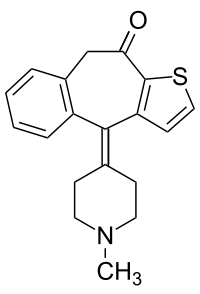
Ketotifen
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, Eye Drops |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Protein binding | 75% |
| Metabolism | Hepatic |
| Elimination half-life | 12 Hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.348 |
| Chemical and physical data | |
| Formula | C19H19NOS |
| Molar mass | 309.426 g/mol g·mol−1 |
| (verify) | |
Ketotifen fumarate is a second-generation H1-antihistamine/mast cell stabilizer available in two forms. In its ophthalmic form, it is used to treat allergic conjunctivitis, or the itchy red eyes caused by allergies. In its oral form, it is used to prevent asthma attacks. Side effects include drowsiness, weight gain, dry mouth, irritability, and increased nosebleeds.
The drug is marketed as ophthalmic solutions under the brand names Zaditor (Novartis), Alaway (Bausch and Lomb)), Zyrtec Itchy-Eye Drops, and Claritin Eye.
General information
Ketotifen relieves and prevents eye itchiness and/or irritation associated with most seasonal allergies. It starts working within minutes after administering the drops. The drug has not been studied in children under 3. The mean elimination half life is 12 hours.[1]
Besides its anti-histaminic activity, it is also a functional leukotriene antagonist and a phosphodiesterase inhibitor.
Off-Label Use
Ketotifen is used to up-regulate Beta-2 adrenergic receptors down-regulated by Beta-2 adrenergic agonists. It upregulates adrenergic receptors thanks to its phosphodiesterase inhibition, since a phosphodiesterase enzyme is involved in their feedback/omeostatic pathway.
References
This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. (June 2009) |
- ^ Grahnén A (1992). "Pharmacokinetics of ketotifen after oral administration to healthy male subjects". Biopharm Drug Dispos. 13 (4): 255–62. doi:10.1002/bdd.2510130404. PMID 1600111.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)
External links
