Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
February 4
Pterodactyl
Would a pterodactyl be strong enough for a person to be able to ride on its back? --108.225.115.211 (talk) 00:31, 4 February 2012 (UTC)
- Wouldn't you be hit by flapping wings there ? StuRat (talk) 00:33, 4 February 2012 (UTC)
- Very doubtful. What would be the point of having that extra strength? The extra muscle mass would mean they need to eat more, which would decrease their chance of survival. Evolutionary pressure is very strongly towards flying creatures being as light as possible and no stronger than they need to be to get off the ground (and perhaps to pick up prey, but according to pterodactylus only had a wingspan of 1.5m, so they weren't large enough to eat human-sized prey). --Tango (talk) 00:41, 4 February 2012 (UTC)
- Pterosaurs in general grow to be much larger, up to a 10m wingspan (see Pterosaur size). They seem to have a lot of tricks that help keep them aloft at that size, including internal air sacs to reduce their density as much as possible. The article of one of the largest pterosaurs, Quetzalcoatlus, talks about their weight (70-200 kg), them needing to vault themselves to get airborne, and includes an illustration of them doing their feeding on the ground. Most of the mighty predatory birds that need to do some impressive lifting feats (like the mighty eagles dropping goats off of cliffs) start lifting mid-flight, having a lot of momentum built up to work with, but is not sustainable flight - they lose altitude continuously.
- To answer your question, at the high-end estimated weight of a Quetzalcoatlus, it may have more room to spare in terms of carrying capacity than one would think, but a 200kg flyer could certainly never haul a 100kg person with any lift. I can't give a calculated estimate without years of incredible engineering research as these scientists have employed, but I can tell you immediately that each downbeat of the wings has to be at least 25% more powerful than normal and 50% more frequent. That's like me jogging to the grocery at a comfortable 8-minute mile, but then having to carry food back at an impossible 4:30. SamuelRiv (talk) 01:22, 4 February 2012 (UTC)
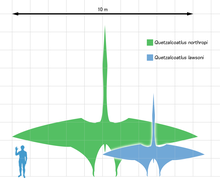
- (e/c) No. The largest pterodactyls (suborder Pterodactyloidea) are the members of the family Azhdarchidae, the azhdarchids. Azhdarchid flight is relatively poorly known, but like SamuelRiv said, they are believed to engage only in limited anaerobic (flapping) flight, and be predominantly soaring/flap-gliding animals that utilized columns of rising air to keep aloft. They launched by a running/flapping start and the addition of half to a third (a human's weight) of their current estimated body weight (~200 kg (440 lb) for an azhdarchid with a wingspan of 10 to 11 m (33 to 36 ft)) would make that quite impossible. Not to mention the aerodynamic drag and balance problems produced by a human rider. Azhdarchids are also believed to be stalkers, hunting their prey on foot like modern storks, not skimmers like some of the more commonly known marine pterosaurs (e.g. Pteranodon). They can not pick up prey while in flight because of the drag produced. A human riding them would produce similar if not even more catastrophic drag.-- OBSIDIAN†SOUL 01:43, 4 February 2012 (UTC)
- But according the article, many of those claims of large-animal flight, consistent with today's birds, are under dispute for pterosaurs. Surprisingly to me, there are apparently models for flight that is athletic and dynamic like a swallow, not soaring like an owl. As an analogy this might be improper, but one might consider in human flight, the size and shape of a plane varies greatly with what it has to carry - in no sense can one make a plane "scale up". So to make a small one-person recreational airplane carry 20 people, you can't simply make the wingspan 20 times bigger and/or the engine 20 times stronger - you have to redesign it. SamuelRiv (talk) 18:45, 4 February 2012 (UTC)
- Swallows are dynamic soarers actually. They do not engage in much anaerobic flapping. While azhdarchids are more likely to be such, given their shape and the velocities they can achieve, and not like the heavy poor flyers of today (like pelicans); also consider the fact that the faster they fly, the greater the effect of parasite drag. An extraneous human clinging on its back would produce such a drag. The paper points out the inappropriateness of the comparison with birds. The shape, lack of feathers, bend location, muscle structure, wing membrane (with matted pycnofibers that give it additional structural integrity) and bone strength of azhdarchids are too different from birds for any accurate comparisons. Thus the older estimates of azhdarchids only having an estimated weight of 80 kg (180 lb) for an individual with a 36 m (118 ft) wingspan used for the flying model is now considered wrong. But that argument is really only useful in refuting the assertions that azhdarchids were flightless. 70 to 100 kg (150 to 220 lb) human is still a third to half of the largest pterosaur's weight. Unless they had propellers, they wouldn't even be able to launch, much less keep aloft.-- OBSIDIAN†SOUL 01:34, 5 February 2012 (UTC)
Cycloaddition Mechanism
Hello. What is the reaction mechanism for the addition of dichlorocarbene to cyclohexene? Cited sources are appreciated. Thanks in advance. --Mayfare (talk) 06:35, 4 February 2012 (UTC)
- We have an article about dichlorocarbene, with a section entitled "Reactions" that tells you the product but not the mechanism. But it also suggests that the "dichloro" and the "cyclohex" details might not be important (they are not altered by the change taking place in the given reaction). So take a look at the more generic carbene article and maybe in particular the "Reactivity" section. DMacks (talk) 15:18, 4 February 2012 (UTC)
Most numerous vertebrate species
I recently heard a naturalist claim that humans are the most numerous vertebrate species. Is this true? If so, which other vertebrate species have populations in the billions? I would have thought that the brown rat and some domesticated animals would challenge humans for numbers. Warofdreams talk 13:47, 4 February 2012 (UTC)
- That definitely sounds wrong. Biomass (ecology)#Global biomass says there are more chickens. Chicken says it is the most common bird. I guess there are other more common vertebrates. PrimeHunter (talk) 14:23, 4 February 2012 (UTC)
- I don't think anyone really knows. He's wrong, but probably not by much, we'd probably be in the top 50 or something, few species are really readily adaptable to a wide range of ecological conditions like humans are. And to be fair, the most likely candidates (domestic and synanthropic animals) only attained their population sizes and distribution because of humans. On the opposite end of the spectrum, passenger pigeons which was believed to have once reached an estimated population of 3 to 5 billion were singlehandedly decimated to extinction within a century by humans.-- OBSIDIAN†SOUL 14:34, 4 February 2012 (UTC)
- The brown rat article claims there are 1.3 rats in the UK per person (which, if it's true, would obviously push the rats ahead of the humans). But it cites an hysterical piece in, of all sources, The Sun, which takes its info from Rentokil (Britain's largest ratcatcher, in whose interests it surely is to embiggen the "rat threat"). Snopes cites rather better sources for New York City claiming a ratio of only 1:36. -- Finlay McWalterჷTalk 15:00, 4 February 2012 (UTC)
- Rodents aren't limited to cities, they also do quite well in farm fields. In the U.S., we have around 220 million acres just under corn, soybeans, and wheat, and, even at a conservative estimate of 40 rodents/acre, U.S. grain field rodents are more populous than humans. (They also love fruit orchards and grass, e.g. alfalfa fields.) However, most of them are not brown rats, they are meadow voles.--Itinerant1 (talk) 19:56, 4 February 2012 (UTC)
- Rodentia, however, is not a single species. Few rodent species are truly cosmopolitan, and the ones that are are synanthropic species that stay close to human habitations. Field mice in Pakistan, for example, will be most likely to be a different species than field mice in Iowa. In contrast, a Norway rat in the Hong Kong sewers would be the same species as a Norway rat in New York.-- OBSIDIAN†SOUL 00:56, 5 February 2012 (UTC)
- Rabbits certainly number in the billions, though they aren't a single species either. I'm sure there are some vertebrate fish like herring that would give us a run for our money (according to the herring article there can be up to four billion in a single school)
. And there were 24,000,000,000 chickens as of 2003. 112.215.36.185 (talk) 05:15, 5 February 2012 (UTC)
- fish are vertebrates, right? Surely there are several species of small fish hat have a larger population than 7 billion. --Lgriot (talk) 00:43, 6 February 2012 (UTC)
- Gonostomatidae (a family of deep-water marine fish) says: "Cyclothone, with 12 species, is thought to be (along with Vinciguerria), the most abundant vertebrate genus in the world." See also http://www.nefsc.noaa.gov/faq/fishfaq1.html#q5. I guess it's hard to estimate how many billion small fish there are in deep water around the world. PrimeHunter (talk) 03:01, 7 February 2012 (UTC)
back muscles
When I lean backwards whilst standing, my abs and (to a lesser extent) chest muscles flex to help me maintain position. When I lean forwards my back muscles do the same. So why, after carrying a heavy backpack all day, do my back muscles hurt and not my abdominal/chest muscles? This seems counter-intuitive. The Masked Booby (talk) 14:43, 4 February 2012 (UTC)
- DIdn't you just answer your own question? With a backpack on, you lean forward. 79.122.74.63 (talk) 15:21, 4 February 2012 (UTC)
- Both muscle groups would have to act with each other to stabilize your backpack, so both would be working. A backpack is fairly efficient, though, so the group to feel the most soreness would be the group that is least used to this type of heavy work. For me at least, my that mid-lower-back area doesn't see a lot of heavy or long-term exertion, even in any sport I play. SamuelRiv (talk) 18:52, 4 February 2012 (UTC)
Is it possible to improve vision with eye exercises
my friend told me that it is possible. if it is then please tell me some eye exercises RahulText me 15:08, 4 February 2012 (UTC)
- Bates method. (Googling "eye exercises" leads you to loads of pages, including a wikihow site.)--TammyMoet (talk) 15:31, 4 February 2012 (UTC)
- I personally used to use the Bates method, because my initial experience with it was surprisingly good. I tried palming, and afterwards my vision was noticeably clearer for a little while (less than a minute). I started believing the claims, but no matter what, it doesn't do much in the long term. Once I did manage to shave about a diopter off my prescription, but there is no way of knowing if that was just because my eyesight was unnaturally bad due to stress, or whether it was some other effect of the treatment. Also, the eyesight eventually went back to what it was. When I finally read the research, I gave up pretty quickly, regardless of the initial experience. IBE (talk) 15:54, 4 February 2012 (UTC)
- If the Bates method is of any value at all, is it that it reminds one that the eyes focus by the means of muscles and if they don't get enough exercise they weaken. I can understand IBE's comment because I can't say that his (Bates) exercises appeared to do me any good when I tried them. Yet, I noticed after I stopped working in an office (where every-day I was only focusing 10 – 15 ft at the most and most of the time a lot shorter and a similar amount in the evening glued in front of the TV) that my eyesight improved to the point where I forgot to wear my specs because I could see well enough without them. Maybe also, because I then needed to take notice of vertical line as well as horizontal that my bad astigmatism became negligible. Thus, I think that just doing a few exercises from time to time it not really enough to make a major improvement, as they don't exercise the muscles enough. Also, as one grows older, atrophy of muscles, due to lack of exercise, takes less time to occur. Extended Bed Rest Accelerates Muscle Deterioration In Older Adults. Some might consider this last comment trite, but older friends of mine that have only spent a few hours in the Pub, focusing no further than the bar-maid, come out, unable to see their way home – and back to the wife. I tell you -this is the truth. Old age is not very kind – so don't go there. --Aspro (talk) 19:59, 4 February 2012 (UTC)
Vitamin D may also be beneficial to eye health. Count Iblis (talk) 00:00, 5 February 2012 (UTC)
- Weakening of the ciliary muscle with age, which regulates the accomodation reflex, tends to result in presbyopia, or reduced ability to focus on near objects. There is definitely variation in ciliary muscle strength between individuals of the same age, so any valid eye exercises may focus (no pun intended) on these muscles. ~AH1 (discuss!) 01:25, 5 February 2012 (UTC)
Why can animals be classified as vertebrates or invertebrates if vertebrates only make up one phylum?
I simply don't understand why scientists have to classify animals as vertebrates or invertebrates. Invertebrates make up several phyla, while vertebrates are in only one phylum? Yes I know humans are vertebrates, and that fact can lead to bias (like the Old World and New World stuff) but remember, not all chordates are vertebrates. Was it done for the sake of convenience, or just because chordates are somehow special? And what current phylum has the most similarities with chordates? Narutolovehinata5 tccsdnew 15:23, 4 February 2012 (UTC)
- Scientists classify things in whatever ways they think will be useful. Vertebrates have a lot of similarities to each other that they don't have with invertebrates, which makes it a useful classification. You won't find scientists talking about invertebrates much since, as you say, they are so varied - you would want to talk about a specific subset of invertebrates. The word only exists to distinguish them from vertebrates, rather than as a classification of its own. --Tango (talk) 15:54, 4 February 2012 (UTC)
- Of course, there is a discussion of this very topic in Wikipedia: Invertebrate#Significance_of_the_group. --Tango (talk) 15:58, 4 February 2012 (UTC)
- (WP:EC)See Invertebrate#History and Invertebrate#Significance_of_the_group. The term was coined by Lamarck, who himself is responsible for making further taxonomic subdivisions (Linneaus apparently divided all invertebrates into insects and worms). The article goes on to say "the (invertebrate) grouping has been noted to be "hardly natural or even very sharp."" Suffice it to say, it is a useful term for casual conversation, and does reflect some bias, but is not really used with much scientific weight. Also, just to clarify: "invertebrate" is not a "classification", it has no place in taxonomy or phylogeny, and as you point out the group is highly paraphyletic. It has the same scientific level of rigor as a term like "Non-ant insect". In short, the term is still used because it is useful, and is fairly well-defined. SemanticMantis (talk) 16:09, 4 February 2012 (UTC)
- Right. Classifying animals as vertebrates and invertebrates is a bit like classifying people as Americans and foreigners. If you live in America the classification is useful, but it is not a very meaningful distinction from a global point of view. Looie496 (talk) 23:57, 4 February 2012 (UTC)
- It's a very old classification system, back when people didn't truly appreciate the vast differences between groups of organisms and were still a bit anthropocentric in that they consider vertebrates to be more important than anything else (after all, they are usually the largest organisms). Today, Invertebrata is not a formal taxonomical rank (note its article does not have a taxobox in contrast to subphylum Vertebrata). It's merely a colloquial grouping meant to quickly distinguish vertebrates from non-vertebrates.-- OBSIDIAN†SOUL 00:51, 5 February 2012 (UTC)
On the similarity thing, echinoderms are the only deuterostomes other than the chordates and were said (at least when I was in high school 15 years ago) to be the phylum most closely related to chordates (and by "most closely related," I think I mean that on a cladistics graph, they branch most closely to or in concert with chordates in some fashion or another). DRosenbach (Talk | Contribs) 06:14, 7 February 2012 (UTC)
Basic iTunes question
Sorry, this is really basic, and please transfer it to Entertainment RD if that's more appropriate. I am gradually ripping all my CDs and putting them onto an iPod, using iTunes. It is taking up more space than I would like on my hard drive. If the music is safely on the iPod, how can I easily take them off the hard drive, or compress them, or whatever? ITunes seems to want me to have everything on both the hard drive and the iPod, "synchronised". Thanks. Itsmejudith (talk) 18:31, 4 February 2012 (UTC)
- You would probably be better off on the Computing desk. --Tango (talk) 19:02, 4 February 2012 (UTC)
- (ec)You might do better at the Computing desk than either! Not having used it, my hazy understanding of iTunes is that it is mostly for copy-locked (DRM) music files rather than for the all-purpose files from a ripped CD, and so a simple general purpose sound playback program might do better. But the extent of my musical forays nowadays is YouTube running Video DownloadHelper (with NoScript to block the VEVO ads ;) ) Wnt (talk) 19:04, 4 February 2012 (UTC)
- This is not true at all. You can use iTunes to manage any MP3s or other media files. They don't have to be DRMed at all, and usually aren't if you are ripping them from your own CDs (which you can do within iTunes easily). They are only DRMed if you get them through the iTunes store, which you're under no obligations to do. I don't think anybody cares about how you pirate music and I'm not sure why you offered that up as an answer. --Mr.98 (talk) 20:59, 4 February 2012 (UTC)
- I thought most/all? music downloaded from iTunes is DRM free by now? Nil Einne (talk) 07:42, 5 February 2012 (UTC)
- This is not true at all. You can use iTunes to manage any MP3s or other media files. They don't have to be DRMed at all, and usually aren't if you are ripping them from your own CDs (which you can do within iTunes easily). They are only DRMed if you get them through the iTunes store, which you're under no obligations to do. I don't think anybody cares about how you pirate music and I'm not sure why you offered that up as an answer. --Mr.98 (talk) 20:59, 4 February 2012 (UTC)
- Pirate? Why, how can I possibly pirate music by downloading it from the authorized publisher publishing from his official YouTube account? Wnt (talk) 03:53, 5 February 2012 (UTC)
- It depends greatly on your local laws. Nil Einne (talk) 07:43, 5 February 2012 (UTC)
- Pirate? Why, how can I possibly pirate music by downloading it from the authorized publisher publishing from his official YouTube account? Wnt (talk) 03:53, 5 February 2012 (UTC)
- You can set iTunes to "Manually Sync" your iPod. That way, it won't attempt to automatically make the two libraries match every time you plug it in. You can then safely delete music from your computer's hard drive, without affecting the iPod as long as you don't try to sync the whole music library manually. Usually, I prefer to set it to Manual and only select specific playlists to sync to my iPod. As long as you keep that music on your computer's hard drive, it won't have any problems. — The Hand That Feeds You:Bite 19:15, 4 February 2012 (UTC)
- You can also just set it to manually manage (not sync at all). Set up in this way, you move music to the iPod by dragging it within iTunes. You can then delete it off of the main computer if you want. --Mr.98 (talk) 20:59, 4 February 2012 (UTC)
- Thanks folks. Itsmejudith (talk) 20:52, 5 February 2012 (UTC)
- You can also just set it to manually manage (not sync at all). Set up in this way, you move music to the iPod by dragging it within iTunes. You can then delete it off of the main computer if you want. --Mr.98 (talk) 20:59, 4 February 2012 (UTC)
Parrots (e.g. Amazon)
Is it true that male Amazon Parrots usually get on better with women and that female Amazon Parrots get on better with men? Something to do with pheromones? Thanks.
Also (I asked this before but I don't think I got an answer, can't find the question now), is it just a coincidence that a baby Goffin's Cockatoo makes a noise that sounds a lot like a human baby? e.g. like http://www.youtube.com/watch?v=zLAp98VVuao --95.148.105.157 (talk) 21:50, 4 February 2012 (UTC)
binary star info
Can someone give the formula including 3 numbers: solar mass of both stars, separation distance, period. So if i was given 2 out of the numbers i can figure out the other one. So a formula that including all of 3 info above. Thanks!Pendragon5 (talk) 22:50, 4 February 2012 (UTC)
- The separation distance isn't necessarily constant, for elliptical orbits. StuRat (talk) 00:07, 5 February 2012 (UTC)
- The deviation of an elliptical orbit from perfectly circular is known as its orbital eccentricity, where a value of 0 is a circle and 1 is a parabola. The average distance between two objects is the semi-major axis, and the centre of gravity between a system of gravitationally-bound objects is its barycenter. You may try finding some 2-body simulators online, as the mass of either object does affect the shape of the orbit, or compare some real-life examples such as Capella versus Epsilon Aurigae. ~AH1 (discuss!) 01:15, 5 February 2012 (UTC)
- I need a formula.Pendragon5 (talk) 02:14, 5 February 2012 (UTC)
- The deviation of an elliptical orbit from perfectly circular is known as its orbital eccentricity, where a value of 0 is a circle and 1 is a parabola. The average distance between two objects is the semi-major axis, and the centre of gravity between a system of gravitationally-bound objects is its barycenter. You may try finding some 2-body simulators online, as the mass of either object does affect the shape of the orbit, or compare some real-life examples such as Capella versus Epsilon Aurigae. ~AH1 (discuss!) 01:15, 5 February 2012 (UTC)
And let assume that the separation distance is constant.Pendragon5 (talk) 02:34, 5 February 2012 (UTC)
- According to Standard_gravitational_parameter#Two_bodies_orbiting_each_other, the relationship is 4π2r3/T2 = G(m1 + m2). 98.248.42.252 (talk) 03:06, 5 February 2012 (UTC)
- I was going to derive it from scratch, but then saw Kepler orbit. Basically looks like the standard Kepler equation, and just set all eccentricity terms (e, E) to zero. SamuelRiv (talk) 03:32, 5 February 2012 (UTC)
- I don't think this equation, 4π2r3/T2 = G(m1 + m2), is what i'm looking for. I'm looking for the equation include the solar mass of the stars, the period of the star (how much time it took for them to orbit each other once), the separation distance.Pendragon5 (talk) 19:51, 5 February 2012 (UTC)
- See below. That's the exact equation you are looking for, as m1 and m1 are the masses, G is the "big G" gravitational constant, r is their seperation distance (as measured from their centers), and T is their orbital period. I've explained in more detail below, with links, before I knew you also asked it up here. --Jayron32 20:49, 5 February 2012 (UTC)
- I don't think this equation, 4π2r3/T2 = G(m1 + m2), is what i'm looking for. I'm looking for the equation include the solar mass of the stars, the period of the star (how much time it took for them to orbit each other once), the separation distance.Pendragon5 (talk) 19:51, 5 February 2012 (UTC)
- I was going to derive it from scratch, but then saw Kepler orbit. Basically looks like the standard Kepler equation, and just set all eccentricity terms (e, E) to zero. SamuelRiv (talk) 03:32, 5 February 2012 (UTC)
February 5
Water decapitiation or being squashed like a pancake?
Suppose the US has the capabilities to construct a 5,000-ton (but only about 1000 tons' worth of space available to the crew) submarine that has a super-strong titanium hull capable of withstanding great pressure. Suppose that the sub is at the bottom of Mariana Trench, and water is leaking through a 5 mm hole in the ceiling. There is only enough food and water supplies to last the single submariner another 24 hours. He has two options -- to wait until the sub fills up and die due to drowning in the cold water and the immense pressure, or instead position his head underneath the leak so the rapid leak could kill him. Now he doesn't know how quick the leak is and how fast the water is travelling, so he hesitates. Can somebody help him out by calculating the water's actual velocity, and the water's required velocity at which the submariner's head will get punctured instantly and die? --Sp33dyphil ©hatontributions 00:05, 5 February 2012 (UTC)
- Note that the rapidly moving water will also rapidly enlarge the hole, so they wouldn't need to get bored waiting to die. :-) StuRat (talk) 00:10, 5 February 2012 (UTC)
- Suppose all the dimensions stay the same. --Sp33dyphil ©hatontributions 00:16, 5 February 2012 (UTC)
- This sounds like a homework problem, no? Well, it would be a good one, and I don't use numbers. We could of course calculate the water pressure at that depth, but the Mariana Trench article provides it: 109x106 pascals (N/m^2). Pressure is force per area, and the area into which the force is being applied is a 5mm hole and A=πr2, which gives the force of the water flowing in. To break a neck, I'll just use the Hanging#Long_drop article for the heck of it: 5600 N. I get 2000 N from the water stream (full calculation left as exercise for the reader). Note that the smaller the hole, the less force, so you might want to widen it to a couple cm before you go a-suicidin'. SamuelRiv (talk) 03:02, 5 February 2012 (UTC)
- What would the water velocity for both cases be? --Sp33dyphil ©hatontributions 03:13, 5 February 2012 (UTC)
The velocity will be affected by three things: 1. The orrifice effect as the water transitions from the sea into the hole, 2. The viscous drag while in the hole, and 3. the orrifice effect as the water transitions from the hole to the air inside the hull. You can get 1 & 3 from tables in hydraulics and/or fluid dynamics texts, and (2) can be calculated by applying the Fanning-Darcy equation arranged to solve for flow given the pressure drop, length of the hole (= hull thickness), and the viscosity of water at the temperature that exists at that depth. Fanning Darcy has 2 forms - one for laminar flow, one for turbulent flow. You need to first calculate the Reynolds Number, dependent of hoile dimensions and viscosity, to decide which Fanning Darcy to use.
So, you need to know the thickness of the hull. Unless you think titanium is so strong the hull is paper-thin - not realistic. Keit124.182.20.59 (talk) 03:43, 5 February 2012 (UTC)
- If the hull is 2 cm thick, what's the velocity? --Sp33dyphil ©hatontributions 03:45, 5 February 2012 (UTC)
Look up "Reynolds Number", look up "Fanning-Darcy equation", and look up a good text for tables on the two orrifice efects, and do the math. I'm happy to point you in the right direction, but not to do your homework. Keit124.182.20.59 (talk) 03:49, 5 February 2012 (UTC)
- Naive velocity estimate is 400 m/s. SamuelRiv (talk) 04:01, 5 February 2012 (UTC)
 This is not h/w. I was just curious. And wow, 400 m/s! --Sp33dyphil ©hatontributions 05:19, 5 February 2012 (UTC)
This is not h/w. I was just curious. And wow, 400 m/s! --Sp33dyphil ©hatontributions 05:19, 5 February 2012 (UTC)
- And (assuming my maths is right, which it may very well not be), it will only take 159 seconds or thereabouts to fill your 1000 M3 space with water anyway, with it coming in through a 5mm diameter hole at 400 m/s. I don't think that food and water supplies are going to matter much here. AndyTheGrump (talk) 05:55, 5 February 2012 (UTC)
I suspect the 400 m/s flow is just a guess. But assuming it's right, 400 m/s thru a 5 mm hole is 0.00785 m3/sec. That will need 35.4 hours to fill the 1000 m3 sub. Keit58.170.173.63 (talk) 07:08, 5 February 2012 (UTC)
- Er, yes. I've probably got this wrong, on consideration. Though I'm not convinced you're right either. Maths was never my strong point... AndyTheGrump (talk) 07:20, 5 February 2012 (UTC)
- ~35 hours is correct, given the assumptions. Your value, Andy, would require the hole to have 800 times the area it does. As for the flow rate itself, if we are allowed to ignore friction, I believe 464 m/s is a more accurate value. Although perhaps Sam's naivete operates on a higher level than my own, and he is able to do guess the affect of friction. Someguy1221 (talk) 07:38, 5 February 2012 (UTC)
- Er, yes. Third time lucky, and I get the same answer: 35.37 hours, give or take. Never ask a bloke with a social science degree to design a submarine ;-) AndyTheGrump (talk) 08:28, 5 February 2012 (UTC)
- 400m/s was not a guess, but calculated using the hole bore volume to get the effective mass being ejected, without consideration for hydrodynamic effects. The water that actually exits will be very much more of a spray than a jet because of its turbulent speed and diffraction off the hole edges, and the work of displacing air will slow down the flow soon, but this is a nice upper limit. SamuelRiv (talk) 22:37, 5 February 2012 (UTC)
- "mass being ejected without consideration of hydrodynamics"??? I would have thought that hydrodynamics, ie.e, Fanning-Darcy equation, is at the core of the calculation - please explain. Displacement of air can be neglected: Starting air pressure inside = atmospheric sea level pressure = 1 Bar, and the water pressure at the bottom of the Mariana Trench = 1000 Bar. Therefore, it's not until the air in the hull is compressed to less than 0.1 % of its volume that its presure will be comparable to the sea. Don't forget to allow for the Ratio of Heats for air (1.404). Keit60.231.241.252 (talk) 01:18, 6 February 2012 (UTC)
- Hm, for the most basic approach I would have used the momentum change induced on the water by the pressure force -- i.e. p*A=v*dm/dt=v*density*A*ds/dt=density*A*v^2 <=> v=sqrt(p/density). For density=1e3kg/m^3 and p=1.1e8 N/m^2 that would be v=331.7 m/s. And from that you'd get the mass flow rate via v=ds/dt=dV/A/dt=(dm/density)/A/dt <=> mass flow rate=dm/dt=A*sqrt(p*density)=6.5 kg/sec. No? Olaf Klischat (talk) 18:43, 9 July 2013 (UTC)
- I don't think displacement of the air can be neglected, since 400 m/s is faster than the speed of sound in air. In other words, it's a supersonic jet ;) Gandalf61 (talk) 08:41, 6 February 2012 (UTC)
- Good point, I didn't think of that. However, upon thinking about it, the ratio of sea pressure to starting air pressure is so great (1000:1) it still won't matter. What might matter is that the resulting shock wave in such a confined space might knock out all the humans forthwith. Though I don't accept (without evidence) that the 400 m/s value has been correctly estimated anyway. Keit121.221.76.67 (talk) 12:42, 6 February 2012 (UTC)
- Bernoulli equation has the water velocity from V=0m/s and p=1000bar to p=1bar and V=450m/s. --145.94.77.43 (talk) 03:27, 8 February 2012 (UTC)
- Good point, I didn't think of that. However, upon thinking about it, the ratio of sea pressure to starting air pressure is so great (1000:1) it still won't matter. What might matter is that the resulting shock wave in such a confined space might knock out all the humans forthwith. Though I don't accept (without evidence) that the 400 m/s value has been correctly estimated anyway. Keit121.221.76.67 (talk) 12:42, 6 February 2012 (UTC)
- Why should this submariner worry? Nitrogen narcosis is a nice euphoric way to go (as the pressure increases). If he is on-board with a mixed crew - all the better. --Aspro (talk) 23:17, 8 February 2012 (UTC)
What kind of spectrum is this?
Hi. One day while glancing over the surface of the water in a partly-full water bottle in a room with windows at the back and fluorescent lights over top, I noticed an unusual spectrum. At least part of the spectrum was likely from the fluorescent lights, but probably not the part you'd expect, since I observed the fluorescent spectrum in water to be very similar to the natural white light spectrum. I will list the colours of this spectrum in order, but keep in mind that italic text signifies what I call "narrowband", bold what I wrote down as the thickest part of the spectrum, taking up almost half of the entire spectrum, and underline for colours seen very narrowly at the edge of some transition, possibly into fluorescent lighting or ambient ceiling.
- Tan
- Pink
- Rouge
- Magenta
- Violet
- Indigo
- Navy
- Blue
- Green
- Teal
- Lime
- Tan
- Haze
- Orange
- White
- Orange
- Red
- Mahogany
- Violet
- Magenta
- Venusian
- Clear
Now, any idea what this spectrum may consist of? There did not be any objects directly contributing, unless there was a ubiquitous green backpack or other container within my line of sight. Thanks. ~AH1 (discuss!) 01:04, 5 February 2012 (UTC)
- How in the world did you come up with those color names? Looie496 (talk) 02:52, 5 February 2012 (UTC)
- You should ask Roy G. Biv. Edison (talk) 03:27, 5 February 2012 (UTC)
- It's hard to picture an image from such descriptions, but the first non-bold colors seem to match those of the supernumerary rainbow depicted in the article. Reportedly this has something to do with interference... but I can't really speculate exactly how that appeared in a water bottle. Wnt (talk) 03:58, 5 February 2012 (UTC)
- You should ask Roy G. Biv. Edison (talk) 03:27, 5 February 2012 (UTC)
The normal spectrum sequence is seen with oil films on water surfaces due to interference within the oil. Your observed effect could be due to viewing this through another oil film on the inside surface of the bottle. This could thru addition provide more colours. Keit124.182.20.59 (talk) 03:59, 5 February 2012 (UTC)
In other words, AstroHurricane is not looking at a "spectrum," but rather a "colorful array of refracted and diffracted light." The root cause for such a colorful display is the same as prismatic diffraction - optical dispersion - but whatever was causing the diffraction was not high-grade, precisely-ground polished optical glass. Consequently, you see a muddied mix of a lot of different light, including some regions where clean spectral lines might have been visible. AstroHurricane, perhaps you can build your own spectrometer, using these ordinary house-hold materials, and let us know if your spectrum looks any cleaner. If you have access to a laboratory-grade prism, you can build an even better spectrometer, and should get even cleaner results. Nimur (talk) 16:37, 5 February 2012 (UTC)
Earth spin and distance to the sun
Sir, suppose if the spinning speed of earth (around its own axis)is doubled what will happen to distance between the sun and earth. will it increse or decrease? — Preceding unsigned comment added by Tobyaickara (talk • contribs) 12:55, 5 February 2012 (UTC)
- Why do you think it would change? I can see no reason why it should. AndyTheGrump (talk) 15:21, 5 February 2012 (UTC)
- Perhaps you should read tidal locking and orbital resonance. The orbit does affect the axial rotation in a very complicated way; the two parameters are, effectively, "coupled oscillators." Objects with larger orbital axis are much more weakly tidally-locked, effectively meaning that the time-constant for the resonant locking imcreases very significantly. We could go through calculations that are outlined in the articles I linked, if you have questions; but the OP will need to specify some details if they want a mathematical answer. As I always mention when people ask questions about cosmological hypotheticals: in order for us to answer correctly, you will need to be more specific: how would the Earth's orbit or axial rotation change speed? For example, would it be due to an impact with another object? In the absence of a cause, conservation of momentum - specifically angular momentum - tells us that there should be no change without outside interference.
- Anyway, here is the equation that defines the most important part of the original question: the relation defining the time constant of axial rotation resonantly locking to the period of orbital revolution. Note the dependence on the semimajor axis of the orbit. If you want a more rigorous, less approximate form, I recommend de Pater and Lissauer's Planetary Science text, which covers orbital parameters very extensively and mathematically. Nimur (talk) 16:27, 5 February 2012 (UTC)
- Another effect might be that the Earth would lose some of it's atmosphere, which would reduce the Earth's mass. However, I wouldn't expect this to be significant (but either would the tidal locking, due to the Sun's distance from Earth). StuRat (talk) 23:12, 5 February 2012 (UTC)
- These effects are small (especially since there is no rotation–orbit resonance for the Earth), so, initially, and to a good first approximation thereafter, AndyTheGrump's intuitive reply of "no effect" on the year or distance if the day length was to be magically halved seems reasonable. In reality, as Nimur explains, magic doesn't happen. Dbfirs 22:47, 6 February 2012 (UTC)
Is amphibia a paraphyletic group?
Kinda related to the invertebrate question, seems like invertebrates are a paraphyletic group since it does not include all of the descendents of invertebrates (like vertebrates), so is this the same for amphibia? I know we evolved from tetrapods, which we are part of, but did we at one point evolve from a lifeform that was for all intents and purposes, an amphibian? ScienceApe (talk) 17:44, 5 February 2012 (UTC)
- See Pederpes, which we describe as "Amphibia sensu lato". In Linnean taxonomy it is an amphibian, but in cladistic taxonomy it is a tetrapod. Other fauna from Romer's Gap may also be interesting to you. Wnt (talk) 17:53, 5 February 2012 (UTC)
- "Invertebrata" is polyphyletic, not paraphyletic.See note And define "amphibian". Do you mean living amphibians (Lissamphibia) or Amphibia sensu lato (all non-amniote tetrapods - Temnospondyli, Lepospondyli, and Lissamphibia, etc.)?
- Amphibia sensu lato (i.e. including all extinct forms) is paraphyletic, since it does not include Amniota (reptiles, birds, mammals, including extinct forms).
- If you meant the Lissamphibia alone. Quick answer is we don't know. See Labyrinthodontia#Origin of modern amphibians. Theories range from a monophyletic Lissamphibia derived from Lepospondyli; to separate origins of Caudata from Temnospondyli; to separate origins of caecilians from other amphibians sensu lato closer to amniote ancestors or even porolepiform fish (!).
- Also see Tetrapod#Phylogeny. Note location of Amniota and Lissamphibia.-- OBSIDIAN†SOUL 18:48, 5 February 2012 (UTC)
- Note: the traditional classification of Invertebrata included [macroscopic] animals from more than a dozen different phyla while excluding common ancestors ("Insecta" and "Vermes"). It even included plants! This is not even taking into consideration the problem of whether Metazoa (Animalia) itself is monophyletic or polyphyletic. But yeah I guess, Invertebrata can also be said to be paraphyletic in relation to vertebrates, but that's a bit superfluous. Like assembling a doll with three arms, two heads, no body, and five legs; and then detaching one leg because it was "different". :P -- OBSIDIAN†SOUL 19:57, 5 February 2012 (UTC)
- See invertebrate. The first sentence of the second paragraph says, "Invertebrates form a paraphyletic group." ScienceApe (talk) 23:14, 5 February 2012 (UTC)
- See above note (I have removed the <small> tags). In Linnean taxonomy, non-vertebrate animal phyla included only two groups: "Vermes" and "Insecta", and they included a mishmash of organisms (again even including Volvox, which is under Plantae). When Lamarck introduced the formal group "Invertebrata", he divided them into "Mollusques", "Crustacés", "Arachnides", "Insectes", "Vers", "Radiaires", and "Polypes". All of which do not include a common ancestor. Thus these groupings are polyphyletic. These were the only instances where Invertebrata was treated as a formal group. In the modern sense, the term "invertebrate" is completely informal with a definition that has become simply "all metazoans, excluding Vertebrata". It is thus paraphyletic to vertebrates, provided that you accept a monophyletic Metazoa.
- And that article still needs a lot of work, which illustrates exactly how problematic the grouping is.-- OBSIDIAN†SOUL 02:21, 6 February 2012 (UTC)
It's important to distinguish between amphibia and amphibians. In modern biology amphibia names a monophyletic group which includes amphibians as well as reptiles, birds, and mammals. Similarly reptilia names a monophyletic group which includes reptiles, crocodilians, and birds.— Preceding unsigned comment added by Looie496 (talk • contribs) 00:47, 6 February 2012 (UTC)
Equation explanation
Can someone explain for me how to use this equation? 4π2r3/T2 = G(m1 + m2). Thanks!Pendragon5 (talk) 19:48, 5 February 2012 (UTC)
- It's a form of Newton's law of universal gravitation as it applies to a body in orbit. G is the Gravitational constant and m1 and m2 are the masses of two bodies, r is the distance between the centers of the bodies, π is 3.14159... and T is the orbital period (i.e. time for the two bodies to return to the same relative position). An alternative form of this same equation is at Orbital_period#Small_body_orbiting_a_central_body. --Jayron32 20:44, 5 February 2012 (UTC)
What is / sign, the one right in front of T stand for? And yes finally, this is the one i'm looking for. I just didn't understand it at first.Pendragon5 (talk) 20:57, 5 February 2012 (UTC)
- Division. 4 times pi squared times r cubed divided by T squared. — Lomn 21:03, 5 February 2012 (UTC)
- WOW, there should be a bracket () around 4 times pi squared times r cubed so people can understand it. And by the way, what number is Gravitational constant? I look in the article i still don't get it. Can someone give me the number that represents G in the formula.Pendragon5 (talk) 21:15, 5 February 2012 (UTC)
- Why? It doesn't make a difference in this case. (4π2)r3/T2 ≡ (4π2r3)/T2 ≡ 4(π2r3)/T2 ≡ 4π2(r3/T2) No point in putting in unneeded brackets, but then I always lean more maths than physics. The '/' as division is a programming convention, I think, which has crept into casual use where people are communicating with mostly plaintext over keyboards. You could use ÷ instead, if you prefer. 86.166.41.126 (talk) 21:22, 5 February 2012 (UTC)
- WOW, there should be a bracket () around 4 times pi squared times r cubed so people can understand it. And by the way, what number is Gravitational constant? I look in the article i still don't get it. Can someone give me the number that represents G in the formula.Pendragon5 (talk) 21:15, 5 February 2012 (UTC)
- There isn't a single number which is "G" - it's a physical quantity, which means it has both magnitude and units. What number you use depends on the units system you are operating under. If you're using standard SI units, then G ≈ 6.674 × 10-11 N×(m2/kg2) . The equation as written is valid, however, for any linear unit system where zeros are appropriately placed. For example, you could use a G ≈ 3.730 × 10-7 furlong3/(troy ounce×(fortnight2)), if those were the units you were measuring the other quantities in. -- 140.142.20.101 (talk) 22:03, 5 February 2012 (UTC)
- I'm just like lost right there. I have no idea which unit i'm suppose to be in. Alright let just make up a problem. 2 stars with the total solar mass is 2, separation distance is 5 AU. What is the period? The answer unit is in second or minutes or hour? Which number is G in this case?Pendragon5 (talk) 22:50, 5 February 2012 (UTC)
- Well, it's up to you what units you want. If you want seconds (easy to convert to minutes or hours), then the easiest way of writing G is 1.124 AU3 Msun s^-2, which means that you can plug the numbers straight in. That said, it's almost certainly better to put the units in SI - 1 solar mass is ~2 x 1030 kg, 1 AU is ~1.5 x 1011 metres. Then you can use the SI version, G ≈ 6.674 × 10-11 N×(m2/kg2) which is the version that you'd be given in most text books. Smurrayinchester 23:43, 5 February 2012 (UTC)
- It is not just converting. I know how to convert and it's easy yea. OK let say i do that problem, at the end i will get a number right? That number will have a certain unit in second, minute, hour? It can not be randomly one of them then you can convert it, it doesn't work that way. "Which means that you can plug the numbers straight in"? I don't get this? I don't understand all of this AU3 Msun s^-2 after the number 1.124. So if i plug the number in my calculator, what number is G? This is just way too confusing, i don't really get any of the explanations.
- OK how about just do one problem as an example for me. It's better than just explaining around. 2 stars with the total mass of 2 solar mass, separation distance is 5 AU. What is the period? <---- answer this question and show me how you did it. Write out the equations with numbers that can be plug in the calculator.Pendragon5 (talk) 02:11, 6 February 2012 (UTC)
- If you're looking for the period anyways, just do the algebra first to get yourself in a form of T= blah blah. If you look at Orbital_period#Two_bodies_orbiting_each_other it's already in that form. The article uses "P" instead of "T", but just plug the numbers in. If we assume a nearly circular common orbit between two bodies of exactly one solar mass each, of a distance of 1AU, use:
- P = what you are looking for
- π = 3.14159...
- G = 6.674x10-11 N m2 kg -2
- (m1 + m2) = 2x(1.9891x1030) kg
- a = 149.60x109 m
- Since the G constant uses SI units, then the time unit is seconds; because N = kg m s-2, so the Newton unit already has seconds in it. If you do the dimensional analysis properly, you get units of P in seconds. So, plug those numbers in the equation, solve, and viola. You're done. --Jayron32 03:33, 6 February 2012 (UTC)
- I think that should be a = 5 x 149.60x109 m. Gandalf61 (talk) 08:57, 6 February 2012 (UTC)
- If you're looking for the period anyways, just do the algebra first to get yourself in a form of T= blah blah. If you look at Orbital_period#Two_bodies_orbiting_each_other it's already in that form. The article uses "P" instead of "T", but just plug the numbers in. If we assume a nearly circular common orbit between two bodies of exactly one solar mass each, of a distance of 1AU, use:
- It's not a complicated concept. Say you want to calculate the distance that light travels in time t. d=ct, where c is the speed of light. If you choose c to be in m/s (standard SI), you have to put t in seconds, and d will be in meters. If you choose c to be in cm/nanosecond, you have to put t in nanoseconds, and d will be in centimeters. --140.180.15.97 (talk) 08:12, 6 February 2012 (UTC)
- Well, it's up to you what units you want. If you want seconds (easy to convert to minutes or hours), then the easiest way of writing G is 1.124 AU3 Msun s^-2, which means that you can plug the numbers straight in. That said, it's almost certainly better to put the units in SI - 1 solar mass is ~2 x 1030 kg, 1 AU is ~1.5 x 1011 metres. Then you can use the SI version, G ≈ 6.674 × 10-11 N×(m2/kg2) which is the version that you'd be given in most text books. Smurrayinchester 23:43, 5 February 2012 (UTC)
- In this problem, it is easier not to go via SI units. We know one orbit for which the equation is fulfilled, that is earth's orbit with , (earth's mass can be safely neglected) and . These are convenient units for this sort of problem, especially since OP's data are given in those units. Observing that
- ,
- we can write the equation as
- With the numbers given by OP, the result is . In those units (AU, yr, solar mass), the gravitational constant is simply . --Wrongfilter (talk) 08:41, 6 February 2012 (UTC)
- So the equation of Wrongfilter will result the answer in year, and the equation of Jayron will result in seconds, right?Pendragon5 (talk) 20:30, 6 February 2012 (UTC)
- Yes, or you could take the answer from my section and divide it by 31,557,600 to get years, or you could take the answer from Wrongfilter's version and multiply it by 31,557,600 to get the answer in seconds. Or, knowing the conversions (1 minute = 60 seconds, 1 hour = 60 minutes, 1 day = 24 hours, 1 year = 365.25 days) you can take any of the answers and put it into any arbitrary time unit you like. --Jayron32 01:49, 7 February 2012 (UTC)
- So the equation of Wrongfilter will result the answer in year, and the equation of Jayron will result in seconds, right?Pendragon5 (talk) 20:30, 6 February 2012 (UTC)
- I'm just like lost right there. I have no idea which unit i'm suppose to be in. Alright let just make up a problem. 2 stars with the total solar mass is 2, separation distance is 5 AU. What is the period? The answer unit is in second or minutes or hour? Which number is G in this case?Pendragon5 (talk) 22:50, 5 February 2012 (UTC)
- There isn't a single number which is "G" - it's a physical quantity, which means it has both magnitude and units. What number you use depends on the units system you are operating under. If you're using standard SI units, then G ≈ 6.674 × 10-11 N×(m2/kg2) . The equation as written is valid, however, for any linear unit system where zeros are appropriately placed. For example, you could use a G ≈ 3.730 × 10-7 furlong3/(troy ounce×(fortnight2)), if those were the units you were measuring the other quantities in. -- 140.142.20.101 (talk) 22:03, 5 February 2012 (UTC)
Center of gravity below the center of mass
Does anybody know how the location of Petronas Towers center of gravity was calculated in the "Young H. D., Freedman R. A., Sears and Zemansky University Physics"? There are different approaches/approximations in calculating CG position in non-uniform field. (Or, at least, how to find our discussion page on CG after the article was merged into the Center of mass article?)--Ilevanat (talk) 21:30, 5 February 2012 (UTC)
Thank you! I would not have found it myself. Still, does anybody know anything about the Sears and Zemansky textbook result?--Ilevanat (talk) 01:17, 6 February 2012 (UTC)
- The variation in the force of gravity over the height of a building is so small that it can probably be ignored entirely. So, I certainly wouldn't worry about which formula they use to compensate for the non-uniform field, as any difference between them is sure to be insignificant. The CG would vary more with the movement of people through the building. StuRat (talk) 08:51, 7 February 2012 (UTC)
- My simplistic model, which incorrectly assumes each floor of the Petronas Towers has the same mass, finds that the center of gravity is less than an inch from the center of mass. Someguy1221 (talk) 09:13, 7 February 2012 (UTC)
It indeed is, according to the textbook (about 2 cm). Surely, nothing significant from the practical point of view (and the observation by StuRat might be true in some sense). But the discussion we had about the former CG page was related to the conceptual and historical differences between CG and CM (and it is partly reflected in the present CM article, but I think this could still be improved). Anyway, Someguy1221, I will probably bother you some more soon at your talk page.--Ilevanat (talk) 02:05, 8 February 2012 (UTC)
lamp starters in series circuit fluorescent lamps e.g. philips lighting s2. how are these different from ordinary lamp starters e.g. philips lighting s10?
when two 20 watt fluorescent lamps were arranged in parallel each with its own lamp starter but series-connected to a single 40 watt choke only one or the other lamp lit up. arranged in series, each lamp parallelled with a philips lighting s2 starter on two lamp terminals and series-connected to the 40 watt choke both lamps lit up as desired.
what is different about an s2 lamp starter compared with the more ordinary lamp starters such as an s10 both manufactured by philips lighting?
thank you for your interest. — Preceding unsigned comment added by Davidlixenberg (talk • contribs) 21:35, 5 February 2012 (UTC)
If you make two flouro tubes in parallel share a common ballast, I would expect only one will light up, and even if both fire, only one will stay lit. This is because when a tube is not ionised (ie not lit up) it is an open circuit. This allows the ballast to resonate with the starter to produce a high voltage to close the starter and provide heater current to the tube. Due to manufacturing variation, one starter will close at a lower voltage - this will prevent the other starter from allowing heater current in its tube. Once one tube is going, it "clamps" the voltage well below that which will fire the its own starter and the other starter - so the other tube cannot start. Keit124.182.1.238 (talk) 02:41, 6 February 2012 (UTC)
February 6
Order of a mixture
Is there an article here in wk about why the order of elements of a mixture matters? (for example, hot oil + water, or acid + water or Nescafe + hot water). — Preceding unsigned comment added by 88.8.79.238 (talk) 00:17, 6 February 2012 (UTC)
- For acid and water mixture, see Sulphuric_acid#Reaction_with_water_and_dehydrating_property99.245.35.136 (talk) 00:29, 6 February 2012 (UTC)
- Yup. regarding the acids, our Sulfuric acid article explains why it matters:
- "Preparation of the diluted acid can also be dangerous due to the heat released in the dilution process. The concentrated acid is always added to water and not the other way around, to take advantage of the relatively high heat capacity of water. Addition of water to concentrated sulfuric acid leads to the dispersal of a sulfuric acid aerosol or worse, an explosion. Preparation of solutions greater than 6 M (35%) in concentration is most dangerous, as the heat produced may be sufficient to boil the diluted acid: efficient mechanical stirring and external cooling (such as an ice bath) are essential.
- As for mixing hot oil and water, if the oil is above 100 C, adding water is going to result in vaporisation, and ejection of hot oil. This is why it is extremely dangerous to try to put out a cooking-oil fire with water - you can end up with a fireball - see Chip_pan_fire#Fire_hazard. AndyTheGrump (talk) 00:34, 6 February 2012 (UTC)
- I've also noticed that you want to add cocoa powder to hot water, not the reverse, or it will stick to the bottom of the cup. StuRat (talk)
- For Nescafe + hot water, you've apparently never seen what happens when you add powdered substances to superheated microwaved water. The temperature of the water can exceed 100 degrees, and adding the powder can trigger a mess, and even dangerous, explosion. Same goes with hot cocoa powder. Dominus Vobisdu (talk) 01:36, 6 February 2012 (UTC)
- I just use hot tap water, no nuking here. StuRat (talk) 01:50, 6 February 2012 (UTC)
- Frankly I imagine that the issue is less about explosive water (which is pretty uncommon) but the fact that if you add the powder last it becomes very hard to mix in. I've done in backwards many times and what you end up with is a lot of powder floating on top, vigorously resisting making a mixed liquid. If you do it the other way, that doesn't happen. --Mr.98 (talk) 03:18, 6 February 2012 (UTC)
- Yes, because it's all stuck to the bottom. :-) I think adding a bit of hot water, then the cocoa powder, then the rest of the hot water, is the best approach. StuRat (talk) 05:05, 6 February 2012 (UTC)
- I concur with StuRat, but additionally stir the initial water + powder mix vigorously with the teaspoon (why do we never say cocoaspoon?) before topping up. This enables you to reverse the first two steps and add some water to the dry powder, which is sometimes more convenient, as you can have the cocoa jar put away again before starting to mess with the kettle, thus decluttering the work surface. It's all in the therbligs! {The poster formerly known as 87.81.230.195} 90.197.66.42 (talk) 12:46, 6 February 2012 (UTC)
- For what it's worth, I treat hot chocolate powder like cornflour: dry stuff goes in mug first, then add splash of water and stir thoroughly to make a thick paste. Then water down the paste, stirring constantly. No clumps at the bottom! Brammers (talk/c) 16:42, 6 February 2012 (UTC)
- For Americans who might be confused — Brammers is talking about cornstarch, which for some odd reason Brits call cornflour. --Trovatore (talk) 16:53, 6 February 2012 (UTC)
- For what it's worth, I treat hot chocolate powder like cornflour: dry stuff goes in mug first, then add splash of water and stir thoroughly to make a thick paste. Then water down the paste, stirring constantly. No clumps at the bottom! Brammers (talk/c) 16:42, 6 February 2012 (UTC)
- I concur with StuRat, but additionally stir the initial water + powder mix vigorously with the teaspoon (why do we never say cocoaspoon?) before topping up. This enables you to reverse the first two steps and add some water to the dry powder, which is sometimes more convenient, as you can have the cocoa jar put away again before starting to mess with the kettle, thus decluttering the work surface. It's all in the therbligs! {The poster formerly known as 87.81.230.195} 90.197.66.42 (talk) 12:46, 6 February 2012 (UTC)
- Yes, because it's all stuck to the bottom. :-) I think adding a bit of hot water, then the cocoa powder, then the rest of the hot water, is the best approach. StuRat (talk) 05:05, 6 February 2012 (UTC)
- The mnemonic I was taught: You're doin' watcha otter when you add the acid to the water. --Trovatore (talk) 16:45, 6 February 2012 (UTC)
- The mnemonic I remember is "don't do what I did that time many years ago when I added water to acid and the flask shattered". DMacks (talk) 17:33, 6 February 2012 (UTC)
- Well, good for you if you can remember for sure which one you did. In junior high school I had a similar experience (sodium thiosulfite solution plus acid --> cloud of yellow gas that sent everyone out of the room) but I thought I had done it right. Maybe I did add the acid to the water but just too much at once, or something. At this remove it's impossible to be sure. --Trovatore (talk) 19:05, 6 February 2012 (UTC)
- The mnemonic I remember is "don't do what I did that time many years ago when I added water to acid and the flask shattered". DMacks (talk) 17:33, 6 February 2012 (UTC)
Boiling points and tripple points for monatomic gasses
Does anybody know where I can find the (notional) boiling points (at standard pressure) and the triple point for monatomic gasses including H, O, N ? I assume these must be calculated/estimated values as they will not be stable at such low temperatures. Thanks Keit124.182.1.238 (talk) 02:50, 6 February 2012 (UTC)
- See our articles on hydrogen, oxygen, and nitrogen, under "physical properties".--Shantavira|feed me 09:01, 6 February 2012 (UTC)
I wish it was that easy, Shantivira. These Wikipedia articles, although describing the elements H, O, & N, actually give the properties for the molecular form, H2, O2, and N2. If you look up the NIST online database for H2, O2, and N2, you get the same values as given in the WP articles. Keit121.221.76.67 (talk) 12:28, 6 February 2012 (UTC)
- Then the only gases you're talking about are the noble gases Helium neon Argon Krypton Xenon and Radon. Dmcq (talk) 14:03, 6 February 2012 (UTC)
- No, definately not talking about the noble gases. Monatomic gasses such as H, O, and N are not stable at low temperatures (and pressures), but they CAN and DO never the less exist - that's why I need values for them. I could find all manner of properties for them, but not boiling and triple points. Keit124.178.157.111 (talk) 15:20, 6 February 2012 (UTC)
- Don't you see a problem with high temperature low pressure solid gas? Dmcq (talk) 16:10, 6 February 2012 (UTC)
- No, definately not talking about the noble gases. Monatomic gasses such as H, O, and N are not stable at low temperatures (and pressures), but they CAN and DO never the less exist - that's why I need values for them. I could find all manner of properties for them, but not boiling and triple points. Keit124.178.157.111 (talk) 15:20, 6 February 2012 (UTC)
- You are essentially asking a counter-factual. Monoatomic H, O, N can never form liquids or solids because long before they reach that state they will first form diatomic molecules. Hence it makes no sense to ask what their boiling points / triple point would be since they will never reach such a state without forming molecules. I don't see how such notional values could be useful. More to the point, I don't think there is anyway to usefully calculate such values. The interatomic forces between these monoatomic gases lead to pair-bonding, but you want to calculate how the interatomic forces lead to the formation of liquids and solids while somehow preventing pair-bonding. Whatever answer you get from such a calculation would depend strongly on the modifications you made in order to prevent the formation of pair bonds, and at that point it wouldn't be representative of any true behavior for the monoatomic gas. Dragons flight (talk) 18:36, 6 February 2012 (UTC)
- It seems valid to ask what the properties are, though maybe there are no published values. Once created, unstable forms can exist, it's just for a limited time - in some cases, years, in some cases, minutes, in some cases picoseconds. But they can exist. Monatomic gasses are extremely unstable at standard temperature (~298K) and pressure (~1 Bar), you can certainly find thermodynamic data (eg specific heat, enthalpy, etc) for them (eg Rose & Cooper, NIST, etc etc) - presumably these are calculated values. And the common forms H2, O2, N2 are quite unstable at 6000K, but you can find calculated properties for them at 6000K. Don't forget that gases unstable below certain temperatures can be made so by increasing pressure. Judging by the marked upward tilt of specific heat at low temperatures and 1 bar, the triple point and boiling point of monatomic oxygen must be close to standard temperature and pressure, that of H must be very low. Ratbone121.221.96.117 (talk) 01:22, 7 February 2012 (UTC)
- Where on NIST did you find thermodynamic data for monatomic gases? The boiling and triple points are statistical properties resulting from the interaction of the molecules. I don't see how you can expect to determine a statistical property caused by interaction if you neglect the most significant aspect of that interaction, the pair bonding. --140.180.7.220 (talk) 06:57, 7 February 2012 (UTC)
- Go to the NIST website http://webbook.nist.gov/chemistry/form-ser.html, key in, say "H" in the chemical formula filed, click the Search button, and up will come a page headed "Hydrogen atom" and showing an index of what they have. Choose "Gas Phase Thermochemistry Data" from this index - you'll then get a page with a table of key thermodynamic data, and the Shomate coefficients so you can calculate specfic heat etc at any temperature your heat desires, at standard pressure, including low temperatures where H is most unstable. If you prefer, you could also go to your local university's library and look up the NIST-JANAF tables. Or look at Rose & Cooper and similar standard texts. The 4th ed of NIST-JANAF has data at standard pressure for all monatomic gases down to 100K where H is extremely unstable. But none have the triple point, unfortunately. You are quite wrong in your comment about calculating boiling and triple points - you have missed a key part of my original question - but it is somewhat complex to explain - I will see if I can give a simplified explanation in a separate post. Keit121.221.73.82 (talk) 13:55, 7 February 2012 (UTC)
- If the reaction you are interested in takes orders of magnitude longer than the substances involved are stable for, then for all intents and purposes the reaction can't happen. That's why, for instance, you won't find any data about hadrons containing top quarks. At first glance, it makes sense to talk about top quark hadrons, but they would take longer to form than the top quark is stable for. That means they don't actually exist and there is no meaningful data, even theoretical, for them. The same is true for liquid monoatomic hydrogen, oxygen and nitrogen. --Tango (talk) 12:44, 7 February 2012 (UTC)
- "If the reaction ... takes...". I'm certainly aware of that, though I know nowt about quarks. The reactions I am interested in may or may not be noticeably affected by the presence of low concentrations of monatomic H, O, N, and perhaps C. I'm NOT interested in their liquid forms. I'm only interested in their gas forms, i.e., their behavior above the line joining the boiling/sublimation point to their triple point (which is a straight line if plotted on a reciprocal progression temperature scale). To do that I need to know (at least approximately) where these points are. Keit121.221.73.82 (talk) 14:43, 7 February 2012 (UTC)
- I meant "reaction" very generally - I was referring to the condensation/evaporation. I don't understand how you can not be interested in their liquid forms. You asked about their boiling points. Boiling is something liquids do. It doesn't make sense to talk about the boiling point of anything that isn't a liquid. --Tango (talk) 22:26, 7 February 2012 (UTC)
- "If the reaction ... takes...". I'm certainly aware of that, though I know nowt about quarks. The reactions I am interested in may or may not be noticeably affected by the presence of low concentrations of monatomic H, O, N, and perhaps C. I'm NOT interested in their liquid forms. I'm only interested in their gas forms, i.e., their behavior above the line joining the boiling/sublimation point to their triple point (which is a straight line if plotted on a reciprocal progression temperature scale). To do that I need to know (at least approximately) where these points are. Keit121.221.73.82 (talk) 14:43, 7 February 2012 (UTC)
- I've just realised that I have been writing "boiling point" where I should have written "critical point". I don't know why I did that - must be not enough sleep or too many parties. My question may make more sense now. My appologies. But those who understand gas phase reactions could probably have seen thru it anyway. Tango is probably still confused, as he now thinks it only makes sense if I am interested in the state at temperatures below the critical temperature, and pressures above the vapour pressure, which is a liquid. No I'm not interested in the liquid state. To see why, consider a broad analogy: the notional absolute zero temperature of metals. To calculate the electrical resistance of conductors, which is approx proportional to temperature, electrical engineers use a simple formula incorporating absolute zero
R2 = R1 (T2 + T0)/(T1 + T0) . T2 is the temperature of the conductor; T1 is the temperature (usually 20C) at which R1 is the resistance listed in tables.
- This formula assumes that the resistance is a straight line relationship and all metals have zero resistance at notional absolute zero (T0). They commonly use a value of -235C, whereas the thermodynamic absolute zero is -273.15C. They use -235 because it compensates for a number of factors that make the relationship depart from an exact straight line. It DOES NOT mean that electrical engineers are interested in what metals do at -235 or -273C, which mostly is not what this usefull practical formula predicts. They want to know the resistance at operating temperature, usually between 0 and 75C. In a broadly similar manner, I am not interested in liquids. But to work out the behavior of monatomic gasses at "low temperatures" (up to around 900K for oxygen) I would like to know what the NOTIONAL state transition temperatures are. Keit124.182.169.5 (talk) 01:38, 8 February 2012 (UTC)
Relife from pain
This question has been removed. Per the reference desk guidelines, the reference desk is not an appropriate place to request medical, legal or other professional advice, including any kind of medical diagnosis or prognosis, or treatment recommendations. For such advice, please see a qualified professional. If you don't believe this is such a request, please explain what you meant to ask, either here or on the talk page discussion (if a link has been provided). --Jayron32 04:48, 6 February 2012 (UTC)
The Ostrich & Disemboweling
The wikipedia article on the Ostrich mentions that they are capable of disemboweling people. Several other websites repeat this fact but without any citations. Are there any confirmed stories of ostrich disembowelment? --188.220.46.47 (talk) 14:56, 6 February 2012 (UTC)
- They're certainly capable of causing eye injuries to people, but I can't find any evidence of ostrich disembowelment in recent history. That doesn't mean it's not completely possible - cassowaries are apparently especially aggressive and do have sharp rear claws on their feet perfectly capable of disemboweling or other painful injuries. A cassowary did kill a boy in 1926 but no bowels were involved. §everal⇒|Times 17:23, 6 February 2012 (UTC)
- What about other predator disemboweling? --CGPGrey (talk) 17:34, 6 February 2012 (UTC)
- Does a locomotive count as a predator? Ostriches will attack all kinds of things but it's not clear that disembowelment is a frequent result. §everal⇒|Times 18:01, 6 February 2012 (UTC)
- What about other predator disemboweling? --CGPGrey (talk) 17:34, 6 February 2012 (UTC)
- Human disembowelment is a quite specific procedure of which very many animals are no doubt capable should they be so minded, but to categorise specific animals in this way seems not to be particularly useful or encyclopedic. It's not as though they routinely go around disembowelling people.--Shantavira|feed me 19:06, 6 February 2012 (UTC)
- I disagree that it is a "specific procedure." We are not talking about a precise and specific procedure. There are many ways that the abdomen could be cut open and some or all internal organs torn out. Edison (talk) 16:18, 7 February 2012 (UTC)
MAGNETIC GENERATORS - Worth using?
I REALLY don't care all that much about long-winded, techincally-termed details of how these things work, or are claimed to work. I'd just like to know in LAYMAN's TERMS: A. Do they work at all?; and B. Are they capable of substantially reducing electric bills or operating any electrical appliance (110 or 220V) independently with little "Supplied Electricity" [wired power companies] use? Much thanks. I don't trust them to refund ALL my money for their books. — Preceding unsigned comment added by Scizottstheb (talk • contribs) 16:53, 6 February 2012 (UTC)
- Well the first link I read, from "thefreeenergygenerator.org" talks about "perpetual motion" and doesn't explain the source of the energy, except they seem to confuse force with energy, so I suspect that the whole thing is a scam, unless, of course, they are selling a device to stop your meter from recording the real energy used, then they are illegal. The same site advertises running your car on water! Draw your own conclusions. (or read this) Dbfirs 17:24, 6 February 2012 (UTC)
- In layman's terms: no and no. §everal⇒|Times 17:28, 6 February 2012 (UTC)
- This saying comes to mind. Roger (talk) 09:01, 7 February 2012 (UTC)
Should I turn off the LCD before switching off the surge protector?
Thanks. — Preceding unsigned comment added by 66.108.223.179 (talk) 17:19, 6 February 2012 (UTC)
- I assume you mean an LCD computer monitor. If it's Energy Star compliant, the monitor will go into standby mode if not used for a few minutes, so there's no need to turn it off in either place. If the surge protector also powers other things you need to turn off, then it's OK to turn it off there first. I also do this with my non-Energy Star LCD monitor (stupid thing shuts off the screen except leaves the back-light on). Also, if the computer is on the same surge protector, then you do need to turn the computer off using the menu first. StuRat (talk) 19:35, 6 February 2012 (UTC)
Diesel hybrid car
Why don't they make hybrid diesel cars? Is it a technical thing? — Preceding unsigned comment added by 166.205.136.209 (talk) 20:08, 6 February 2012 (UTC)
- Diesel-electric transmission#Production-ready cars -- Finlay McWalterჷTalk 20:15, 6 February 2012 (UTC)
Electric transmission (using electric means to convert engine RPM and torque to road-appropriate speed and torque) should not be confused with Hybrid electric drive (using an electric motor for propulsion to assist an internal combustion engine). Electric transmision dates back at least to the 1920's but has never been popular as the conventional hydro-mechanical automatic transmission does the job just as well and is a lot lighter. Recent developments in better magnetic materials is changing this picture. Re hybrid diesel-electric propulsion, it will happen but there is less incentive than hybrid gasoline-electric because diesel engines are more expensive than gasoline engines, and the main advantage - fuel efficiency - is significantly discounted by the much greater efficiency of diesel engines at larger sizes. A diesel engine, more efficient that a gasoline engine at full throttle, is still more efficient at part thottle than is a gasoline engine. But in a hybrid vehicle, if you don't need high power at certain times during driving, the gasoline engine can be simply shut down. Keit121.221.96.117 (talk) 04:30, 7 February 2012 (UTC)
Any US cars with this feature?
I press a button on the dashboard. Later, I press it again, or another button, and the car tells me how many gallons of gas have been used between the two button presses. No, I don't care about the feature of seeing instantaneous calculated mileage, because slope snapshots are useless (to me) when I'm not constantly staying at the same mileage. 20.137.18.53 (talk) 20:45, 6 February 2012 (UTC)
- Some cars have trip computers, which have a button to start the "trip" and another to end it. However, the ones I've seen report total miles traveled and average MPG over the trip, from which you could calculate gallons of gasoline used, but don't actually report the gallons. StuRat (talk) 20:50, 6 February 2012 (UTC)
- A "gas used" readout was on some of the last years of Pontiacs. The dashboard display could select "gallons of gas" and it would keep adding up fuel as it was used. It could be reset to zero anytime, such as when the tank was filled, or when a trip began. The display could alternately show "miles remaining" "miles per gallon, or "% remaining before oil change needed." Edison (talk) 00:45, 7 February 2012 (UTC)
- Also bear in mind that a car fuel gauge is not a precision instrument.--Shantavira|feed me 10:14, 7 February 2012 (UTC)
- Yes, that's why I ask. :) 20.137.18.53 (talk) 15:35, 7 February 2012 (UTC)
- The General Motors gas used readout worked off the fuel pump, and consistently read about 5% less than what the gas station pump said the car had used, when it was filled each time. The needle on the fuel gauge was far less accurate, dropping slowly as the first few gallons were used, then dropping rapidly at the end. It has a sensor in the gas tank consisting of a float on a swivelling arm, which connects to a potentiometer. I've no idea if the inaccuracy came from a failure to allow for varying relationship between fuel level and arm angle, or the varying relationship between the change in fuel level and the varying cross-section of the fuel tank.With computers in the car's electronics, it would have been trivial to use a lookup correction table to make the dial (or digital display) accurately reflect the fuel remaining. Edison (talk) 16:15, 7 February 2012 (UTC)
- What would be good for what I'm talking about would be a flowmeter on the line that carries the fuel from the fuel pump to the engine, because I want to know how much volume of gas has been consumed between time A and time B. This would not be frustrated by tank fill-ups between button presses. 20.137.18.53 (talk) 17:33, 7 February 2012 (UTC)
Saccharides
What is the most stable form of glucose in the crystaline, solid phase - open chain, furanose, pyranose or some other form? How does the chain length affect the stability of said form compared to the others? Plasmic Physics (talk) 21:52, 6 February 2012 (UTC)
- If you read Glucose you can answer much of your homework question yourself. If you can't find your answer, please look closely in the section titled "cyclic forms", especially the last paragraph. But the entire "Structure and nomenclature" section is good reading as well in this regard. You can also follow bluelinks from that article, especially to articles like furanose and pyranose, to find the other answers to your homework problems. --Jayron32 01:45, 7 February 2012 (UTC)
OK, so aldohexoses exist as the pyranose form in the crystaline solid phase. What about pentoses, heptoses, etc. What about ketoses?
P.S. It is not a homework question. Plasmic Physics (talk) 04:22, 7 February 2012 (UTC)
February 7
How big will sun be at first RGB in 5 billion years?
2008 studies show Earth will be swallow up because of the tidal interaction. Without considering loss of sun's mass/gravity to make it expand orbit, how big will sun be at first RGB in 5 billion years? is it roughly size of earth orbit little less than 1 AU or greatly than 1 AU. is it less than 200 solar radius or is is 250 solar radius? Does sun shrink between first and second RGB? My astronomy teacher display sun shrinks after first RGB then expands again at second RGB? How much will sun shrink between first and second RGB? about the size today or less than sun's size today. Becuase second RGB is 1.2 AU, or 260 solar radius. The article didn't mention the first RGB.--69.229.39.25 (talk) 00:37, 7 February 2012 (UTC)
Life form on Europa and Titan few billion years back in the history
I thought some scientist thought Europa was once filled with globes of liquid oceans and Titan might been habitatable at one time.[1] I wonder how they do it. Five billion years back in the history was sun bigger and brighter than now or was it smaller and fainter than now (I never paid attention in class, I just daydreamed). Europa and Titan is too small, I am surprised to hear Europa can handle off an atmosphere. How could Mars be blue and wet 4 billion years ago. Was sun bigger back then or was it smaller. Mars had alot of atmosphere back then was sun smaller, or was it bigger. Why did Mars lose its atmosphere?--69.229.39.25 (talk) 01:49, 7 February 2012 (UTC)
- 5 billion years ago the Sun did not even exist yet, let alone Jupiter and its moons.
- The presence or absence of an atmosphere has little to do with the size or mass of the body. Uranus is 63 times more voluminous than Earth, yet it has almost no atmosphere. Titan is 1/15th the size of Earth, yet its atmosphere is actually denser than Earth's. The critical factor is whether or not there is a magnetosphere to protect the atmosphere from solar wind.
- Atmosphere_of_Mars lists a few possible causes for Mar's current thin atmosphere.99.245.35.136 (talk) 02:47, 7 February 2012 (UTC)
- Venus has no magnetosphere, it can still hold atmosphere. I don't know if Titan and Pluto even have magnetosphere. When Pluto is closer to sun it actually have more magnetosphere, that is weird. Yes, Uranus has atmosphere.--69.229.39.25 (talk) 05:10, 7 February 2012 (UTC)
- Uranus has no atmosphere? It's a gas giant. All of it is atmosphere. --140.180.7.220 (talk) 05:16, 7 February 2012 (UTC)
- I don't think magnetosphere may entirely protect the atmosphere. Venus has no magnetic field, its atmosphere is 90xs stronger than Earth. Titan's gravity is like one-seventh that of Earth compare to moon which is one-sixth of earth. I thought the discussion is gravity holds the atmosphere, I forgot we talked about it before. I thought all the sources I found on Europa is reliable, could be the author is just biased about something, sometimes I am just not careful, they may just privode speculations to trick dummies.--69.229.39.25 (talk) 06:49, 7 February 2012 (UTC)
- The early sun was fainter, about 70% of modern. See faint young sun paradox. The presence or absence of an atmosphere is influenced by several factors: the mass of the planet/moon, the distance from the sun (affecting both surface temperature and solar wind), the composition of the atmosphere (heavier gases are more persistent than light gases), and the presence or absence of a magnetosphere. See atmospheric escape. Both Mars and Earth probably had a thicker atmosphere in the early solar system, but the Earth is much better at holding on to it due to its higher mass, so most of the Earth's atmosphere is still here while most of Mars' early atmosphere has been lost. Dragons flight (talk) 08:15, 7 February 2012 (UTC)
Special relativity
Does anyone know a good easy (but rigorous) introduction to special relativity? Money is tight (talk) 03:26, 7 February 2012 (UTC)
- Tipler's Modern Physics pulls no punches, but only covers elementary relativity. On the other hand, it does so in an application-centric way, rather than a purely theoretical derivation from first principles; so it's immediately useful to the experimentalist or engineer (as much as any relativity knowledge can be useful to the applied sciences). It reads at a level suitable for an advanced freshman or sophomore (university-level) physics student. It also covers many other topics besides relativity. Jackson's Electrodynamics also has a chapter where he derives Lorentz transforms, but it's more suitable for the advanced physics student. Personally, I think the best way to "rigorously" learn special relativity is to "rigorously" learn classical electrodynamics; after this foundation, special relativity is the obvious consequence. Relativistic gravitation, on the other hand, is a whole different subject (...that is, not special relativity); there's not a lot you can do to prepare for it, but it does help to have an advanced background in the analytic mathematical tools of classical physics. Nimur (talk) 05:11, 7 February 2012 (UTC)
- We do have an article Introduction to special relativity here. Graeme Bartlett (talk) 12:10, 7 February 2012 (UTC)
I learned it when I was in high school from Lillian Lieber's The Einstein Theory of Relativity, which I still think is wonderful if you can find a copy. Looie496 (talk) 18:49, 7 February 2012 (UTC)
- I was pretty impressed by Ray d'Inverno's Introducing Einstein's Relativity, although I didn't go far with it, since I don't know anything about Maxwell's equations. It seemed fairly rigorous, although it started with easier introductions (the initial proof of the Lorentz transformations was somewhat simple, then it covered the orthodox method, afaicr). Also the Schaum's guide, but that one leaves you to do most of the work (proving E=mc^2 is one of the exercises). IBE (talk) 22:05, 7 February 2012 (UTC)
Blue star-like object
On a walk tonight, I saw a "star" in the western sky; given that it was brighter than anything in Orion, visible behind me, given that it was visible at all even though it was the Las Vegas sky and slightly overcast in that direction, I'm guessing it was a planet. (I don't think it was Sirus, as it was opposite Orion in the sky.) My question is that I could see a blue light from the top of it. It wavered in and out, disappearing for a few minutes, and was never clearly distinguishable from the star, but it was visible for most of an hour. It and the star were stable in position for that hour, so it wasn't a plane or anything of the like. It was maybe 30 degrees up in the sky, well above the mountains, so it wasn't a land based light. Am I correct in assuming it was some sort of chromatic aberration of the atmosphere or something? That's the conclusion I'm left with. (Yes, I thought UFO; besides all the other problems with that conclusion, it was stable, and you'd think a bright flying object a few miles from Nellis would get intercepted in the time I watched it. Unless the Air Force had someone hover in place for over an hour... but I don't regard that as a serious possibility.)--24.120.231.24 (talk) 03:38, 7 February 2012 (UTC)
- Um, Venus? In tonight's Las Vegas sky, Venus would be to your west, pretty near the horizon, between Pisces and Aquarius. Someguy1221 (talk) 04:33, 7 February 2012 (UTC)
- Venus, Jupiter, and Mars are all visible in the night sky in the Northern Hemisphere right now, and all are as bright or brighter than Sirius (the bright star that Orion's Belt points at, and the brightest star in the sky). Planets are pretty easy to spot if you can find the ecliptic, which is roughly the plane of the solar system, and corresponds to the path the sun seems to follow as it goes through the sky. At night, the moon and planets also lie roughly along the ecliptic, so they're easy to spot, especially if you are spotting one of the three planets brighter than Sirius; Venus is currently quite bright, and obviously the brightest thing after the moon. --Jayron32 05:23, 7 February 2012 (UTC)
- Okay, thanks; but I wasn't really asking about the object itself. It seemed to be white, but the top of it sparkled with a bright blue light. That's why I thought it was an airplane at first glance, because that blue light isn't part of the natural hue of the night sky and it was so close to the other light (presumably Venus). I'm guessing the blue light was about a 2 magnitude by itself.--Prosfilaes (talk) 05:49, 7 February 2012 (UTC)
- Hot air balloon \ or weather balloon? \ Chinese lantern? SkyMachine (++) 07:11, 7 February 2012 (UTC)
- No, it was almost certainly Venus. The real question is why the top edge looked blue. Did the bottom edge look red? If so, that's a sign of chromatic aberration of your glasses and/or eyes. --140.180.7.220 (talk) 07:25, 7 February 2012 (UTC)
- I didn't notice a red bottom edge, though I wasn't looking. My glasses are pretty thick high-index plastic. I tried looking at Venus from different angles, and didn't notice the change in effects I usually see in chromatic aberration. It doesn't thrill me as an answer, but it does seem like the best fit.--Prosfilaes (talk) 13:11, 7 February 2012 (UTC)
Would a "warranty cutoff switch" exist?
You have heard/read of dealer cutoff switches - placed on cars purchased by customers with bad credit so that the dealer (or one in charge of the lien) can disable the car if the customer falls behind on payments (or start making annoying beeping sounds, disable the radio, etc. to entice the delinquent to pay up). Natch, they are triggered remotely, or even on a preset timer.
So, could "warranty cutoff switches" be placed in expensive equipment so that shortly after the warranty runs out, the timer running out causes the "switch" to foul up a critical component that is expensive to replace. (Perhaps the timer would choose a random time to toggle the switch in order to not create too many coincidences, but still take place shortly after the warranty ends.)
Why haven't I heard of such a switch? Wouldn't various manufacturers stand to make a killing on either expensive replacement parts, or just an upgrade to a newer product? I thought planned obsolescence would perhaps involve it, wouldn't you?
So on what products would there be "warranty cutoff switches" and yet, why haven't I heard of them on news and consumer reports? --70.179.174.101 (talk) 07:57, 7 February 2012 (UTC)
- "Why haven't I heard of such a switch"? Two possibilities: (A) they don't exist. Or (B) they don't want you to hear about them. Why do you think that we'd be able to say which alternative was the right one? AndyTheGrump (talk) 08:01, 7 February 2012 (UTC)
- You may be interested in Planned obsolescence 157.193.175.207 (talk) 08:23, 7 February 2012 (UTC)
- If this wasn't disclosed, and you own the item outright, I believe they could be charged with a crime. After all, they are intentionally damaging your property. This would be "willful destruction of property", would it not ? This assumes that you own the item, versus the car which you don't own until you complete payments. And, of course, once it got out that they did such a thing, nobody would ever buy from them again. StuRat (talk) 08:42, 7 February 2012 (UTC)
- It would be almost impossible to keep such a device secret long-term from the flapping lips of disgruntled workers, ex-employees, probing repairmen and techies, and the like. The manufacturer responsible would also gain a reputation for unreliability and poor quality if their "expensive equipment" repeatedly failed just after the warranty expired. Combine that progressive loss of face with their shattered reputation once they were inevitably outed, and why would any manufacturer with anything but the shortest possible timeframe in mind ever consider doing such a thing? As Stu says, no one would ever buy from them again, and their business would quickly fail through market forces, if not through government intervention. --jjron (talk) 10:01, 7 February 2012 (UTC)
- Are you sure you don't own the car until you complete the payments? If you have a loan secured to something, you still own it unless you default on the payments. Are car loans different from normal secured loans? --Tango (talk) 13:20, 7 February 2012 (UTC)
- The Quicken program stops working after a certain number of months, in that it will no longer download any data from banks or credit cards. You could, in principle, manually enter every transaction, but downloading is a major part of the functionality.This brute force way of getting you to buy the newer version is not present on most software, although vendors have historically cut off any tech support of old software, and have not provided free updates so the software works with newer operating systems. If a device has an internal clock, it is possible for it to shut down automatically after some "lifetime." This is common in carbon monoxide detectors,with a 5 year sensor life, as described in the instructions for this model: "Sensor Life Monitor: Internal clock starts once lithium battery is activated. Visual and audible signals notify you when sensor life has expired. " Edison (talk) 16:06, 7 February 2012 (UTC)
- Right, and there they have a justification for the action and notify the consumer, making it all legal. Another example is a toothbrush with bristles designed to dissolve over time, supposedly to let you know when it's germy and needs to be replaced. This is a rather iffy justification, as, of course, any toothbrush can be sterilized by soaking it in bleach or by several other methods. StuRat (talk) 20:30, 7 February 2012 (UTC)
- I don't know where you got that "germy" business from: a toothbrush's germ load will reach a steady-state level a few days after the first time you put it in your mouth. The reason to replace a toothbrush every few months is mechanical wear causing the bristles to lose their scrubbing power. --Carnildo (talk) 02:09, 8 February 2012 (UTC)
- I've seen ads specifically claiming the reason to replace a toothbrush frequently is "germs". The bristles seem capable of lasting for years, provided they aren't designed to wear out quickly. StuRat (talk) 03:09, 8 February 2012 (UTC)
- I'm no lawyer, but it seems to me there'd be some serious liability issues if the device were to be disabled at a particularly bad time, e.g. resulting in injury or death. Clarityfiend (talk) 21:57, 7 February 2012 (UTC)
science.chemistry
does ink and salt dissolve at the same rate and different temperature — Preceding unsigned comment added by 119.235.88.180 (talk) 11:20, 7 February 2012 (UTC)
- no. Ink has much larger molecules and may even be a suspension. Graeme Bartlett (talk) 12:22, 7 February 2012 (UTC)
- And ink molecules may be soluble in liquids in which salt is not soluble. But I think we've done enough of what sounds like your science homework for now. DMacks (talk) 15:25, 7 February 2012 (UTC)
- Did the OP mean to ask the same rate at different temperatures? This seems plausible to me, unless there's some sort of cap or floor on rate of dissolution. 216.197.66.61 (talk) 23:17, 7 February 2012 (UTC)
lines of field
I wanted to derive the equation (like, in Cartesian coordinate system) for electric field lines (for dipoles)and it turned out to be huge and not elegant at all. I just wanted to check if there's any online source to check it, or anybody here who knows the answer...-Irrational number (talk) 15:43, 7 February 2012 (UTC)
- ...please?--Irrational number (talk) 18:09, 7 February 2012 (UTC)
- Does Dipole#Field from an electric dipole help? I'm pretty sure the field around a dipole is never going to look elegant in any conventional co-ordinate system since it has an annoying asymmetry in it - this is why for some applications it's best to use [dipole co-ordinates]. Smurrayinchester 18:27, 7 February 2012 (UTC)
- My main reason to look for that was to be able to draw the lines of field for a dipole in "graph"(the program I mean), which was not successful... I had tried p orbital cross section before, so I guess I was underestimating the dipole?Is there a way for me to do that?--Irrational number (talk) 03:17, 8 February 2012 (UTC)
- Does Dipole#Field from an electric dipole help? I'm pretty sure the field around a dipole is never going to look elegant in any conventional co-ordinate system since it has an annoying asymmetry in it - this is why for some applications it's best to use [dipole co-ordinates]. Smurrayinchester 18:27, 7 February 2012 (UTC)
Hawking radiation and Black hole
Having re-studied your exhaustive articles on "Black hole" and especially the segment on evaporation, cross checking the article on "Hawking radiation", having studied both the DVD on "Hawking's Universe" as well as the corresponding book, especially his "The Universe in a Nutshell", and having studied Kip Thorne's "Black Holes & Time Warps", I have noted a possible discrepancy between the sources and the published articles in Wikipedia: Stephen Hawking himself noted, especially in his book "The Universe in a Nutshell" that the discussed radiation just LOOKS as if the black hole would emit that radiation, but that this is just a picture where the mathematics happen to describe this specific behaviour of virtual particles near an event horizon, seemingly being radiated away by the black hole.
However, in physical reality, the black hole behaves "as usual" (or remaining "hairless"), especially not emitting any kind of radiation (or virtual particle). The consequence would be that a black hole would never evaporate, at least not via this kind of process.
My suggestion would be to expand your publications along those lines, provided you concur with the a.m findings. 87.184.34.215 (talk) 18:00, 7 February 2012 (UTC) edited for correction of main source 80.132.241.226 (talk) 20:14, 7 February 2012 (UTC)
- I am not aware of any currently-available observational techniques that could detect Hawking radiation, so whether any theory predicts its existence is moot: it's below the noise-floor of what we can currently measure using 2012 equipment. On the other hand, we can measure all sorts of other effects of black holes (rather, relativistically supermassive objects): among these, we have pulsar radiation due to accretion; we have several observational signatures associated with active galactic nuclei (usually postulated to be related to relativistic effects of the massive nucleus), and of course, we have gravitational lensing. In fact, the lack of direct observationm of Hawking radiation is still consistent with theoretical predictions: the theory predicts it should be a magnitude much smaller than these other effects. What this means: every time astronomers look at a distant active galactic nucleus or other massive deep-sky object - and don't see Hawking radiation - they are confirming that our theoretical understanding of general relativity is pretty good. Nimur (talk) 20:12, 7 February 2012 (UTC)
- It does not really matter if the Hawking radiation comes from "inside" the black hole, or from "just outside". There are two alternative "simplified to my level" explanations for it - the "virtual particle" explanation, and the "uncertain speed of light" explanation. The fist one depends on virtual particle pairs, one escaping, and one dropping into the black hole. It's hard to understand how that would lead to evaporation, but it does. The reason is that the particle that drops into the hole has a negative total energy (i.e. the negative potential energy it has from materializing just inside the Schwarzschild radius is larger than its positive rest mass/energy). So, by falling into the black hole, it decreases the energy (and, amazingly, by just the energy of the escaping particle, so that the conservation of mass/energy is maintained). The second explanation is that particles in the black hole have to follow the uncertainty principle. Since their location is constrained by the Schwarzschild radius, their impulse has to be uncertain. But stuff falling into a black hole will travel (in the limit) at the speed of light. So, by applying the uncertainty principle, some of the particles will have a slightly higher speed, and can escape. Unsimplifying this to Hawing's or Thorne's level requires a good approximation of the TOE ;-) --Stephan Schulz (talk) 20:42, 7 February 2012 (UTC)
It may be the other way around, i.e. that real black holes don't exist. Count Iblis (talk) 23:41, 7 February 2012 (UTC)
Clenbuterol, doping or food poisoning
If someone {like Alberto Contador eat food contaminated with Clenbuterol, would this Clenbuterol show up in a blood test? 212.170.181.95 (talk) 20:46, 7 February 2012 (UTC)
- Clenbuterol#Use as performance-enhancing drug mentions people testing positive in blood tests in that way, so I guess the answer is "yes". --Tango (talk) 22:36, 7 February 2012 (UTC)
- Perhaps the OP meant to ask, could such excuses be true? After all, they only allege their food had been poisoned. 216.197.66.61 (talk) 23:16, 7 February 2012 (UTC)
- The report on Contador did say that it could be true in theory, but they discounted this explanation because clenbuterol contaminated meat is rarely found in Europe. Count Iblis (talk) 23:34, 7 February 2012 (UTC)
- Perhaps the OP meant to ask, could such excuses be true? After all, they only allege their food had been poisoned. 216.197.66.61 (talk) 23:16, 7 February 2012 (UTC)
- What I thought about this case is that Contador indeed used Clenbuterol, however, he tried to cover it/eliminate it with another substance, which didn't work perfectly, therefore, thorough testing could still find some traces of it. 88.8.79.238 (talk) 23:50, 7 February 2012 (UTC)
does gelatin formation in chicken soup start during simmering?
Is there a way to speed it up? Or does it really have to incubate overnight in the fridge to taste better? 216.197.66.61 (talk) 23:05, 7 February 2012 (UTC)
- Gelatin forms when animal connective tissue (collagen and stuff like that) is heated to near boiling in a water solution. Gelatin takes time to form and disolve into the water, so it definately has to simmer for a while. The gelatin also does have to cool to form proper cross-linkages that give that unctuous mouthfeel associated with gelatin; that's why soups and stews and chilis always taste better after being refrigerated overnight and then reheated; the solution has to cool and rest for a while so the gelatin can "set up". Unfortunately, it's not a step that can be rushed or skipped, if gelatin formation is what you are going for. --Jayron32 06:00, 8 February 2012 (UTC)
Not eating before surgery
A family member of mine recently underwent a fairly modest surgery. He was asked not to eat for about a day before the surgery and wasn't fed in the hospital for that period. I've never understood why this is. Surely you want people as strong as possible for surgery, not weakened by a short fast? Thanks, 130.88.172.34 (talk) 23:42, 7 February 2012 (UTC)
- Under general anaesthesia a patient can vomit (or just leak) stomach contents, which they may then breathe (ref). -- Finlay McWalterჷTalk 23:47, 7 February 2012 (UTC)
That clears that up, thanks. 130.88.172.34 (talk) 23:48, 7 February 2012 (UTC)
- Relevant Wikipedia articles: Preoperative fasting, nil per os,pulmonary aspiration, aspiration pneumonia. --Carnildo (talk) 02:15, 8 February 2012 (UTC)
- It is a problem though, especially with diabetics, who really shouldn't fast. StuRat (talk) 02:49, 8 February 2012 (UTC)
- I believe that diabetics have the option of taking glucose tablets to maintain blood sugar while keeping their stomach contents relatively empty. Doctors should know of diabetes and be able to advise on what diabetics can do before going under general anesthesia. --Jayron32 05:58, 8 February 2012 (UTC)
February 8
Embryological Skeleto/muscular development
Hello. I've been looking to find out information on whether in a developing human embryo, does the skeleton/bones or the muscle develop first? My understanding is that both are produced simultaneously through differentiation of the mesoderm, but I have surprisingly been unable to find much on this. Any references (preferably online) that would shed light on this matter would be greatly appreciated. 114.77.39.141 (talk) 03:45, 8 February 2012 (UTC)
Brownian motion as perpetual motion
Could Brownian motion be actually considered the perpetual motion in a closed system?--46.204.55.76 (talk) 05:37, 8 February 2012 (UTC)
- If you do, you might as well include planetary orbits, too. StuRat (talk) 05:41, 8 February 2012 (UTC)
- When it comes to energy, there ain't no such thing as a free lunch. See Maxwell's demon and Brownian ratchet. Red Act (talk) 05:46, 8 February 2012 (UTC)
- To make things a bit simpler: Perpetual motion is not an impossibility; there's lots of things in the universe which are essentially in perpetual motion; the molecules in the air around you are in perpetual motion. However you cannot use such motion to do work; any attempt to use a system in perpetual motion to do work will result in the system "winding down", and losing energy until it reaches some state of equilibrium and becomes unable to do more work. --Jayron32 05:50, 8 February 2012 (UTC)
Sun leaving main sequence between 1 and 5 billion years from now
How much bigger suppose the sun to get between 1 and 5 billion years from now. Our article said 10% luminosity in 1.1 Gyr, 3.5 Gyr is 40%. What is 10%, 20% mean by growing luminosity. Does sun get 10 times larger in size, every billion years, 20 times larger in size every billion years. Is sun's RGB 250xs current radius 5000 times more luminosity it means 5000 times brighter, or 2000 times brighter. If 5000 times brighter means habitable zone surface temperature everywhere in our solar system is too hot,even 100 AUs away. 2000 times brighter is habitable zone at Saturn system, Neptune system, or Pluto system? I am all messed up confused right now.--69.229.39.25 (talk) 06:06, 8 February 2012 (UTC)








