image at right really explains it well. It's called "The Ebbinghaus illusion". Imagine that the orange dot is the moon - but with the largeness of the sky it looks smaller than with the small details of the horizon. SteveBaker (talk) 23:32, 26 November 2009 (UTC)
- I wouldn't call it relativistic, I would call it relative - just to avoid ambiguity about the actual effect. Nimur (talk) 22:38, 26 November 2009 (UTC)
How big can a Thanksgiving turkey be?
The Wikipedia articles on turkeys don't say how big a Thanksgiving turkey can be, and searching around on the Internet did not tell me, either. I know that the supermarket has turkeys larger than 24 pounds, but how big can they be? --DThomsen8 (talk) 14:56, 26 November 2009 (UTC)
- Is there some reason Thanksgiving turkeys are different from normal turkeys? Googling suggests a world record was set at 86 pounds - Wikipedia blocks the link because apparently it's spam. Are you perhaps talking about specific weights at which turkeys are commercially sold? Vimescarrot (talk) 15:34, 26 November 2009 (UTC)
- The human variety may weigh more. 71.100.11.112 (talk) 15:52, 26 November 2009 (UTC)
- Googling around suggests that birds as large as 40lbs can be bought commercially - but almost all of the "How long does it take to cook" guides top out at 30lbs. Many of those guides recommend buying two smaller turkeys when you need more than 18lbs because it's easier to control the cooking process. SteveBaker (talk) 17:21, 26 November 2009 (UTC)
- Turkeys have been bred over generations to be larger and larger. Today they are so big they can no longer mate on their own, and must be artificially inseminated. They are also starting to have problems walking. To make them larger would probably require too much human intervention to make it cost effective. Ariel. (talk) 19:30, 26 November 2009 (UTC)
- With the above mentioned cooking control issue it seems like even large kitchens might prefer smaller turkeys. Perhaps GM will find a way to make legs stronger or to eliminate unwanted parts. I dare to imagine what kind of "turkey" science could come up with if it really went to work. 71.100.11.112 (talk) 19:39, 26 November 2009 (UTC)
- Wouldn't that simply be growing meat in a lab. Remove all the unwanted parts - the stuff that isn't meat. You are left with meat. It isn't too hard to imagine. It also means that no turkeys would be killed to produce Thanksgiving dinners. I know - that is purely evil GM thinking. Thanksgiving without killing turkeys - I dare to imagine what kind of freak holiday that would be. -- kainaw™ 23:10, 26 November 2009 (UTC)
- The Space Merchants (1952) features 'Chicken Little' which is exactly what Kainaw suggests (but the article is currently under maintenance, and presumably for that reason doesn't mention Chicken Little at present). --ColinFine (talk) 00:02, 27 November 2009 (UTC)
- When I was a kid, my family grew a turkey that weighed 50 lbs dressed out. A turkey that size will not fit into a standard oven. Googlemeister (talk) 15:34, 30 November 2009 (UTC)
- The Space Merchants (1952) features 'Chicken Little' which is exactly what Kainaw suggests (but the article is currently under maintenance, and presumably for that reason doesn't mention Chicken Little at present). --ColinFine (talk) 00:02, 27 November 2009 (UTC)
hoax? Bulgarian scientist claims alien contact
So what is this all about? There is a scientist called Lachezar Georgiev Filipov, who has a CV here, hosted by the Space Research Institute of Bulgaria. Today he is in the news for claiming that "aliens are already among us". Is this Bulgarian National Tease the Rest of the World Day? Or is it a reverse practical joke, designed to extract more funding -- "see, the public will believe anything, so we need mass education"? Or has his name been taken in vain? Or could it be that he actually believes this? BrainyBabe (talk) 16:22, 26 November 2009 (UTC)
- How very interesting, I wonder if we should have messages relayed to us via 'the power of thought' as another one of the WP:Reliable sources Wikipedia uses? ;-) Dmcq (talk) 16:37, 26 November 2009 (UTC)
- Is it wrong that I laughed at
- "PROFESIONAL TRAINING
- [...]
- 1981 Language qualification - England
- in his CV? 86.144.145.238 (talk) 16:41, 26 November 2009 (UTC)
- Yes, I noticed that too. The website of the institute looks c. 1997 -- in fact, I thought it might be a fake, but if so, someone has gone to many many pages of trouble. BrainyBabe (talk) 16:45, 26 November 2009 (UTC)
- Lots of reputable science figures end up promoting crackpot ideas. Initially starting with a scientific-like approach, these people usually devolve into disreputable science figures, by way of ignoring factual evidence and the scientific method. Take a look, for example, at Edgar Mitchell. His outlandish views have put him outside the main fray of NASA and at odds with most scientific thought. Nimur (talk) 16:55, 26 November 2009 (UTC)
- A little tracing of sources shows that everyone is ultimately reporting something that appeared in Novinar, a Bulgarian newspaper. You can read Novinar's interview with Filipov in broken English using Google translate: here. The blurb at the beginning suggests there is an earlier (Monday?) Novinar article publishing his thoughts, which sparked all of this. I cannot find this earlier article online, but then I have no grip on Bulgarian or the Cyrillic script. 86.144.145.238 (talk) 17:06, 26 November 2009 (UTC)
- It also seems possible that there's an active and malicious effort to mistranslate key words and construe the interview in a different way than it was originally intended. Nimur (talk) 17:08, 26 November 2009 (UTC)
- In which case, should we rope in some Language Desks regulars to comment on the English reporting compared to what was said in Bulgarian? 86.140.174.66 (talk) 17:16, 26 November 2009 (UTC)
- How could the translation be intentionally mistranslated or misconstrued? Google translate is a purely automated tool, so there's no people involved in the translation process that could be intentionally malicious in the process.
- Having read the automated English translation of the Bulgarian article, I see no evidence that would suggest that this is a hoax, or a joke, or that he doesn't genuinely believe it. He's just a scientist who wound up getting caught up in some bullshit. I think scientists as a whole are much less likely than the general public to get lured into believing nonsense like this, but it certainly does happen. Scientists are still just human. Red Act (talk) 17:59, 26 November 2009 (UTC)
- Well, he has carefully examined 130 crop circles and asked the Vatican about it - you can only take 'due diligence' so far.
- But to address the OP's question: I don't think we can accurately research his motives behind this announcement. If it's a hoax or a joke - it's a pretty pathetic one - maybe it was funnier in the original Bulgarian? He's claiming the messages are telepathic - and hearing voices in ones head (See Auditory hallucination) is a sign of schizophrenia or mania. It's perfectly possible that this guy has a real medical problem. As an effort to extract more funding? Well, if that was the intent - I think it may have backfired - the linked article says that the finance minister is discussing it with the prime minister...I don't think that's going to end positively. SteveBaker (talk) 17:14, 26 November 2009 (UTC)
- From the Bulgarian interview, I don't get that Filipov thinks that he himself is getting telepathic messages through the crop circles, it's a woman by the name of Mariana Vezneva. Filipov has just become one of Vezneva's followers. Vezneva's web site says that the "Teachers" materialized twice in her home, so if she's having hallucinations, they're not just auditory ones. Red Act (talk) 20:38, 26 November 2009 (UTC)
- "Mr Filipov said that even the seat of the Catholic church, the Vatican, had agreed that aliens existed." Indeed, they agree, but they call them God, seraphim, cherubim, etc. --Cookatoo.ergo.ZooM (talk) 18:09, 26 November 2009 (UTC)
Translation of Bulgarian article: An anonymous Bulgarian editor has kindly and amazingly translated the article into readable English on the Language desk. Wikipedia:Reference_desk/Language#Bulgarian_Scientist. You can go there to read precisely what he said, including where he says some very strange things and also where he seems to have tried to investigate a fringe area vaguely scientifically. All very odd. Also, tantalising hints of at least one earlier article laying out some of this bizarre theories in more detail.
Props to the translator, who really exceeded expectations translating something so bizarre into something so accessible. 86.140.171.80 (talk) 05:31, 27 November 2009 (UTC)
- Side note - it is great that Wikipedia can link up talent and interest to people in need. Thanks for the translation.
- Anyway, from a reading of the article, it looks like the scientist is being blacklisted for investigating a paranormal claim - it doesn't appear that he has taken a stance to believe or disbelieve any of the paranormal claims. Furthermore, it appears that he did it on his own time, and not through the auspices of the Bulgarian Academy of Science. As I mentioned earlier, I think it's possibly a slander campaign to make this out to be much more sinister, and paint the guy as a "nut-job". Clearly there's a political and financial angle to such a maneuver. I guess the moral of the story is that any scientist who wants to keep their job should avoid so much as mentioning the paranormal, lest the media should find out about it. Nimur (talk) 16:08, 27 November 2009 (UTC)
- It is very important that you do not panic. Be assured that reliable scientists with white coats and horn rimmed spectacles have the situation under complete control. So keep calm. There is absolutely no substance in rumours you may hear that the all-encompassing mental field is sucking the brains out of our children. Crop rings do not exist. They have been proved to be hoaxes perpetrated by malicious farmers seeking to inflate the price of wheat, and they will be dealt with by
commando death squadsthe proper authorities. Furthermore, Bulgaria is a friendly country somewhere in a foreign place that is famous for its sunflower seeds and volleyball players, and it could never be taken over by aliens posing as mad professors without someone noticing. Even as we speak the CIA is only waiting for the clouds to clear so our Spy satellites can find exactly where Bulgaria is. Not only do telepathic aliens not exist, no telepathic alienamong those now held at Area 51 underground Sector 4shows any sign of understanding Bulgarian language. So there is no reason to panic. Wikipedia has an article on Bulgaria that may be helpful. Cuddlyable3 (talk) 10:20, 27 November 2009 (UTC)
- I was asked by BrainyBabe to comment on the general situation here. In my very humble opinion, the whole story is not notable enough for us to discuss it. It is typical for the media in Bulgaria that they should include materials on aliens, paranormal phenomena and events, etc. from time to time. See for example this almost humorous reportage by bTV, the most influential television channel in Bulgaria, telling of an alien creature reportedly noticed in Varna. There are many people everywhere who claim to have communicated with extraterrestrials, and professor Filipov is just one of them, with my greatest respect to him. Searching Google for his name gives mostly interviews with him and articles by him, such as: Lachezar Filipov: UFOs have been witnessed in Bulgaria several times, Professor Lachezar Filipov, astrophysicist: Our civilisation is not yet ready for a contact, Bulgarian cosmologist: There is nothing to worry about as long as we are under the control of God and so on. --62.204.152.181 (talk) 16:03, 27 November 2009 (UTC)
- So fringe rather than nutter. My apologies for the aspersions, I should take more care about newspapers. You need people who'll investigate fringe ideas sometimes even if most people wouldn't be seen dead doing so. Dmcq (talk) 17:05, 27 November 2009 (UTC)
- Thank you again, o anonymous Bulgarian! Your putting the hoo-ha in context is valuable, especially the new links you provide. Your good work is appreciated. BrainyBabe (talk) 21:48, 29 November 2009 (UTC)
- There have been many compilations of reputable alien sightings, but many UFOs could be secret government millitary craft such as the Avrocar. One such compilation is Project Blue Book, and the British and Belgian government recently released information on possible alien sightings. There was also a 1996 case where a large 2/3 wide spacecraft was indpendently reported by about two dozen witnesses. ~AH1(TCU) 02:06, 2 December 2009 (UTC)
How does the body sense temperature?
I don't mean, what does it do with the information, but the physical measurement used. I read Thermoregulation, Thermoception, and Homeostasis, and none of them mention it.
Temperature changes the speed of sound, the size of objects, the kinetic speed of gases, melting things, the speed of reactions, pressure in a container, electrical resistance. And yet none of those would seem to be measurable by the body. And accurately too - to less that .1 of a degree, and it's also tunable (fever) - how is it calibrated?
What physical thing does the body measure? I assume thermoregulation is tuned for accuracy, and thermoception for speed. Ariel. (talk) 18:53, 26 November 2009 (UTC)
- To quote the Thermoception article, "The details of how temperature receptors work is still being investigated." :( If anyone does find refs for research on that, please add to the article. DMacks (talk) 21:00, 26 November 2009 (UTC)
- There are specific neurons that sense heat -- they are cross-sensitive to capsaicin; there are also cold-sensing neurons, and these are cross-sensitive to menthol. That's why these and other items give us a "hot" or "cold" sensation. Perhaps what needs to be worked out and is still under investigation is how the body senses finer changes in temperature than basic hot + cold. It's possible, though, that the body just senses relative warmth of lack of warmth, just like the case of the three buckets (one with hot water, one with cold water, still a foot in each and after a minute, pour both contents into 3rd bucket, put both feet in and it feels hot for the foot that was in the cold and cold for the foot that was in the hot). DRosenbach (Talk | Contribs) 23:45, 26 November 2009 (UTC)
- Even just basic hot/cold (without any fine measurements), and even simpler: simply measuring if something is hotter or colder than the body - how? What physical thing does it measure? And accurately measuring body temperature with no reference, or method calibration has to be even harder, and I wonder both how it does that, and what physical change does it measure? Ariel. (talk) 00:32, 27 November 2009 (UTC)
- It really isn't known (and, given somatosensation is the last sensory system where very little is understood at the molecular level, when it is fully worked out it will probably earn the scientists a Nobel Prize). Scientists are largely focusing in Transient receptor potential ion channels, such as the TRPV and TRPA (channel)s, as the candidate proteins that detect temperature. I happen to have some colleagues that are at the forefront of this research. Frustratingly, the detection of actual temperature appears to be mechanistically different from the detection of chemicals that we perceive as having temperature (such as menthol or capsaicin), which complicates matters. But, without giving away too much unpublished data, they appear to have narrowed down the precise parts of these proteins that are temperature sensitive. They are now trying to work out what exactly happens to these specific amino-acids when you change their ambient temperature. That should provide a clue to the exact physical characteristic that precipitates the molecular change. Come back and ask this question in about 5 years, and I expect we will be able to answer it more fully. Rockpocket 01:55, 27 November 2009 (UTC)
- Thank you. At least now I know that it isn't known, and I should stop trying to find someone who knows. And I shall do just that, I'll put an entry in my calendar for 5 years from now, and I'll email you from your wikipedia page and ask how things are going. :) (Similey, but I really will.) Ariel. (talk) 04:14, 27 November 2009 (UTC)
- It really isn't known (and, given somatosensation is the last sensory system where very little is understood at the molecular level, when it is fully worked out it will probably earn the scientists a Nobel Prize). Scientists are largely focusing in Transient receptor potential ion channels, such as the TRPV and TRPA (channel)s, as the candidate proteins that detect temperature. I happen to have some colleagues that are at the forefront of this research. Frustratingly, the detection of actual temperature appears to be mechanistically different from the detection of chemicals that we perceive as having temperature (such as menthol or capsaicin), which complicates matters. But, without giving away too much unpublished data, they appear to have narrowed down the precise parts of these proteins that are temperature sensitive. They are now trying to work out what exactly happens to these specific amino-acids when you change their ambient temperature. That should provide a clue to the exact physical characteristic that precipitates the molecular change. Come back and ask this question in about 5 years, and I expect we will be able to answer it more fully. Rockpocket 01:55, 27 November 2009 (UTC)
- Even just basic hot/cold (without any fine measurements), and even simpler: simply measuring if something is hotter or colder than the body - how? What physical thing does it measure? And accurately measuring body temperature with no reference, or method calibration has to be even harder, and I wonder both how it does that, and what physical change does it measure? Ariel. (talk) 00:32, 27 November 2009 (UTC)
- There are specific neurons that sense heat -- they are cross-sensitive to capsaicin; there are also cold-sensing neurons, and these are cross-sensitive to menthol. That's why these and other items give us a "hot" or "cold" sensation. Perhaps what needs to be worked out and is still under investigation is how the body senses finer changes in temperature than basic hot + cold. It's possible, though, that the body just senses relative warmth of lack of warmth, just like the case of the three buckets (one with hot water, one with cold water, still a foot in each and after a minute, pour both contents into 3rd bucket, put both feet in and it feels hot for the foot that was in the cold and cold for the foot that was in the hot). DRosenbach (Talk | Contribs) 23:45, 26 November 2009 (UTC)
harmful aspiration
Are societies which now are able to provide an abundance of food to everyone growing overweight due to the psychological effect of the aspiration to be wealthy and the fact that the wealthiest have become overweight? In other words if we aspired to break-even so to speak instead of aspiring to have wealth beyond our need would we find acquisition of normal weight to be the result of our ambition or the rule? 71.100.11.112 (talk) 20:36, 26 November 2009 (UTC)
- Research has been published both indicating and refuting your assertion; e.g. Food insecurity is associated with increased risk of obesity (2003); and Obesity: the emerging cost of economic prosperity (2006). I searched Google Scholar for results with obesity and prosperity as my query. Nimur (talk) 22:57, 26 November 2009 (UTC)
- I believe that the best explanation is simply that we evolved in a world without farming - where food was not abundant and where high-calorie foods were especially valuable to day-to-day survival. Hence, nothing in our biochemistry turns off the desire to eat or moderates our attraction to foods that are (in abundant quantities) harmful to us - but which were crucial to survival in early hominids. SteveBaker (talk) 23:27, 26 November 2009 (UTC)
- An important point I think is that it is not the wealthiest who have become overweight — not within a given society. Comparing countries we might find prosperous countries more overweight than economically ineffectual countries. But I would doubt that the wealthiest Americans represent the most overweight group of Americans. Bus stop (talk) 02:55, 27 November 2009 (UTC)
- Also, our dogs and cats are overweight too - it's safe to assume that they don't have some psychological aspiration to become wealthy. They get obese because they are fed more of the wrong foods than their bodies need - and there is no biological imperative to stop eating after having eaten a healthy amount. Pet birds don't seem get obese (at least, I've never heard of an obese parrot!) - presumably because they DO have a built in biological mechanism to prevent them from over-eating and becoming to heavy to fly. Evolution does things like that! SteveBaker (talk) 03:27, 27 November 2009 (UTC)
- Some studies have found that in affluent countries, the poor have the highest rates of obesity. For instance [20] Possibly because they are less able to buy fresh vegetables, more likely to eat highly processed foods. Or perhaps because they are less likely to sit down to carefully prepared meals at all. 75.41.110.200 (talk) 07:04, 27 November 2009 (UTC)
- Also, our dogs and cats are overweight too - it's safe to assume that they don't have some psychological aspiration to become wealthy. They get obese because they are fed more of the wrong foods than their bodies need - and there is no biological imperative to stop eating after having eaten a healthy amount. Pet birds don't seem get obese (at least, I've never heard of an obese parrot!) - presumably because they DO have a built in biological mechanism to prevent them from over-eating and becoming to heavy to fly. Evolution does things like that! SteveBaker (talk) 03:27, 27 November 2009 (UTC)
November 27
Does blood really thicken in cold weather?
I have heard people say that their blood thickens in colder climates, and that this is why one can be more accustomed to the cold if one has lived their whole lives in, say, Canada versus visiting after spending their whole lives in Mexico.
Is this true? Or, is it just a joke, and something else causes one to become more accustomed to the cold? I suppose that it *could* be true, in that cold contracts things and heat expands it, but there are so many other things, such as nerve cells, that impact how we feel heat and cold. 209.244.187.155 (talk) 02:23, 27 November 2009 (UTC)
- See Acclimatization. There is a real biological/psychological process whereby someone becomes accustomed to (acclimated to) their environment, to the point where they become more comfortable with the conditions where they live. Blood itself doesn't become "thicker", per se, but such phrases are merely a "figure of speech" which is understood to refer to acclimatization to temperatures. --Jayron32 03:41, 27 November 2009 (UTC)
- Dehydration sets in if the amount of water in the blood is just 2% below normal. At 5% below normal, one may become groggy, dizzy, tingly and get serious headaches and such. At 10% to 15% below normal - you're dead. So if there were any real "thickening" - it would have to be by a very small amount. I agree with Jayron32 - this is just a figure of speech that describes some more complex acclimatization. SteveBaker (talk) 04:07, 27 November 2009 (UTC)
- Short-term exposure to cold can significantly "thicken the blood" (as measured by blood viscosity): PMID 17929604; however, longer term acclimatization to cold weather results in a compensatory thinning of the blood: PMID 10627870. Such studies aren't conclusive, but they do seem to make intuitive sense. -- Scray (talk) 04:14, 27 November 2009 (UTC)
- Water doesn't thicken until it freezes, while oils and fats do thicken gradually when they cool. Since blood contains both water and (a small amount of) fat, I'd expect it to thicken some when it cools. However, note that the core body temperature doesn't drop much in cold weather (unless you go into hypothermia, that is). However, the blood temp in the extremities, like fingers and toes, could drop considerably during cold weather, if you aren't dressed properly. So, blood in those areas could become significantly thicker. StuRat (talk) 05:47, 27 November 2009 (UTC)
- Water's viscosity does almost double when its temperature drops from 37 degrees C (body temp) to 20 degrees C, though the viscosity is still low compared to oils for example. -- Scray (talk) 06:58, 27 November 2009 (UTC)
- Blood viscosity is much higher than that of water, even though blood is mostly water, because proteins (e.g. fibrinogen) and other constituents of blood have a big effect on viscosity, as noted in the blood viscosity article and Pubmed references I cited in my prior edit. Hence, blood really is thicker than water, even though it's mostly water. -- Scray (talk) 16:55, 27 November 2009 (UTC)
- Water's viscosity does almost double when its temperature drops from 37 degrees C (body temp) to 20 degrees C, though the viscosity is still low compared to oils for example. -- Scray (talk) 06:58, 27 November 2009 (UTC)
- Blood is a non-Newtonian fluid - it behaves in very strange ways and conventional measures of viscosity are not entirely meaningful. SteveBaker (talk) 19:50, 28 November 2009 (UTC)
quantum mechanis
How much quantum mechanical is experimental or a theoritycal subject thats means scope for thinking?Supriyochowdhury (talk) 03:24, 27 November 2009 (UTC)
- The computer you are sitting in front of wouldn't work if it were not for Quantum tunnelling. So-called "NAND flash memory" - such as you find in USB memory sticks use a technique called tunnel injection for writing and tunnel release for erasing. Neither of those things would be possible if quantum theory were not true. No - it's safe to say that quantum theory is very real - and quite applicable to all sorts of real-world applications. SteveBaker (talk) 04:15, 27 November 2009 (UTC)
- Thae article Quantum mechanics is your friend. Cuddlyable3 (talk) 09:23, 27 November 2009 (UTC)
- Even some of the parts that appear on the face of it to be pure philosophy—like the debates over the EPR paradox—can turn out to have not only testable results, but practical importance (see quantum cryptography). --Mr.98 (talk) 13:47, 27 November 2009 (UTC)
theoretical chemistry
will u give me information about theoritical chemistry.Supriyochowdhury (talk) 03:30, 27 November 2009 (UTC)
- We can give you the information in the article Theoretical chemistry. There is also the professional journal Theoretical Chemistry Accounts which presumably discusses cutting-edge work in the field. --Jayron32 03:37, 27 November 2009 (UTC)
- I have worked with theoretical chemists; their discipline runs across a loosely-defined boundary. You might also want to read about applied physics, specifically molecular physics; also, chemical physics, computational chemistry, molecular dynamics are commonly synonymous. In some cases, protein folding or biophysics can all be classified as "theoretical chemistry" (I have known self-described theoretical chemists in all of these disciplines). Most often, the theoretical chemists I know are using mathematical and computer-based models to predict chemical properties and the aggregate behaviors that result from microscopic chemistry. In some cases, there are no applications. My personal exposure is more applied; so the theoretical chemists I have worked with have also had applications in biochemistry, petrochemistry, atmospheric chemistry, and photochemistry. "Theoretical" can mean a lot of things to different people, ranging from thought-experiments and chalkboards, all the way to lab-work for next-generation materials that don't exist yet. For comparison, see applied chemistry or chemical engineering, which are categorically the opposite of theoretical chemistry. Nimur (talk) 16:55, 27 November 2009 (UTC)
which are the compounds in which boron has -3 oxidation state?
which are the compounds in which boron has -3 oxidation state? —Preceding unsigned comment added by Danishmanzar (talk • contribs) 05:03, 27 November 2009 (UTC)
- I am not sure there are any; there are a class of compounds called Borides, which have nominally negative oxidation states for boron. However, these are network solids for which concepts like "oxidation state" has little meaning. You could probably find some "boride"s with a nominal -3 oxidation state, but this has little meaning when discussing compounds of this type. --Jayron32 05:13, 27 November 2009 (UTC)
Are there any compounds in which boron has -4 or even -5 oxidation state? --84.61.167.221 (talk) 12:13, 27 November 2009 (UTC)
- Again, it may be possible to find compounds where you could calculate a nominal oxidation state of Boron at just about any negative number; however the nature of such compounds is such that those are meaningless oxidation numbers. Many boron-containing compounds are network solids, for which "oxidation state" is a meaningless statistic. Many borides, in fact, have fractional oxidation numbers, which again is a meaningless reality, since you cannot have a part of an electron. There may be, or there may not be, boron-containing compounds which could be calculated to have -3 or -4 or -5 oxidation states, but the chemistry of these compounds is such that such calculations aren't going to be useful. --Jayron32 04:48, 28 November 2009 (UTC)
Are there any compounds in which boron has +6 oxidation state? --84.62.199.19 (talk) 22:02, 28 November 2009 (UTC)
- No, Boron only has 5 protons. Most commonly, if one really wants to count it, it has a +3 oxidation state, as in Boric Acid. I'm not sure that, under normal room conditions, one would find boron in any positive ion except +3, since those two core electrons are bonded rather tightly. --Jayron32 02:13, 29 November 2009 (UTC)
Deep sea fish: do we know how the buggers cope with the insane pressures?
Well I'm curious if the mechanisms by which a small puny fish survives while machanical behemoths may fail have been understood. Also do we know how do they breath? They must have special adaptations in there too right?Bastard Soap (talk) 07:58, 27 November 2009 (UTC)
- "Mechanical behemoths" have to survive both standard atmosphere and deep-sea pressures, and everything in between. Deep-sea fish only have to survive one, which is a lot easier. (Similarly, I've heard that designing something that can go at Mach 2 isn't difficult - the difficulty is making something that can reliably do Mach 2 and Mach 0.2.) Vimescarrot (talk) 09:10, 27 November 2009 (UTC)
- Deep sea fish (see article) are aclimatised to their environment by having their internal pressure matched to the external pressure so there is no differential stress on the body structure. That is unlike a submarine in which the hull must resist external pressure to maintain comfortable air pressure for humans. A deep sea fish would experience difficulty if it were to ascend rapidly to shallow water, comparable to The Bends known to divers. We have no reliable sources to confirm or deny the occurence of buggery or insanity at deep sea depths. Cuddlyable3 (talk) 09:20, 27 November 2009 (UTC)
- Or bastardy, for that matter. ;-) --Heron (talk) 20:32, 27 November 2009 (UTC)
- Please do not make fun of an OP's name from Heron. Cuddlyable3 (talk) 09:19, 28 November 2009 (UTC)
Unmanned submarine design
This brings up a design question I thought of before. In an unmanned submarine (one with just cameras and such), could the pressure be kept equal inside and out, since there's no human the pressure changes would kill ? I doubt if you'd want seawater inside the submarine, so you could use pressurized air tanks (or some other gas), instead. Of course, the electronic and mechanical components inside the sub would need to be able to withstand pressure changes, but the hull would no longer need to be thick. Has anyone tried such a design ? StuRat (talk) 07:52, 28 November 2009 (UTC)
- In principle yes but the submersible would be loaded with an extra pressurised gas tank, a pressure regulator and a compressor pump. Omit the compressor pump and you have to vent the gas on returning to the surface, which means you need a compressor to refil the tank anyway. However why should a submersible need a controlled pressure compartment at all? Cuddlyable3 (talk) 09:26, 28 November 2009 (UTC)
- Some electronics don't do well when wet. There are options, such as coating everything in epoxy or waterproofing everything, but this is not always possible; it's especially notable that deep oceans are pressurized and saline - so you really need to be careful about where seawater can be allowed to flow. Most often, the electronics cabin is inside a pressure hull, which is filled with air at or above atmospheric pressure. It's not totally unheard of to have wet electronics, e.g. no "interior", but it's less common. Nimur (talk) 21:06, 28 November 2009 (UTC)
- You don't need tanks, or a pressure regulator or anything like that. A simple flexible bladder filled with air (a big bag o' air) outside the submarine would work just fine. As the pressure went up, the bladder would get smaller, but the pressure inside and out would be the same, and no saline would enter. Ariel. (talk) 03:35, 29 November 2009 (UTC)
- Some electronics don't do well when wet. There are options, such as coating everything in epoxy or waterproofing everything, but this is not always possible; it's especially notable that deep oceans are pressurized and saline - so you really need to be careful about where seawater can be allowed to flow. Most often, the electronics cabin is inside a pressure hull, which is filled with air at or above atmospheric pressure. It's not totally unheard of to have wet electronics, e.g. no "interior", but it's less common. Nimur (talk) 21:06, 28 November 2009 (UTC)
is it hard to make vacuums?
Is it hard to make a large area of vacuum on Earth, for example the size of a football stadium etc? I would think it would be really easy, because you could just have a bunch of layers, for example 100, then each one would only have to be able to support the pressure difference of 1/100th of an atmosphere... but maybe I'm not thinking clearly... so anyway: would it be hard to cover a football stadium, and make a vacuum out of it, using many layers or any other technique? Thanks. 92.230.68.102 (talk) 13:02, 27 November 2009 (UTC)
- There are several problems here. But the main one is coping with atmospheric pressure, which is about 101 kN/m2, or the equivalent of 10 tons per square meter. So, roughly, every part of your construction must be strong enough to carry that weight - unless I missed a zero somewhere, that means that if you pack your construction with cars, one next to the other, with so space in between, it would need to be able to withstand the weight of about 60 layers of (empty) Toyota Corollas. --Stephan Schulz (talk) 13:11, 27 November 2009 (UTC)
- This is a moot point, and it's pretty much categorically wrong (sorry Stephan Schulz). DOT-rated tanks (the sort you see every single day) are rated to 3000 psi gauge pressure. A theoretical perfect vacuum only has 14.7 psi gauge pressure. It'd be a piece of cake to make a tank or structure of aluminum that can withstand this - and in fact, they are commercially available. Anver or Fisher sell all the parts you need. If you felt like building a vacuum system the size of a football stadium, some unique engineering challenges would arise; but the total material stresses would probably be less severe than, say, a large crane or cantilever bridge. Sorry Stephan Schulz, your calculations are very wrong. For comparison, think of an average tire - it's inflated to a ballpark of around 30 psi - which means that the contact force of a Toyota Corrolla is ... 30 psi. This is actually twice atmospheric pressure, on the road surface, everywhere the tire contacts the road. Nimur (talk) 17:05, 27 November 2009 (UTC)
- Actually, it's three times atmospheric pressure, as the gauge only registers pressure difference to the outside. A Corolla's weight is 1300 kg, divided by 4 that's roughly 400 kg per tire. Assuming a contact patch of 200 cm2, that is 400*50kg per square m, or 20 tons - with 2 times overpressure that is exactly compatible with my computation. The other way round: The Corolla covers 4.3 times 1.7 m, so the atmospheric load on a Corolla-sized patch is 7.3 times 10 tons, or 56 Corollas. What you have shown is that we can make containment vessels that can withstand this force (see below for the submarine examples), not that my calculation is wrong somewhere. --Stephan Schulz (talk) 17:32, 27 November 2009 (UTC)
- This is a moot point, and it's pretty much categorically wrong (sorry Stephan Schulz). DOT-rated tanks (the sort you see every single day) are rated to 3000 psi gauge pressure. A theoretical perfect vacuum only has 14.7 psi gauge pressure. It'd be a piece of cake to make a tank or structure of aluminum that can withstand this - and in fact, they are commercially available. Anver or Fisher sell all the parts you need. If you felt like building a vacuum system the size of a football stadium, some unique engineering challenges would arise; but the total material stresses would probably be less severe than, say, a large crane or cantilever bridge. Sorry Stephan Schulz, your calculations are very wrong. For comparison, think of an average tire - it's inflated to a ballpark of around 30 psi - which means that the contact force of a Toyota Corrolla is ... 30 psi. This is actually twice atmospheric pressure, on the road surface, everywhere the tire contacts the road. Nimur (talk) 17:05, 27 November 2009 (UTC)
- There is one thing you didn't address - my question about layers. If you have a 1 meter-per-side cube, then if you empty it you are right, you create 10 tons (101 kN) of force on each side. Okay, 10 tons is too big, I want to reduce it to 500 pounds. So, I only create a 2% vacuum, so that the pressure differential is 0.02 atmospheres. Now, my question is, why can't you create that pressure differential even if immediately inside the large cube there is a SLIGHTLY smaller cube, that also supports 500 pounds of force on each side -- and, you guessed it, inside of it you put another 0.02 atmospheres of pressure difference. So the difference between the outside air and the inside of the small cube is now 0.04 atmospheres, but each one only has to support 0.02 atmospheres. The difference in size between the two cubes could be negligible: a millimeter is plenty. Now you repeat it for 50 cubes (like Matryoshka dolls), getting down to a perfect vacuum. If there is a millimeter of buffering each one (2 millimeters per side total), then the 50th cube should be 100mm, ie 10 centimeters smaller than the largest one. So it will be 0.9 meters per side, instead of 1 meter. But inside it it will have a perfect vacuum, and none of the intermediate cubes have to support more than 500 pounds per face. 500 pounds is not so hard to support, I bet styrofoam can do it (if there's no sudden jerk but the pressure is slowly laid on). My question is why this couldn't be done on a larger scale -- why couldn't you have a football stadium inside 100, 1thousand, 100thousand, however many layers it took to get the pressure differencial so slight that it is very easy to build nested structures that each support it. What is wrong with my reasoning here? 92.230.68.102 (talk) 13:53, 27 November 2009 (UTC)
- For one, you neglect the thickness and the weight of your layers. 500 pounds per square meter still is significant loading. Styrofoam is not airtight, and to hold 500 pounds on it you would need a very significant thickness - certainly more than 10 cm. And whatever you build, each layer must have completely independent structural integrity. You cannot rest the outer layer on the inner layer, as that would also transfer the force. So you are talking about building 100 complete enclosures around your stadium, each one a little bit bigger than the one before. I don't think that is even technically feasible - it's certainly not feasible economically. --Stephan Schulz (talk) 14:32, 27 November 2009 (UTC)
- Thank you, but I was not asking not in a technical, or economical sense, but a physical sense. Nice catch that you can't in any way rest the outside ones on the inside ones, as that would transfer force. Unless of course you somehow had "pylons" go through seals (it's easy to seal 0.02 atm) directly to the center, if you can imagine what I'm talking about: I mean like a Kline bottle. So, again, in a physical sense (not really an economic one), imagine if cost wasn't an issue. So, I want a football-stadium size vacuum, so the technique is:
- like this (I don't know how to embed this so it appears inline -- if someone else can, please replace this with an embedded version! Thank you.)
- where you imagine these are spheres, the pressure difference between each one is small, and when you see one going "through" another it means a seal lets it through without equalizing the pressure. This is only 4 layers, but I don't see why a thousand spheres couldn't be put one inside the other, again the only constraint for me would appear to be the total thickness of each sphere. You are right that styrofoam is probably a bad idea -- way too thick. But, again if cost were not an issue, would this general idea work to set up a vacuum in a very large (say football-stadium sized) cavity without bearing the enormous atmospheric pressure on a single surface? Thanks for any further analysis! and if you can put my image inline somehow. 92.230.68.102 (talk) 15:19, 27 November 2009 (UTC)
- Now you talk about 100 (or even 1000) sets of pylons. I don't know if that's impossible, but certainly very complex, and there wont be much space for the vacuum, with, say, 10000 pylons in the way. Why not just build one thick containment vessel instead? If you are interested only in the physical principle, and not in technical and economic plausibility, just create a sufficiently large and thick steel sphere. A Los Angeles class submarine at depth withstands 20 times the pressure, and it already is 110 m long/10m wide. The Russian Typhoon class submarines are 175 times 23 m, and can go down 400m, for 40 times atmospheric pressure (but they have 2+1 separate pressure hulls). Neither is quite stadium-sized, but they are not too far off. --Stephan Schulz (talk) 16:17, 27 November 2009 (UTC)
- I second What Schulz said above. There is no reason not to build a single hull strong enough to hold the atmospheric pressure. Dauto (talk) 16:28, 27 November 2009 (UTC)
- Is there ANY limit on the size of the cavity inside that could be produced in this way (outside technically/economically)? Is there a size that the steel sphere would collapse on itself or something due to the limit on how much compression it could take?
- also: is there any difference then between one large sphere and the pylons solution above, in terms of the total amount of material you would end up using? Is it the same to have 10,000 or 100,000 matryoshka dolls getting the pressure down to zero by degrees, versus melting them all together and making one large one with the pressure going from atmosphere straight to zero? What is the mathematical/physics analysis of this equivalance? (if it guaranteed that the total structure would have as much mass, and it's not possible to save on weight by doing it this way). By the way thanks for your answers.
- and finally: what would the approximate thickness be for a large hollow steel sphere with a vacuum inside, with a cavity of a volume equivalent to the size of a stadium. How much would it weigh? Is the sphere the best shape for this? (as opposed to, say, a dodecahedron).92.230.68.102 (talk) 16:35, 27 November 2009 (UTC)
- The problem here isn't going to be material properties. We have very strong metals and we know how to engineer good trusses to support large semi-hollow spaces. The hard part will be pumping. It will take a lot of very powerful pumps to get this much air out of a big chamber. Even small (10-cm diameter cylinder) vacuum chambers suffer from a variety of interesting phenomena - first, of course, is leakage and backflow through the pump system; so you switch off the roughing pump and turn on the bigger roughing pump or the turbo pump (which you can't even operate until you're at pretty low pressure!). Next, leakage through the real, non-ideal seals, flanges, and gaskets; and then when you get down to ultra high vacuum, weird things begin to happen. Your pumps have set up a steady state, you're accounting for leakage; but the pressure doesn't go down any more! Weird! Where did all these gas atoms come from! Every time you pump them out, something still registers. By now, your pressure is so low that hydrogen atoms which used to be embedded into the metal lattice of your structure start to outgas. So you turn off the turbo and turn on the ion pump; and you get most of the hydrogen out. But now some really weird things happen. Helium and Neon start percolating through the two inches of solid, hermetically sealed steel. Because these noble gases are sort of small and they don't really interact with the electrons of transition-metal atoms, they just "squeeze" through the pore spaces. And, because they aren't ions, you can't get rid of them with the ion-pump! So, you turn down the cryo and liquefy everything that is gas; and you can get rid of most of the neon... but the Helium won't go away! Well, your cryo is made of helium, so you can't get it much colder than that; and something about the Second Law of Thermodynamics is kicking in. No worries - turn on the optical laser system and start plucking any of the remaining atoms out of the vacuum chamber - ... you say you want this for a foo tball-sized vacuum-chamber arena? The logistics problems will be in the pumping, not the material strength. Nimur (talk) 17:15, 27 November 2009 (UTC)
- Wow, thank you for the really interesting read! I really didn't mean to imply anything about needing anything near a perfect vacuum though... what percent of a vacuum can you reach with just the initial pumps? Will it just take a really long time, or do they need to be very special to pump out air even when the pressure differential is close to 1 atmosphere? (which doesn't seem THAT much, especially since you can pump out over as small an area as you want...) —Preceding unsigned comment added by 92.230.68.207 (talk) 19:30, 27 November 2009 (UTC)
- Different pumps have different operating regimes. A roughing pump can operate from atmospheric pressure down to pressures on the order of millibars. Some are even more powerful than that. Our article says 1x10-3 torr which is about 1 millionth of atmospheric pressure. (That's still a lot of atoms per cubic centimeter). The other types of pump I mentioned require a pretty good vacuum already (e.g. require a roughing pump behind them); these ultra high vacuum pumps can not operate from room-pressure and would be damaged if you tried. It's been a while since I played with vacuum systems, and I can't remember who made our roughing pumps, but you can buy simple pumps for under $1000 (here's a nice pump table from University of Alberta). Here's some product literature on top of the line rotary-vane roughing pumps. It takes a different amount of time to pump down, depending on a lot of factors - size of your chamber, size of your pump, material in the chamber, any residues or oils left over in the chamber, etc. If you are actually building a vacuum system, I would suggest finding an expert at your local university and getting some experience working with them before you try on your own. Be aware that there are a variety of unique hazards when dealing with pressurized (and unpressurized) gases. Watch out for moving parts and electrocution, make sure your system is pressure rated for what you want to do; don't mix oil-lubricated roughing pumps with ox-clean plumbing or experiments. Nimur (talk) 21:02, 27 November 2009 (UTC)
- I think the answer to the original question is that the idea would work just fine. However, after you have gone to the trouble of designing 100 thin, strong, concentric cubes or spheres, each of which can withstand a pressure difference of 0.01 atm without collapsing under the pressure or its own weight, you will find that you have just designed, the hard way, a 1 atm pressure hull. So, philosophically speaking, you will not have gained anything. Just as Schultz and Dauto said. --Heron (talk) 20:30, 27 November 2009 (UTC)
- Can you tell me in physics or math terms why 100 hulls with a pressure difference of 0.01 atmosphere are equivalent to one big hull that can stand 1 atm? I just don't understand what of necessity makes the two possibilities need to have the same sum mass or in any other way represent no preference for one over the other (except for the preference for 1 large hull because of the engineering challenge of making a hundred separate parts). I'm just not grasping the key thing that makes them equivalent. —Preceding unsigned comment added by 92.230.68.207 (talk) 21:07, 27 November 2009 (UTC)
- They would not be equivalent. Monolithic pieces of metal have different properties than individual pieces of metal, of the same size, that are "stuck together". This is a crucial bit of material science. You might want to read about metal lattice structure if you're into the gory details. Heron is making a simple approximation, suitable for a limited range of properties. I am highly suspicious of this approximation - especially since estimating pressure tolerances is very important for establishing safety margins. Even metal welded together is not the same as a single piece of metal. Even polycrystalline metal that is forged together is not the same as monocrystalline metal that is slowly cooled. There's no reason to assume that a metal sphere suitable for 1 atm gauge pressure would be equivalent to 100 concentric spheres each suitable for 1/100th atmosphere gauge pressure. I would expect the single hull to be made of much less material. The only real way to know is to consult an empirical table of material pressure strengths; e.g. Metal Chamber Pressure Standards from the AIAA. Don't mess around with approximations when it comes to pressure-tolerance. Consult a reliable reference. NASA, DTIC, and AIAA all publish similar standards handbooks. Nimur (talk) 21:15, 27 November 2009 (UTC)
- thanks, but this is theoretical. I actually don't have anyplace to put the football-stadium-sized structure at the moment, so it's just sitting out back waiting to be put together. 92.230.68.207 (talk) 21:34, 27 November 2009 (UTC)
- I might suggest Sandusky, Ohio. You might have some competition. Nimur (talk) 03:13, 28 November 2009 (UTC)
- thanks, but this is theoretical. I actually don't have anyplace to put the football-stadium-sized structure at the moment, so it's just sitting out back waiting to be put together. 92.230.68.207 (talk) 21:34, 27 November 2009 (UTC)
- They would not be equivalent. Monolithic pieces of metal have different properties than individual pieces of metal, of the same size, that are "stuck together". This is a crucial bit of material science. You might want to read about metal lattice structure if you're into the gory details. Heron is making a simple approximation, suitable for a limited range of properties. I am highly suspicious of this approximation - especially since estimating pressure tolerances is very important for establishing safety margins. Even metal welded together is not the same as a single piece of metal. Even polycrystalline metal that is forged together is not the same as monocrystalline metal that is slowly cooled. There's no reason to assume that a metal sphere suitable for 1 atm gauge pressure would be equivalent to 100 concentric spheres each suitable for 1/100th atmosphere gauge pressure. I would expect the single hull to be made of much less material. The only real way to know is to consult an empirical table of material pressure strengths; e.g. Metal Chamber Pressure Standards from the AIAA. Don't mess around with approximations when it comes to pressure-tolerance. Consult a reliable reference. NASA, DTIC, and AIAA all publish similar standards handbooks. Nimur (talk) 21:15, 27 November 2009 (UTC)
- Can you tell me in physics or math terms why 100 hulls with a pressure difference of 0.01 atmosphere are equivalent to one big hull that can stand 1 atm? I just don't understand what of necessity makes the two possibilities need to have the same sum mass or in any other way represent no preference for one over the other (except for the preference for 1 large hull because of the engineering challenge of making a hundred separate parts). I'm just not grasping the key thing that makes them equivalent. —Preceding unsigned comment added by 92.230.68.207 (talk) 21:07, 27 November 2009 (UTC)
- Wow, thank you for the really interesting read! I really didn't mean to imply anything about needing anything near a perfect vacuum though... what percent of a vacuum can you reach with just the initial pumps? Will it just take a really long time, or do they need to be very special to pump out air even when the pressure differential is close to 1 atmosphere? (which doesn't seem THAT much, especially since you can pump out over as small an area as you want...) —Preceding unsigned comment added by 92.230.68.207 (talk) 19:30, 27 November 2009 (UTC)
can you make it out of anything? (non-porous)
here is another question: could you make a structure to house a football-stadium sized vacuum cavity out of ANY material (that was non-porous), including different types of steel, plastic, fiberglass, even treated wood? (so as not to be porous), with the only difference being how thick you would need to make it? If you can't make it out of ANYTHING (even plastic) then what must the material's properties be, and which materials would and wouldn't qualify? Would some kind non-porous styrofoam be possible, so long as you had ENOUGH of it?
- I mean this question purely on a theoretical-level; maybe you would need a half-mile-thick sphere to get a cubic-meter vacuum ... if the sphere is made of chocolate. Is it possible? Can you make it out of chocolate? (so long as you ahve enough, again only theoretically). If not, why not?92.230.68.207 (talk) 21:37, 27 November 2009 (UTC)
- No, you can't make it out of chocolate. Sturdiness notwithstanding, chocolate walls would erode and oily residues would gassify. In fact you should not put chocolate in a vacuum system, let alone build the walls from it. I don't even have any idea how you might hermetically seal chocolate; it might actually be pretty decent if it is thick enough. Again, "sealing" from outside to inside is usesless if the material is going to erode and gassify into the vacuum chamber. Nimur (talk) 03:10, 28 November 2009 (UTC)
- A good thing to remember whenever you are thinking about the structural requirements of containing a vacuum is that the pressure difference between sea level atmosphere and a perfect vacuum is approximately the same as the pressure difference between sea level atmosphere and 10 metres underwater. If we ignore the weird outgassing and erosion that Nimur talks about (which you can't for chocolate, but probably can for metal or plastic) then anything which can be sealed and taken 10m underwater without being crushed can also have all the air sucked out of it while at sea level - it is exactly the same problem (in terms of material strength - you may need different types of seals and things to keep water out vs keeping air out). --Tango (talk) 10:15, 28 November 2009 (UTC)
- I'm not sure but I don't think so. The main problem, is to stop the weight of the material itself crushing the material. The way I'd go about it is to use a number of domes within each other. Each would be supported by lots of thin struts forming triangles and the outside of each dome covered with a thin sheet of material. The question then boils down I believe to whether one can make a dome framework of plastic or even steel of any size say 20 miles round. I'm not sure what the precise problem would be though. Dmcq (talk) 15:22, 28 November 2009 (UTC)
- As long as you are allowed to include internal supports or some kind, it shouldn't be too difficult. If you aren't allowed internal supports then the problem is very similar to building a single-span bridge. --Tango (talk) 15:43, 28 November 2009 (UTC)
- I totally don't understand - you guys seem to be in disagreement, as you're talking about a series of domes but above someone else said there is NO advantage to have a series of domes one inside the other versus having one large dome with a thickness equal to the individual domes combined - in fact, they said the monolithic (single-domed) structure would probably end up THINNER! So who's right? If there is no advantage to a series of concentric domes, why are we even talking about it anymore? 85.181.144.200 (talk) 15:52, 28 November 2009 (UTC)
- I'm not talking about concentric domes, just some kind of pillars or girders to hold up the structure. --Tango (talk) 15:56, 28 November 2009 (UTC)
- The part about concentric domes vs. single dome was strictly with regard to pressure tolerance. I mentioned earlier about the need for trusses or internal support structure if the dome got sufficiently large - as Tango said, this would be kind of like girders for a bridge. Nimur (talk) 16:19, 28 November 2009 (UTC)
- I'm not talking about concentric domes, just some kind of pillars or girders to hold up the structure. --Tango (talk) 15:56, 28 November 2009 (UTC)
- I'm not sure but I don't think so. The main problem, is to stop the weight of the material itself crushing the material. The way I'd go about it is to use a number of domes within each other. Each would be supported by lots of thin struts forming triangles and the outside of each dome covered with a thin sheet of material. The question then boils down I believe to whether one can make a dome framework of plastic or even steel of any size say 20 miles round. I'm not sure what the precise problem would be though. Dmcq (talk) 15:22, 28 November 2009 (UTC)
Moon Landing
Who was the youngest person, at time of landing, to set foot on the moon and who (currently) is the youngest person to have ever done so? What about oldest for both questions? TheFutureAwaits (talk) 13:20, 27 November 2009 (UTC)
- List of Apollo astronauts#Apollo astronauts who walked on the Moon has everything you want. Charles Duke was the youngest when he stepped, and Alan Shepard the oldest. Both would have retained their titles, but since Shepard died, Buzz Aldrin is the oldest moonwalker. Shepard was the only one not born in the 30s. ~ Amory (u • t • c) 14:14, 27 November 2009 (UTC)
- Cool thanks! TheFutureAwaits (talk) 16:19, 27 November 2009 (UTC)
quantum mechanics
I want to research on theoretical chemistry specially on quantum part in future ? how do I start and go for it?Supriyochowdhury (talk) 15:18, 27 November 2009 (UTC)
- The answer to that question depends on how much you already know about the subject. Why don't you start by telling us wheather you have a degree in chemistry or related subject? Dauto (talk) 16:34, 27 November 2009 (UTC)
- If you want to work on quantum mechanics, I recommend boosting your math skills very significantly. Take several university-level courses on calculus. Follow this with several university-level courses in differential equations. Also take several courses in linear algebra. When you take your quantum class, be sure to take at least one quantum course from a physics department and one quantum course from a chemistry department. A standard chemistry curriculum is sometimes lax in the mathematical requirements, because most chemists do not need the gorey details of quantum mechanics, but if you intend to make it your research focus, you will need to get into the math-heavy parts. Nimur (talk) 17:19, 27 November 2009 (UTC)
- To what Nimur said I would add at least one course in complex variables and analysis. Note that if you really want to do research, you will most likely be required to take some graduate level classes as well. Dauto (talk) 19:19, 27 November 2009 (UTC)
- If you want to work on quantum mechanics, I recommend boosting your math skills very significantly. Take several university-level courses on calculus. Follow this with several university-level courses in differential equations. Also take several courses in linear algebra. When you take your quantum class, be sure to take at least one quantum course from a physics department and one quantum course from a chemistry department. A standard chemistry curriculum is sometimes lax in the mathematical requirements, because most chemists do not need the gorey details of quantum mechanics, but if you intend to make it your research focus, you will need to get into the math-heavy parts. Nimur (talk) 17:19, 27 November 2009 (UTC)
statistical testing for a feeling of being watched
all right, I've had it enough with this pseudoscience. I want to test whether I can feel being watched. I propose the following methodology. I will print a lot of random "1. watch" and "2. watch", "3. don't watch." etc and then, without looking at the person, have someone do the required action (either look at me or don't) while I call the appropriate number. I will then write down whether, after I called out 1, I felt watched (or didn't feel watched), and repeat on and on. at the end I will compare my results with the instructions, statistically. My problem is: how should I do this? How many numbers should I even have? The real problem isn't that the effect might be a weak one -- my problem is that there is a little thing called prior probability. And the prior probability that I can feel where someone's eyeballs are directed without any further sensory input is precisely 0. But if the prior probability is 0, then even if I can feel 100/100 of the gazes (ie 100% match the random instructions) it will still fail to show that there is a feeling of being watched, due to the prior probability of this which is zero. What do you all suggest? How many trials should I do, and what percent should I set the threshold at? I am at a loss, as prior probability to me implies that it is literally impossible to test for something like this, because of its probability 0 of being true. Thanks for any advice you might have. —Preceding unsigned comment added by 92.230.68.102 (talk) 16:09, 27 November 2009 (UTC)
- Have you contacted Rupert Sheldrake, who has published research on this subject? --TammyMoet (talk) 16:46, 27 November 2009 (UTC)
- I believe there's a story of Feynman being rejected for the army because they asked him if he thought he was being watched and he said yes. He had a look around and half of the people waiting for the test were actually looking at him. :) Dmcq (talk) 17:17, 27 November 2009 (UTC)
- I've read that anecdote in his biography - and I think you're explaining the story in a misleading manner. He didn't say that because he 'felt' that he was being watched. He simply reasoned that with nothing else interesting going on during the long, boring wait in line to see the army recruiter - that a lot of people would indeed be watching him. So he replied logically. That being the case, Feynman's story has zero relevance to this question. SteveBaker (talk) 19:18, 28 November 2009 (UTC)
- If you say the prior probability is zero, there is no reason to do the experiment. But you don't really think it is zero, you just think it's very small, say one in a trillion. Statistically, the exact number you assume doesn't have a huge effect on the number of trials needed. (To put it more precisely, the number of trials needed increases in proportion to the logarithm of the prior probability.) What matters a lot more is the effect size. To resolve a 10% difference between watched and unwatched, you need a couple of hundred trials, and the number of trials needed increases in proportion to the inverse square of the effect size—the article on Pearson's chi-square test will give you more information. It is also critical, if you really want to do an experiment like this, to make sure that the person being watched has no information whatsoever about what the watcher is doing. Looie496 (talk) 19:47, 27 November 2009 (UTC)
- I believe (without proof) that this feeling comes about from subliminal cues we're picking up from the environment. If someone tiptoes up behind you - they'll still subtly block ambient light - and reflect light - and absorb or reflect ambient sounds and emit smells in ways that are perhaps so subtle as to be below our conscious ability to detect - but plenty enough for our subconscious mind to pick up on. Since no definite information is available - all your consciousness gets is a vague feeling of unease. This seems like an entirely reasonable thing - after all, our ancient ancestors would have had to be very attuned to predators sneaking up behind them - and any teeny-tiny cue that we could pick up would have evolutionary benefits - so it's quite possible that we've evolved this ability. So any experiment you did would have to very carefully test for this effect because it's presence would prove something - and it's absence would be hard to set up controls for because we don't know how subtle that effect is. SteveBaker (talk) 19:15, 28 November 2009 (UTC)
- so basically, you want me to change my test from "can I feel being watched" to "is my wife any good at sneaking up behind me"? :) 92.230.69.34 (talk) 20:49, 28 November 2009 (UTC)
- No - I just maintain that on those occasions when we think we're successful at intuitively feeling we're being watched, we're actually picking up on very subtle cues with perfectly normal senses. An overly tightly controlled experiment (eg where a "watcher" randomly either closes their eyes or does not) might come up with a negative result - when in reality there is something super-subtle going on that is worthy of note. It's hard to comment on the quality of your experiment because it's not clear what hypothesis it's trying to demonstrate. I think you need a much clearer statement of your hypothesis before you attempt to devise an experiment to prove or disprove it. SteveBaker (talk) 01:06, 29 November 2009 (UTC)
- so basically, you want me to change my test from "can I feel being watched" to "is my wife any good at sneaking up behind me"? :) 92.230.69.34 (talk) 20:49, 28 November 2009 (UTC)
hydride donors and nitrogen
So if you use sodium borohydride (or forbid, LiAlH4) on say, an imine in aprotic solvent, you really get an anionic amide species until you expose the reagent to protons, right? Or how else does the amine get protonated (to neutral) form? I guess I'm surprised at the reactivity because RNH- is a considerably worse anion (conjugate acid pKa 35) than RO- in the hydride donation to carbonyls.
Also, does the electron-donating effect of nitrogen in some nitrogeneous aromatic rings make the rings more susceptible to non-catalytic hydride donors (LiAlH4?) -- cuz imidazole only has a resonance stabilisation of 14 kcal/mol, right? John Riemann Soong (talk) 16:31, 27 November 2009 (UTC)
- After reactions are done, during the "work-up" phase, water or other proton sources are added to neutralize anionic positions, destroy and/or remove unreacted reagents, etc. I can't make any sense of thse cond part of the first paragraph...the relative reactivity of something in two separate reactions does not affect whether those reactions works, especially since there are other components involved as well. As long as there is enough instability in "all the reactants in a certain reaction", the reaction works.
- To get good reaction with a hydride donor, you need an electron acceptor, not just a "not extra-stabilized π system". DMacks (talk) 17:32, 28 November 2009 (UTC)
- There's a significant dipole moment in imidazole... so I think that would set up a sort of Michael-ish addition right there. Basically I'm wondering since the RNH- anion is less stable than RO-, whether this causes problems for some hydride donation schemes... John Riemann Soong (talk) 03:38, 29 November 2009 (UTC)
- Hydride attack is almost always irreversible. But the whole reason one chooses a certain donor is to guarantee that situation (which is why you learn about differences of reactivity, etc.) for a reaction being studied. Some hydride donors are not strong enough to attack some carbonyl-like compounds. More reactive compounds are more easily attacked and a weaker donor is "good enough". Remember you have to consider "all reactants → all products" not just "stability of one product". There are definitely reactions involving nucleophilic attack on aromatic rings, but it's hard and not usually the ultimate product because loss of aromaticity is such a large energy cost. The product often re-aromatizes by tetrahedral collapse/ejection of a leaving group. This is one of the few situations where hydride actually can be a viable leaving group. DMacks (talk) 11:52, 29 November 2009 (UTC)
- There's a significant dipole moment in imidazole... so I think that would set up a sort of Michael-ish addition right there. Basically I'm wondering since the RNH- anion is less stable than RO-, whether this causes problems for some hydride donation schemes... John Riemann Soong (talk) 03:38, 29 November 2009 (UTC)
Seagull ID needed
Just watched this video:
http://www.youtube.com/watch?v=LjgmXxxM6zM
What sort of gull is that? Never seen one like it before. --84.64.96.34 (talk) 22:36, 27 November 2009 (UTC)
- Have you asked this guy - he's our resident expert in these matters. hydnjo (talk) 06:26, 28 November 2009 (UTC)
- Nice story, I'm intrigued with the presence of the tennis ball, did it go fetch it when you threw it ;-)). Come on Karl, we're all waiting. Richard Avery (talk) 08:27, 28 November 2009 (UTC)
- I have read that ducklings will adopt the first creature they see as "mother" and follow him/her/it around. I wonder if that would have happened if the bird had been taken in as a hatchling and/or hand fed. Cuddlyable3 (talk) 09:16, 28 November 2009 (UTC)
- I think that this may be an immature Northern Fulmar, actually a petrel, rather than a gull. Mikenorton (talk) 12:12, 28 November 2009 (UTC)
- Looking a bit further, it could also be a dark morph adult. Mikenorton (talk) 12:36, 28 November 2009 (UTC)
November 28
Time dilation and multiplayer games
Would it be even theoretically possible for a multiplayer game to accurately model time dilation for one player relative to another? 24.235.164.10 (talk) 06:10, 28 November 2009 (UTC)
78.139.5.39 (talk) 07:36, 28 November 2009 (UTC)On the page "Speed of Light" I am reading the following sentence: "In models of the expanding universe, the farther things are from Earth, the faster they move away from us". It seems to mean the we are, after all, living in a geocentric universe, right? And it seems to me that this kind of sentence appears everywhere (not only on this particular page), when we talk about the expansion of the universe. How about directions?
- For a real time game I think the answer is no in the general case. For example if the players are supposed to be able to interact in real time and then one of them goes away quickly and comes back (as in the twin paradox), their times will be out of whack with each other and they won't be able to interact in real time with each other properly. One work around would be for the game to slow down when you start moving quickly relative to some reference frame, but then you really lose the magic of relativity. For a turn based game you could for example give out extra turns or bonus movement or whatever.
- It's pretty unfortunate. I would love to see a multiplayer space shooter or something like that which models special relativity. Rckrone (talk) 08:00, 28 November 2009 (UTC)
- No, that statement does not imply that we live in a geocentric universe. No matter where you are in the universe, the farther things are from you, the faster they move away from you (on cosmological scales). Picture a bunch of dots drawn on a balloon that's getting steadily inflated. Pick one of the dots. The other dots are moving away from the chosen dot, and the farther a dot is from the chosen dot, the faster it moves away from the chosen dot. But the same thing is true no matter which dot you choose to take the perspective of. Red Act (talk) 09:11, 28 November 2009 (UTC)
- I wonder if this question would be better addressed on the computer desk. I should first clarify my understanding of special relativity and time dilation is limited, in fact I wasn't sure whether to post at all but decided to post based on above posts. While not time dilation, some FPS games have allowed one (or multiple) players to have some sort of powerup (skill/weapon) which works in multiplayer, that is able to 'slow down' time or allows the player to go 'faster' then 'normal' time. This allows them to travel at faster then normal speed (I think) while making other players slower then normal (I think), so the players using the powerup appear to be going very fast relative to the other players. I'm not sure of the precise difference, it obviously isn't 10x, I think it's closer to 2x. For example, F.E.A.R.#Multiplayer [21] (technically in the game story the player is going 'faster' then 'normal' time) & TimeShift#Gameplay [22] (if I understand it correctly, technically in the game story time is slowed down and the player is going at normal speed) & also I think some Source (engine) mods. I presume a similar thing could be used for some aspects of time dilation, e.g. one player at relative rest and the other moving close to the speed of light. Nil Einne (talk) 09:49, 28 November 2009 (UTC)
- Don't forget time dilation is symmetric - every player would have to see time passing more slowly for every other player that was moving at a constant velocity relative to them. Then you would need general relativistic corrections for players and objects that were accelerating relatve to one another. Very difficult to achieve in a real-time MPG, I would think. Gandalf61 (talk) 10:33, 28 November 2009 (UTC)
I admit, this is one thing that confused me after reading the article and hence my reluctance to post. Perhaps I should make a seperate topic but it seems this is relevant to understanding the question and answers... Let's say the typical space flight example, someone takes a ship from earth at close to the speed of light and goes somewhere and later come back. For them it's been 50 years, but a billion years has passed on earth and as it turns out it survived but cats are now the dominant species. I can understand how to the point of the people and cats on earth, it seems that time is passing very slowly on the space ship. I'm confused how it seems to the person on the space ship that time is passing slowly on earth since it seems to me if they are observing all their loves ones dying in a minute and then cats taking over in a few days, time is passing rather fast on earth? (I understand how the observation would get complicated since it will take longer and longer for the information to reach them as they are going away and shorter and shorter as they are coming back but although I haven't completedly thought that part true as with a number of areas of special and general relativity it confuses me no end and I can't see any way it would 'work'.)Nevermind found this is actually dealt with in Twin paradox linked above (sorry neglected to read it earlier) Nil Einne (talk) 12:08, 28 November 2009 (UTC)- Yes, that is, indeed, the Twin paradox. A lot of people talk about the Twin paradox and forget to mention in what way it is apparently paradoxical. Twins ending up different ages is not a paradox, it is just weird. It really annoys me when people claim that that is the Twins Paradox - it isn't, it's just time dilation. Twins both thinking the other twin is older than them is a paradox (which is resolved, of course, by one of the twins not being in an inertial frame the whole time). </rant> --Tango (talk) 12:20, 28 November 2009 (UTC)
- This is a tangent, but there's a good depiction of time dilation effects in The Forever War. Fences&Windows 17:26, 28 November 2009 (UTC)
- There is a simulator of relativistic effects: http://realtimerelativity.org/. It's single player though. Fences&Windows 18:35, 28 November 2009 (UTC)
- Well - yes and no. Obviously if two players of the game are identical twins in the real world - and also in the game - then one of them climbs into a spaceship in-game - and shoots off at a large fraction of the speed of light - then returns home, in the game world, we can loudly assert that the two twins now have different ages - even though they don't in the real world. We could handle that merely by drawing their avatars looking appropriately ancient and youthful. But the problem is that the experience of that happening in the real world would not be reasonable. The twin who is older in-game would have spend his/her time being involved in many more adventures, racking up more 'stuff' (gold, weapons, abilities, etc) than the more youthful twin. But the only way to handle that in-game would be to somehow prevent the younger twin from playing so quickly - or somehow cram more experiences into the in-game experience of the older one. If they each encounter one deep-space battle per day of real-world time, then they'd both end up with the same amount of experience in-game - and that would be wrong. Speeding up the rate at which battles happen for the older twin might make the game unplayable - reducing the number of battles the younger twin would encounter would make the game terminally boring. So it's possible - but it would make for a crappy game, so it's very unlikely anyone will do that in the fully 'general' case. We could of course do it easily if all of the players are travelling around in the same giant spaceship at the same speed. I suppose we could also do it if the maximum speed of spacecraft was limited sufficiently to make the time distortion not too destructive to gameplay. Of course if we did that - and somehow made it work - then the game experience would be more intense for the people who stay home and don't fly off at high fractions of the speed of light - and more boring for those who do. That's not the kind of game mechanic that's going to be hugely attractive to developers because it would tend to be a dis-incentive for high speed travel - which would be the one thing that would make the game seem interesting! SteveBaker (talk) 18:58, 28 November 2009 (UTC)
- The problem with the slower end of relativistic travel is that is it completely useless. It's too slow to be useful for interstellar travel and unnecessarily fast for travel within a solar system (Earth to Saturn in 2 hours rather than 20 just isn't worth all the difficulties). You can add in incentives to travel fairly easily, though - quests that require travel, interstellar trade, different difficulty levels in different places, etc.. --Tango (talk) 19:08, 28 November 2009 (UTC)
- You can't correctly model the twin effect, but you can model redshift and blueshift without the twin effect. It could look a lot like a relativistic world, with redshifted clocks appearing to run slower and blueshifted clocks appearing to run faster (along with everything else on board the ships, including actions by the players), but when two ships separated and met up again, the occupants would always have had the same amount of play time in between. This would require a lot of buffering of old game state, but it is possible. -- BenRG (talk) 08:02, 29 November 2009 (UTC)
- If this could be done, the person who would have done it is Greg Egan. He is a well known Science Fiction writer but also a competent programmer by trade. He takes great pride in ensuring that everything in his novels are scientifically absolutely accurate and doable (or at least, do not contradict any known scientific principle). In one book, there is a mention of a game of quantum football where the players move the ball, not by kicking it, but by adjusting the quantum wave function around the ball by running around the pitch. This game was incidental, not even central to the plot, so I was quite stunned when I found Egans website years later and right there was a Java applet playable version of the game online following all the rules of real quantum mechanics. If anyone has a good strategy for this game by the way, please let me know, I am completely baffled (I have scored goals, but only by complete random flukes). Sadly, although there are many articles on time dilation on his website, there is no multiplayer game. SpinningSpark 12:11, 29 November 2009 (UTC)
Betelgeuse
Did anyone glance Betelgeuse last night from the UK? It seemed unusually bright and particularly red, especially compared to the other stars. I know it's a red supergiant, but the colouration seemed particularly noticeable last night. For what it's worth, I was observing at about 2215 from Oxford. Brammers (talk) 09:56, 28 November 2009 (UTC)
- It is a variable star, so it is possible it was genuinely brighter than usual (which would make the colour more obvious due to the way the human eye works). It is also possible it was some kind of optical illusion. I'm not sure where to look to find details of its brightness at given times, so I can't determine which possibility is correct. --Tango (talk) 10:23, 28 November 2009 (UTC)
- Well Betelgeuse is most likely a supernova already, and it's light might reach us pretty soon. Pretty soon means anywhere within a few centuries, so there are chances that we might see it ourselfs. --131.188.3.21 (talk) 14:22, 28 November 2009 (UTC)
- a) To avoid unnecessarily convoluted language we usually use the present tense to refer to events for which the light is currently reaching us, b) I think "most likely" is overstating the probability. It is certainly possible, but far from certain. Our article says it "may" go supernova (by which it means the light from the supernova will reach us) in the next 1000 years and it is 640 light years away, so that is a long way short of it being "most likely" to have already happened. --Tango (talk) 15:18, 28 November 2009 (UTC)
- Pardon me for not being clear enough. Assuming a symmetrical probability distribution for the event happening here (I mean, light reaching us) in the next 1000 years places the probability of it already been happened there above 0.5 in case of it being farther than 500 light years away. Of course, this might be just a wild speculation. Betelgeuse shrinking 15% in the last few decades and accelerating, however, shifts (at least I think) the mean of the probability distribution to a closer time point. —Preceding unsigned comment added by 131.188.3.20 (talk) 16:16, 28 November 2009 (UTC)
- "May happen" is very different to "will happen". --Tango (talk) 17:42, 28 November 2009 (UTC)
- Ok, then mentally replace "most likely" with "above 50% chance" and "will happen" with ... erm.. where did I wrote this? --131.188.3.20 (talk) 17:49, 28 November 2009 (UTC)
- I think Tango means that since the SN only may happen during the next 1000 years, we can't say that the probability of it happening within 640 is > 0.5. Assuming a uniform distribution: P(t<640) = 0.64P(t<1000) < 0.64, but we have no lower bound on P(t<640) since all we know is that P(t<1000) is greater than 0. Olaf Davis (talk) 21:01, 28 November 2009 (UTC)
- Why are we whispering? LOL --Tristan. Crap, I've got to work on my paper! —Preceding unsigned comment added by 76.235.111.185 (talk) 23:20, 28 November 2009 (UTC)
- I think Tango means that since the SN only may happen during the next 1000 years, we can't say that the probability of it happening within 640 is > 0.5. Assuming a uniform distribution: P(t<640) = 0.64P(t<1000) < 0.64, but we have no lower bound on P(t<640) since all we know is that P(t<1000) is greater than 0. Olaf Davis (talk) 21:01, 28 November 2009 (UTC)
- Ok, then mentally replace "most likely" with "above 50% chance" and "will happen" with ... erm.. where did I wrote this? --131.188.3.20 (talk) 17:49, 28 November 2009 (UTC)
- "May happen" is very different to "will happen". --Tango (talk) 17:42, 28 November 2009 (UTC)
- Pardon me for not being clear enough. Assuming a symmetrical probability distribution for the event happening here (I mean, light reaching us) in the next 1000 years places the probability of it already been happened there above 0.5 in case of it being farther than 500 light years away. Of course, this might be just a wild speculation. Betelgeuse shrinking 15% in the last few decades and accelerating, however, shifts (at least I think) the mean of the probability distribution to a closer time point. —Preceding unsigned comment added by 131.188.3.20 (talk) 16:16, 28 November 2009 (UTC)
- a) To avoid unnecessarily convoluted language we usually use the present tense to refer to events for which the light is currently reaching us, b) I think "most likely" is overstating the probability. It is certainly possible, but far from certain. Our article says it "may" go supernova (by which it means the light from the supernova will reach us) in the next 1000 years and it is 640 light years away, so that is a long way short of it being "most likely" to have already happened. --Tango (talk) 15:18, 28 November 2009 (UTC)
- Well Betelgeuse is most likely a supernova already, and it's light might reach us pretty soon. Pretty soon means anywhere within a few centuries, so there are chances that we might see it ourselfs. --131.188.3.21 (talk) 14:22, 28 November 2009 (UTC)
Military Equipment
Greetings! How do you call this piece of equipment carried by the soldier in front? Thanx! Grey Geezer Grey Geezer 10:02, 28 November 2009 (UTC) —Preceding unsigned comment added by Grey Geezer (talk • contribs)
- It's an M224 mortar without the support or base - BTW not sure this shouldn't have been on the miscellaneous desk. Mikenorton (talk) 11:28, 28 November 2009 (UTC)
- Thank you for the answer! (I followed "engineering and technology"). 62.241.104.212 (talk) 01:04, 29 November 2009 (UTC)
Isomorph only in Developmental Biology?
I've posted this as a query on the Talk page for Isomorph. I'd appreciate a confirmation and/or explanation of whether this word can serve as a noun for non-living structures whose substance is unchanged when its shape is altered. Context: a descriptor of the Tower of Hanoi puzzle. -- Deborahjay (talk) 14:23, 28 November 2009 (UTC)
- Whether or not "isomorph" is a valid word in English in different fields is a question better put to the language desk. Wikipedia articles should not be, or have added to them, dictionary definitions. Wiktionary is the project that likes that stuff. Isomorphism has a meaning in mathematics and a number of other fields, see Isomorphism (disambiguation). Alternative meanings of the term "isomorph" should be explained in the appropriate article, if there is one, and the isomorph article left as isomorph (biology). For the Tower of Hanoi example, I would think that the relevant article would be group isomorphism. SpinningSpark 17:10, 28 November 2009 (UTC)
Are there people with two anuses?
I just watched this porno and the girl in it appeared to have two anuses. Upon Googling, there does seem to be something called 'duplication of the anus' and it's and established medical phenomenon. Why then is there no information about this in Wikipedia?--Damriteido (talk) 14:49, 28 November 2009 (UTC)
- You've answered your own question (per the heading) by searching on Google and finding it an established medical phenomenon. As Wikipedia content is written by people with reliable information and citable sources, go right ahead and add it to the Human anus article, possibly right after "birth defects." If you're not sure how to edit directly, start by adding this information (as you did here) with your sources in a New Section on the Talk:Human anus page and follow the responses. -- Deborahjay (talk) 15:05, 28 November 2009 (UTC)
- We get these kinds of questions fairly often - and in every case (so far) we've established that what you see is just a movie-special-effect - generally latex and makeup. So, no - do not add it to any Wikipedia articles - Porn movies do not constitute a reliable source! SteveBaker (talk) 18:40, 28 November 2009 (UTC)
- Well the porn movie is obviously bullshit but the medical condition does really exist [23] [24] [25] [26] [27] et al (had planned to offer Bing result but it wasn't particularly useful). I'm not convinced this belongs in the human anus article though. There are many very rare medical conditions which likely don't belong on such central articles (perhaps they belong on some other article). And yes, per the sources "congenital double anus are very rare" & "Anal canal duplication (ACD) is the most distal and the least frequent digestive duplication" it is very rare. That's probably also one of the reasons no one has written about it yet. Incidentally, don't expect it to be anything like the porn movie. Nil Einne (talk) 20:56, 28 November 2009 (UTC)
- Some learners of Italian (or Spanish) seem to report such things, though. Italian makes a clear distinction between single and double consonants. If you want to say you're twenty-seven years old, say clearly ho ventisette anni and definitely not ho ventisette ani. It's important.
- When I studied in Italy, there was an intensive language course for a couple of months prior to the school year, and one day we had an exercise where one student role-played someone ordering in a restaurant. She ordered pene con pomodoro. --Trovatore (talk) 18:53, 28 November 2009 (UTC)
How exactly would FTL violate causality?
I think I know quite well most of realtivity, but there is still one part which I can't figure out completely. Let us try a Reductio ad absurdum method: Supposed we had a small spaceship capable of faster than light travel, how would it be possible to send matter, or at least information to the past? What would be an example of an exact scenario which would lead to this? --131.188.3.21 (talk) 15:29, 28 November 2009 (UTC)
- See Tachyonic antitelephone#Sending signals into one's own past. Red Act (talk) 16:29, 28 November 2009 (UTC)
- Even more thorough than our article is the source our article cites: The Tachyonic Antitelephone, published in Physical Review (1970) by the American Physical Society (a decidedly reliable source on such matters). The conclusion is that experimental study of faster-than-light phenomena will yield negative results or result in contradiction. Nimur (talk) 22:09, 28 November 2009 (UTC)

- See also the diagram here, which shows it quite vividly. "Therefore an object moving faster than light, say from O to A in the adjoining diagram, would imply that, for any observer watching the object moving from O to A, there can be found another observer (moving at less than the speed of light with respect to the first) for whom the object moves from A to O. The question of which observer is right has no unique answer, and therefore makes no physical sense. Any such moving object or signal would violate the principle of causality." --Mr.98 (talk) 00:07, 29 November 2009 (UTC)
- Implicit in these kinds of explanations is the idea that the thing that happens at the earlier coordinate time is the cause. I don't understand why anyone would assume that; there's no physical justification for it. I'd assume that the thing that happened at the earlier cosmological time would be the cause, since the low entropy of the big bang is supposed to be the reason for the second law of thermodynamics, which defines the arrow of time. Using cosmological time avoids the logical incoherence of using coordinate time. But it has its own problem, namely that there are spacelike geodesics that reverse direction in cosmological time. The Phys. Rev. D paper seems like a waste of space to me. It spends pages on an obvious contradiction that should have been a one-sentence footnote, and it never talks about the nature of causality, which is the essence of this question and is still very poorly understood 40 years later. -- BenRG (talk) 07:45, 29 November 2009 (UTC)
Optimum positioning of bookshelf brackets?
I'm putting up some bookshelves. These consist of brackets which support chipboard or contiboard 'planks'. The brackets can be positioned anywhere. In my experience the heavily loaded chipboard shelves tend to sag over time. Where would be the optimum place to position the two brackets to minimize the amount of sagging? If, for example, the brackets were placed right at the ends of the shelves, then it would sag in the middle. Moving the brackets in a quarter of the shelves length, so that there was half the length of the shelf between them, would be better: the ends of the shelves would be cantilevered, and nowhere on the shelf would be more than a quarter of its length from the brackets. But the two quarters of the shelf between the brackets would get some support in the middle from each other - so the brackets could be moved further apart. Has anyone ever calculated the optimum place to position two brackets under a bookshelf? 92.27.169.45 (talk) 17:28, 28 November 2009 (UTC)
- That would depend on the material (of the plank), one would have thought. Whether they've worked it out for some things, I don't know. - Jarry1250 [Humorous? Discuss.] 17:34, 28 November 2009 (UTC)
I do not think the material the plank was made of would affect the optimum position, although it would affect the amount of sag seen. That suggests that a spline of cardboard could be used as a test material. 92.27.169.45 (talk) 17:45, 28 November 2009 (UTC)
- Are you assuming an equal weight of books on each part of the shelf? --Phil Holmes (talk) 17:38, 28 November 2009 (UTC)
Yes. 92.27.169.45 (talk) 17:42, 28 November 2009 (UTC)
- With equal weight all along the shelf I think it would bend according to the cube of the length from a support, square for the length and another bit for the weight of the length. Thinking of the bit in the middle as being rather like four ends stuck together my guess would be you want the middle bit to be 2 times the cube root of 2 long compared to 1 for each end. So about 2:5:2 for the lengths. You really need somebody who has done this sort of thing rather somebody who sticks their finger in the air, an engineering student for instance would I'm sure be able to do it no problem. Dmcq (talk) 18:09, 28 November 2009 (UTC)
- If I was just sticking up a bookshelf I believe I would have put the brackets further apart than what I've calculated there. So it'll be interesting to see if my unthinking part is being cleverer or not :) Dmcq (talk) 18:21, 28 November 2009 (UTC)
- There are three different failure modes to consider:
- A bracket pulling off of the wall or snapping or something because the load is too great.
- The plank snapping because the load is too great.
- An unbalanced weight on one extreme end of the plank causing the opposite end of the plank to flip upwards.
- The last one is a bit tricky - if the plank is just resting on the brackets, that's a different situation than if the plank is screwed to the brackets.
- For the first failure mode - it actually doesn't matter how the brackets are situated - so long as everything is symmetrical. Each bracket supports half of the weight and it doesn't matter whether they are closer to the center or out at the ends because symmetry guarantees that they are each bearing the same weight.
- For the second failure mode. If you positioned the brackets right out at the ends - or both at the center(!) then the center of the plank is what will break. But if you place the brackets at the 25% and 75% positions (as you suggest) then the force on the center of the plank is zero - a perfectly evenly overloaded plank would theoretically break in two places - right over the brackets. That's better because you've halved the amount of leverage and effectively doubled the strength of the thing. It seems to me that moving the brackets either closer or further from that position increases the leverage.
- For the third case - there is no problem when the shelf is perfectly evenly loaded - but if it's asymmetrically loaded then there are two cases to consider:
- If the shelf is just layed onto the brackets without being fixed down then if (say) the brackets are at the 25%/75% position and you put a heavy book on one end of the plank - then the opposite end might flip up. The fix for that is to put the brackets as far apart as possible.
- If the shelf is fixed down onto the brackets then when there is a heavy weight on one end of the plank - the bracket at the opposite end is helping to hold the plank down and stopping it from flipping. But still, the best case is when the brackets are at the extreme ends.
- So there is a trade between the risk of an asymmetric flip (best minimised by putting the supports at the ends) and the risk of a plank snap (best minimised when the brackets are closer to the center...maybe 25%/75%). This is a contradictory requirement - so we know that the optimum position depends at least in part on the firmness with which the plank is attached to the brackets and the weight of the plank itself - contrasted with the strength of the material from which the plank is made. It follows that we can't answer the question of optimum positioning until we know something about the strength-to-weight ratio of the plank. A light-but-strong plank is unlikely to break but more prone to flipping under asymmetric loads - so the brackets need to be further apart. A heavy-but-weak plank would be more prone to breaking than flipping - so the brackets need to be closer together.
- Hence we can't come up with a definitive answer without resorting to some ugly questions of material science!
- SteveBaker (talk) 18:24, 28 November 2009 (UTC)
- You have to be a bit careful with the first case — the position of the brackets can matter because the shelf (as analyzed below) can deform when loaded. (And there are static and dynamic loads; consider the effect of dropping a CRC Handbook or the like from even a short distance up.) In the simplest case, deflection of the beam applies a torque to the mounting brackets. There can be additional, interesting forces if the shelf is fairly deep and the front-to-back loads are uneven. TenOfAllTrades(talk) 02:11, 29 November 2009 (UTC)
Thanks, but the risk of failure does not concern me here - the problem is whetre to position the two brackets to get the least deformation in the plank/beam. 92.27.169.45 (talk) 21:32, 28 November 2009 (UTC)
- Here is a very "theoretical" solution. Assuming a uniform load, ignoring the other modes of failure, and assuming the shelf only deforms elastically, then we can apply the Euler–Bernoulli beam equation. Solving this for a uniform beam with a uniform load tells us that the deflection u(x) must be a quartic in x. Boundary conditions are that u = 0 and du/dx = 0 at each of the supports. If the supports are at a and -a then
- Deflection at centre of shelf is ka4. If optimum configuration is such that deflection at each end is equal to deflection at centre then
- So length of beam is , and distance between supports is of the length of the shelf. Gandalf61 (talk) 18:37, 28 November 2009 (UTC)
- Is du/dx = 0 at the supports a reasonable model of a board simply resting on top of a bracket? Intuitively it feels like you'd be likely to get non-zero slope at the brackets. Olaf Davis (talk) 20:55, 28 November 2009 (UTC)
- I hadn't considered that in my version either. I think you're right, the bracket would not hold against the small twist even if it was screwed on. Plus this solution doesn't consider the condition between the brackets and the ends which because it is considered fixed at the brackets should be modelled by another equation. I guess you mean the bracket should be considered as a pin in the terminology of the article. Must go and get some paper and work yet another version out. Dmcq (talk) 09:08, 29 November 2009 (UTC)
- Is du/dx = 0 at the supports a reasonable model of a board simply resting on top of a bracket? Intuitively it feels like you'd be likely to get non-zero slope at the brackets. Olaf Davis (talk) 20:55, 28 November 2009 (UTC)
Does that mean that the supports ought to be 15% of the length of the shelf in from the end? I'd like the average absolute deflection to be as little as possible. 92.27.169.45 (talk) 21:32, 28 November 2009 (UTC)
- Yes, to the nearest %. However practically my experience with making wooden bench seating is that it matters a lot exactly whether the supports are screwed into the shelf (there is a screw hole in the underside of many of them) and how rigid the supports are anchored. --BozMo talk 21:56, 28 November 2009 (UTC)
- Particularly make sure the brackets are anchored into the wall at a stud, and not just into drywall. Here's a tutorial; if you don't know how to find wall studs, you can buy an electronic stud finder tool which helps detect them. Nimur (talk) 00:41, 29 November 2009 (UTC)
- Henry Petroski's The Book on the Bookshelf. New York: Alfred A. Knopf. 1999. ISBN 0-375-40649-2. may be of interest to the OP.John Z (talk) 08:27, 29 November 2009 (UTC)
- Particularly make sure the brackets are anchored into the wall at a stud, and not just into drywall. Here's a tutorial; if you don't know how to find wall studs, you can buy an electronic stud finder tool which helps detect them. Nimur (talk) 00:41, 29 November 2009 (UTC)
- Yes, to the nearest %. However practically my experience with making wooden bench seating is that it matters a lot exactly whether the supports are screwed into the shelf (there is a screw hole in the underside of many of them) and how rigid the supports are anchored. --BozMo talk 21:56, 28 November 2009 (UTC)
Disease question
Is there a name for the phenomenon where one is sick, and lights seem dimmmer? P.S.:No medical advice, I am writing a paper for my first-year medicine course. --Tristan —Preceding unsigned comment added by 76.235.111.185 (talk) 23:17, 28 November 2009 (UTC)
- Hypoesthesia? See also photosensitivity and light sensitivity, and photophobia (which is sort of an opposite effect). Nimur (talk) 00:33, 29 November 2009 (UTC)
I mean, in an objective sense it sounds like you're talking about Miosis, but that is something physical that happens to the eye, so may just be one cause of the symptom you are describing. The article might be a good jumping off place for you though. --Shaggorama (talk) 08:23, 29 November 2009 (UTC)
November 29
Solutes
When I dash salt into a bowl of water, it seems as though the salt falls and 'drags water with it down into the water' upon hitting the surface, but when I dash paprika, it seems as though the paprika falls and 'pulls the water' along its own surface, causing the paprika to rapidly spread along the surface. Does this happen because the salt is immediately ionized and falls into the water, while paprika doesn't ionize and isn't nearly as readily brought into solution, and perhaps because of surface tension, fails to be incorporated? DRosenbach (Talk | Contribs) 01:10, 29 November 2009 (UTC)
- Paprika will not likely dissolve appreciably at all. Most of the compounds in paprika are hydrophobic and/or insoluble in water. If you shake the mixture, you can generate a Suspension, but that is not at all the same thing as a solution. As far as the visual effect of the mixing of the two, well, I can't make much comment on that as I really don't understand what you are getting at (the difference between "drag" and "pull".) But for sure, the way in which salt mixes with water (which is a true solution, see solvation for more details) and the way in which paprika mixes with water are very different. --Jayron32 02:03, 29 November 2009 (UTC)
- Salt being dropped into water "falls into the water," while paprika dropped into water does not "fall in," but spreads out onto the surface. Try it in a white vessel and you'll see the effect. DRosenbach (Talk | Contribs) 02:28, 29 November 2009 (UTC)
- What you're probably seeing is most likely a surface tension effect. Individual grains of salt are larger and more massive than individual specks of paprika powder; table salt is also a denser material than paprika. Consequently, each grain of salt exerts a substantially greater force per unit area on the water's surface and is more likely to fall through. The other factor is that I expect paprika (being dried plant matter) is probably appreciably more hydrophobic than salt; the salt is much more readily wetted by water. TenOfAllTrades(talk) 02:56, 29 November 2009 (UTC)
- May be some component of the Paprika has a surfactant effect? That would explain the phenomenos described. Dauto (talk) 03:44, 29 November 2009 (UTC)
Coffee problem
I was given the following problem: if you have a cup of coffee that's too warm to drink, and you want to cool it as quickly as possible, should you add cream immediately or after?
My first thought was that the cream should be added later, because the coffee will cool down faster when it is warmer (owing to the greater difference in temperature between the coffee and the air). But then I thought, the cream will do a better job of decreasing the temperature of the coffee if it is added immediately, because that is when the difference in temperature between the coffee and cream is greatest. So which is: immediately or later? —Preceding unsigned comment added by 24.200.1.37 (talk) 03:30, 29 November 2009 (UTC)
- Haha, my first concern would be more of preventing precipitation due to solubility issues (and keeping everything supersaturated), but I think adding it first would be more rapid, if your target temperature is above ambient temperature. Of course, adding creamer later will help to achieve lower cooling temperatures, but I don't think that's your goal. (You could also perform an empirical experiment...) John Riemann Soong (talk) 03:45, 29 November 2009 (UTC)
This question is a classic. It's such a classic in fact, that I think it's a homework question. But you could always google for the answer, there are probably hundreds of sites that explain it, and would do a better job than a few paragraphs here. Ariel. (talk) 03:47, 29 November 2009 (UTC)
- The question isn't a homework one, but yes I did try to google it. The problem is, everyone seems to ignore the fact that that the cream will be a more effective cooler if it is added immediately, thereby making the correct answer ambiguous.
- Well, what do you mean by "cool down faster". Do you mean the total time between the starting temperature and the final temperature OR do you mean the instantaneous rate of change of the temperature at any given point along the way. Depending on which definition of "faster" you mean, will result in a different approach to finding the answer. --Jayron32 03:49, 29 November 2009 (UTC)
- Total time.
- I think adding it later works better exactly for the reasons you provided. Dauto (talk) 03:51, 29 November 2009 (UTC)
- But the reasons I gave work against each other...
- No, think about it in terms of energy. Mixing the cream and the coffee doesn't change the total heat energy in the cream and coffee system. The only way the heat of the system changes is by the coffee getting rid of it to the environment as it cools. Rckrone (talk) 07:37, 29 November 2009 (UTC)
- But the reasons I gave work against each other...
- Add the cream immediately to the hot coffee because that increases the volume of liquid in contact with the cup hence reducing the thermal resistance between liquid and atmosphere. But add that cream gently without stirring to avoid heating by Turbulence kinetic energy. OTOH if you have a very cold spoon you know where to stick it. Cuddlyable3 (talk) 11:28, 29 November 2009 (UTC)
Cope elimination
Can you do it with a six-membered transition state? (I'm guessing not, because of the electron-pushing?) John Riemann Soong (talk) 03:48, 29 November 2009 (UTC)
Molecular structure
Does anyone know what the wiggly line means in the molecular structure diagram in the top right-hand corner of Amphetamine? —Preceding unsigned comment added by 86.138.105.112 (talk) 04:00, 29 November 2009 (UTC)
It means that both enantiomers prolly have similar properties on the body -- e.g. chirality doesn't matter. John Riemann Soong (talk) 04:13, 29 November 2009 (UTC)
- Thanks, do you mean that there are "left-handed" and "right-handed" versions of the molecule, but the structure diagram is not distinguishing between them (it could represent either)? 86.138.105.112 (talk) 04:29, 29 November 2009 (UTC)
- That's exactly right. There are two non-superimposible mirror image versions (i.e. "left" and "right" handed), the wavy line indicates that the actual version doesn't matter to the discussion at hand, or that there is a racemic mixture of the two versions under discussion. --Jayron32 04:38, 29 November 2009 (UTC)
- Got it, thanks! 86.138.105.112 (talk) 04:46, 29 November 2009 (UTC)
- That's exactly right. There are two non-superimposible mirror image versions (i.e. "left" and "right" handed), the wavy line indicates that the actual version doesn't matter to the discussion at hand, or that there is a racemic mixture of the two versions under discussion. --Jayron32 04:38, 29 November 2009 (UTC)
Earth's season
Hi! my question is how the season change occur in earth? and a season is same all around the world? i.e same season occur in all countries? —Preceding unsigned comment added by Nirmal kumaran (talk • contribs) 07:36, 29 November 2009 (UTC)
- No, at any given time, the entire world does not experience the same season. What the current season is depends on where you are. See Season. Red Act (talk) 08:12, 29 November 2009 (UTC)
- Seasons are reversed in the Northern hemisphere vs the Southern hemisphere (winter in one is summer in the other, spring is autumn, and vice versa). The nearer to one or other pole you are, the more extreme the seasons are. There basically aren't any seasons in the tropics (although you do get wet season and dry season in some places). The seasons are caused by the axial tilt of the Earth, which means sunlight hits a particular place on the Earth at different angles at different times of year (or, put another way, the sun gets higher in the sky during summer than winter). Sunlight shining directly on the ground heats it up more than sunlight hitting the ground at a glancing angle. --Tango (talk) 11:41, 29 November 2009 (UTC)
Evaporating water
When evaporating water , are you creating new oxygen?
- Evaporating water doesn't create oxygen or anything else. The water molecules (H2O) stay exactly the same. 86.134.90.230 (talk) 12:12, 29 November 2009 (UTC).











