Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
April 10
Unknown drug powder ID
How can I find out what a packet of unknown drug is made of? I'm not sure it's one drug or several, in powder form. It is extremely unlikely to be an illegal drug. I'm more concerned it is a normal prescription drug. Is the Unknown Substance test here [1] the kind of test I should be looking for? Would a doctor or hospital have access to such a service? How would I find a cheap one? How much would it cost? Thanks. 66.108.223.179 (talk) 00:23, 10 April 2012 (UTC)
- Not directly addressing your question: It is of course your own business what you do with this mysterious substance (unless you manage to break any laws by possessing or using it, and are caught), but (and I am not suggesting that this is your intention) it is generally considered extremely unwise to use pharmaceutical drugs (other than "over-the-counter" ones) unless they have been specifically prescribed to treat a condition one currently has, have been kept in the correct conditions where applicable, and are within their "use-by" date (after which they may lose efficacy), which from what you have said doesn't apply. It's also unwise to keep unused and unneeded drugs around (even if their identity is known) in case someone accidentally or through ignorance (a child, an aged adult with dementia) uses them (though again this may not be applicable in your situation), so the prudent course would be to hand it in to the nearest Pharmacist, Doctor's Surgery or Hospital Outpatients Department for safe disposal (tipping it down the drain may have harmful effect on the environment and may well be illegal), which is a routine duty of theirs (in the UK, at any rate). I used to be employed in the pharmaceutical industry (in a minor administrative role) so I'm sensitised to such matters.
- It's possible that further circumstances you haven't disclosed may make your retention and investigation of this presumed drug, perhaps purely to satisfy your intellectual curiosity, a reasonable course, but I can't tell that from what you've said. {The poster formerly known as 87.81.230.195} 90.197.66.44 (talk) 02:57, 10 April 2012 (UTC)
- In the UK you are supposed to take such items to a pharmacist who will dispose of them for you. There are public analysts who will, for a fee, undertake the work you are thinking of, but I'm not sure what they will do if they identify that it is an illegal or controlled substance such as diamorphine. --TammyMoet (talk) 09:34, 10 April 2012 (UTC)
it sounds to me from "I'm more concerned it is a normal prescription drug" that this could be someone "concerned" about this. Whether a teacher or family member, etc, it seems the poster has good reason to say it's "highly unlikely" to be an illegal drug; meaning they know the person or situation in which the bag was found and what the person or people who could be associated with that are likely and unlikely to have access to. It sounds like the worry/concern is prescription drug abuse. 134.255.115.229 (talk) 10:10, 10 April 2012 (UTC)
- I'm not sure what the best option is for you economically for the test, but if you have a significant quantity of the stuff, you could rule out some silly things right away. To do that, try dissolving a trace of it in water, alcohol, acetone, toluene ... whatever chemicals come easily to hand, with one being quite hydrophobic. (Be careful about potential fumes or fires during mixing, if, say, it turns out to be an oxidizer for homemade fireworks!) If the stuff won't dissolve in anything then it might be something like talc/clay/etc. rather than something to worry about. If the stuff has a really, really strong color, even when you add more of your solvent to a drop, then it's more likely (not guaranteed though) to be an art supply. You can also try burning a trace of it in a flame (well ventilated area...) to see if it is flammable; if not, it might be, say, borax to kill roaches. Most modern drugs are carbon-based and would catch on fire. If it gives intense color in a flame (flame test) this might tell you more about it - lithium salts would be red, sodium yellow and so forth. Detergent powder (sodium lauryl sulfate) also has an intense awful smell. Maybe after a few ultra low tech tests like this you'll feel more comfortable to just come out and ask what the stuff is, and find out if it's nothing. Wnt (talk) 16:53, 10 April 2012 (UTC)
- Without an idea of what it might be, such a commercial service is likely your only chance. Checking whether it is a specific substance is much easier. Solubility in different solvents, shape of crystals formed, color etc can give indications, purifying of the active ingredient is done by dissolving, filtering and recrystallising; combined with a melting point measurement would give reasonable certainty. If you're thinking about prescription drugs with abuse potential, it depends on you're experience with and knowledge of drugs and/or chemistry, really. Pharmacies may sell test kits, can't think of a colour test with home chemicals but solubility, crystallisation and melting point usually get the job done. Ssscienccce (talk) 14:11, 12 April 2012 (UTC)
To clarify the question: The substance is unlikely to be one that would be abused, although obviously I wouldn't rule it out until I know what it is. I got it from a Chinese medicine man. It has significantly alleviated a sinus problem that doctors have been unable to diagnose, let alone solve. I've been looking at commerical services but they focus on abuse substances and I don't know how to find one that'd consider all possibilities. 66.108.223.179 (talk) 14:56, 12 April 2012 (UTC)
- In that case, I would simply ask my doctor to have it analysed. But that's in my situation in belgium. Your IP is somewhere in NY, I don't know what doctors can and cannot do over there... Ssscienccce (talk) 22:17, 12 April 2012 (UTC)
- In this case the substance is probably organic and you're just wondering what species are involved. Start, of course, by getting the name of the formulation and any ingredients listed - Chinese herbal medicines are specific mixtures sold in international trade. It might not be what they say, but they may not know that... see [2] Supposedly for $35 you can do this, but that's not a retail price. Wnt (talk) 23:45, 13 April 2012 (UTC)
- I bet the labor doesn't come cheap. I doubt it's herbal medicine because it's been so effective and started working so quickly. 66.108.223.179 (talk) 01:14, 14 April 2012 (UTC)
- Mass spectrometry and related techniques can identify most chemical substances, Most tests are automated, and for a pure substance, little work would be needed. You could contact one of the commercial services, ask about the possibilities. Interpreting the results or deciding what tests to run requires some knowledge, more than just how to insert a sample and mail the results... Ssscienccce (talk) 04:46, 14 April 2012 (UTC)
- I bet the labor doesn't come cheap. I doubt it's herbal medicine because it's been so effective and started working so quickly. 66.108.223.179 (talk) 01:14, 14 April 2012 (UTC)
- In this case the substance is probably organic and you're just wondering what species are involved. Start, of course, by getting the name of the formulation and any ingredients listed - Chinese herbal medicines are specific mixtures sold in international trade. It might not be what they say, but they may not know that... see [2] Supposedly for $35 you can do this, but that's not a retail price. Wnt (talk) 23:45, 13 April 2012 (UTC)
postcognition
what is the minimum time between when something occurs and when we can react to it? (meaning behave in a way that is statistically differentiable from if it did not occur, based only on normal sensory input and our behavior to it). subconscious reactions OK. 188.157.112.202 (talk) 00:30, 10 April 2012 (UTC)
- The human reaction time depends on the stimulus and activity performed. See the article for a variety of different measured values. --Mr.98 (talk) 01:01, 10 April 2012 (UTC)
Could a satellite really pack the energy to knock out the entire US's power grid?
See from 1:35 on this game trailer, a satellite knocking out the entire grid for the US.
After, in the Homefront reality, Korea reunifies under the Northern regime, annexes several countries on the Pacific Rim, and our infrastructure / society already deteriorates due to massive national debt and hyperinflation, we get our power knocked out by a single satellite.
(Then we get invaded by their forces up to the western side of the Mississippi River.)
Is our power grid really not that robust? How much energy must be packed in an EMP blast to take out our power from sea to shining sea?
Moreover, what safeguards can we put on our electrical grid today so that we do not go dark from an EMP attack in the future? Thanks. --Tergigress (talk) 06:14, 10 April 2012 (UTC)
- I would expect that multiple nuclear detonations in the ionosphere would be needed. The best defense is probably just to bury all our electrical lines, as shielding them all would be prohibitively expensive. (Burying also has other advantages, like protecting them from weather.) Individual buildings might still be vulnerable, but the surge typically needs to build up over a long length of exposed wire to cause damage. Surge protectors would help, too. StuRat (talk) 06:19, 10 April 2012 (UTC)
- (edit conflict)As far as safeguards go, there is the Farraday cage, which is basically an enclosing mesh or box made of conducting metals. It eliminates any danger from EM pulses, though I suspect it would be difficult (and very, very costly) to implement such a protection on the entire United States power grid. But I would say that the chances of a solar flare-induced EMP causing problems with our electrics is far greater than any hostile attack, seeing as how the GBMD exists and stuff. I actually heard once that a single detonation could wipe on US-based electrics if it went off fairly high up above Kansas. As far as the North Koreans go, the worst they could do (assuming they were suicidal) with their current missile technology is, with luck, cause a
rollingblack-out or two on the west coast, best-case scenario being as far east as Las Vegas (I would guess). Burying power lines would certainly help, and I've often wondered why this isn't standard practice like it is for water infrastructure. Evanh2008 (talk) (contribs) 06:32, 10 April 2012 (UTC)
- (edit conflict)As far as safeguards go, there is the Farraday cage, which is basically an enclosing mesh or box made of conducting metals. It eliminates any danger from EM pulses, though I suspect it would be difficult (and very, very costly) to implement such a protection on the entire United States power grid. But I would say that the chances of a solar flare-induced EMP causing problems with our electrics is far greater than any hostile attack, seeing as how the GBMD exists and stuff. I actually heard once that a single detonation could wipe on US-based electrics if it went off fairly high up above Kansas. As far as the North Koreans go, the worst they could do (assuming they were suicidal) with their current missile technology is, with luck, cause a
- Shallow burial offers little protection against EMP (except perhaps if you are burying the cables inside some other conductor like a metal pipe). During Soviet era tests, buried power lines exposed to EMP also experienced severe surges. Dragons flight (talk) 16:42, 10 April 2012 (UTC)
For the reasons why most high tension electrical infrastructure is not underground, see Underground transmission — Preceding unsigned comment added by 112.215.36.182 (talk) 08:24, 10 April 2012 (UTC)
- I get that there would extra costs associated with it, but it just seems like those costs would be made up for eventually by not having to replace lines every time a major storm blows through. I dunno. Evanh2008 (talk) (contribs) 08:27, 10 April 2012 (UTC)
- Underground cables can be more expensive to maintain than above-ground lines because you have to dig them up to investigate faults. --Colapeninsula (talk) 08:43, 10 April 2012 (UTC)
- In areas with a risk of salt-water flooding and areas with frequent freeze-thaw cycles, it is typically much more expensive to keep your lines underground because these are factors which can damage underground lines, and as mentioned above there is an extra cost (not to mention extra repair time) when they must be maintained and/or replaced. Also in areas with dense soil such as clay the initial installation cost goes up even further. -RunningOnBrains(talk) 16:35, 10 April 2012 (UTC)
- I think the main disadvantage is that underground lines cannot dissipate heat as effectively as overhead line which get significant air cooling. Hot wires have much greater resistance. 112.215.36.178 (talk) 04:16, 11 April 2012 (UTC)
- In areas with a risk of salt-water flooding and areas with frequent freeze-thaw cycles, it is typically much more expensive to keep your lines underground because these are factors which can damage underground lines, and as mentioned above there is an extra cost (not to mention extra repair time) when they must be maintained and/or replaced. Also in areas with dense soil such as clay the initial installation cost goes up even further. -RunningOnBrains(talk) 16:35, 10 April 2012 (UTC)
- Underground cables can be more expensive to maintain than above-ground lines because you have to dig them up to investigate faults. --Colapeninsula (talk) 08:43, 10 April 2012 (UTC)
- For a long discussion of the effects of an EMP and the fact that our civil infrastructure is largely unprotected from it, see, if you have not already, Electromagnetic pulse. Depending on the size of your weapon and the height at which you detonate it, assuming you can put it where you want, you could do quite a lot of damage to the civilian infrastructure. Whether that is worth spending billions to defend against depends on how likely you think that sort of attack would be and how risky it is. Most non-alarmist commentators think it is a pretty unlikely thing, given that setting off a nuke above the United States in such a way — even firing a nuke on such a trajectory — would be a recipe for massive retaliation, and it would not lessen our ability to retaliate (in part because military technology is shielded from EMP, but also because a lot of our nuclear and conventional forces are not located in the continental United States). --Mr.98 (talk) 19:45, 10 April 2012 (UTC)
- Some general responses to comments: Over a span of years, the installation and maintenance costs for an underground transmission or distribution line far exceed those for overhead. Underground high voltage transmission lines are typically in steel pipes, and buried quite a ways below ground, while distribution lines are typically closer to the surface, often installed by "plowing" them in. The steel pipe and deep burial of transmission lines provide some protection against lightning strikes, if not EMP from nukes. The underground transmission pops up out of the ground every few miles for substations or forced cooling pumping plants, and an EMP could fault them there. A gas insulated substation would provide more lightining/EMP protection than an older style substation with outdoor oil circuit breakers, but an EMP would likely still take out the relay protection and communication equipment required for the line to be in service. Such a line is far more expensive per unit length than overhead. If the underground line insulation fails, it is an expensive and time consuming task to locate the fault and repair it, compared to overhead. Air and porcelein insulators are the insulation of overhead lines. The insulation of an underground transmission or distribution line would fail long before the increased resistance from the heated copper or aluminum became a concern. Underground distribution is often struck by lightning, because it is close to the surface and rarely in steel pipe. A pinhole flaw is all it takes to render an underground line faulted and in need of repair. Edison (talk) 19:08, 12 April 2012 (UTC)
- I disagree that the lines would have to get hot enough for the insulation to fail before the increase in resistance would be a concern. When you're transmitting power across hundreds of kilometers, it won't take a very great change in temperature to make a concerning loss of efficiency. Because of the difficulty cooling the lines underground, there will be stronger positive electrothermal feedback. In copper wiring, a 10°C increase in temperature causes a 3.9% increase in power loss due to resistance. Over the useful life of the transmission line, that will be a significant disadvantage compared to air cooled, overhead lines. 203.27.72.5 (talk) 22:36, 13 April 2012 (UTC)
- But that must be compared with ground temperatures averaging around 60F, and varying little down below the frost line, while above ground temperatures vary dramatically, with many days above 100F in many locations. StuRat (talk) 23:28, 13 April 2012 (UTC)
- Well that depends on where the transmission line is located. My point was that the change in resistance due to the reduced ability to radiate heat is an important consideration (probably more so in an environment like the one where I live; a tropical archipelago of high islands). 203.27.72.5 (talk) 00:21, 14 April 2012 (UTC)
Sexual vs. asexual reproduction vis-a-vis early evolution
Just a thought that I had regarding the theory of evolution and hypothetical applications to theories regarding the origin of life: Are there any theories among biologists as to at what point (and why) sexual reproduction would have come about in the proverbial "primordial ooze"? In other words, what evolutionary pressures would have caused individuals who developed sexual reproduction to become a dominant faction of life? What are the actual benefits of sexual reproduction as opposed to asexual? In addition, in another world, would there have been any fundamental law of science to prevent asexually-reproducing multi-celled organisms from becoming dominant? Thanks! Evanh2008 (talk) (contribs) 08:25, 10 April 2012 (UTC)
- The article Evolution of sexual reproduction discusses this. --Colapeninsula (talk) 08:44, 10 April 2012 (UTC)
- Indeed it does! I should have looked a little harder before posting this question. Many thanks! :) Evanh2008 (talk) (contribs) 08:47, 10 April 2012 (UTC)
- Sexual reproduction allows mixing of genetic material from different individuals to occur, this results in genetic variation at a far higher rate than in the case of asexual reproduction. Genetic variety in asexually reproducing organisms is purely a result of random mutation in individuals, viable cases of such random mutations are very rare. Compare that to the mixing in sexual reproduction which results in a practically unique genetic makeup for every individual organism (except for identical twins of course). More variation allows faster, more effective adaptation to changing environmental pressures. Roger (talk) 09:03, 10 April 2012 (UTC)
- Note that the article points out that many of the benefits of sexual reproduction can be had from "sexual non-reproduction", e.g. bacterial conjugation. The origins of this latter phenomenon (horizontal gene transfer, really) are probably ancient indeed - I would think that they should predate the existence of species and individuals entirely, in some era when genetic material still freely reacted and interacted in the open before some means was invented to partition it. Sexual reproduction simply combines a thorough horizontal gene transfer with the process of reproduction, so that the process is only needed and effects are only tested in progeny at the earliest stage of development. Wnt (talk) 16:37, 10 April 2012 (UTC)
- And I believe that the that longer time between generations of larger organisms also plays a role. That is, with the short time between generations in single-celled organisms, evolution proceeds at a rate quick enough to adapt to changes in the environment, even by asexual reproduction. However, with large, multi-cellular organisms, with a much longer period between generations, asexual reproduction would not be quick enough for them to adapt, and they would go extinct. Similarly, relying on a high mutation rate alone produces a large portion of defective organisms. While this is acceptable for single-celled organisms, it is not for large organisms, which may not have enough offspring to survive so many deaths. StuRat (talk) 16:49, 10 April 2012 (UTC)
What are the colors of human cone cells?



I haven't formally studied color vision, but I've read Ware's Visual Thinking for Design as well as various Wikipedia articles. Often, in diagrams, we treat the L, M, and S cone cells as if they simply detect the colors red, green, and blue. Those colors are used for the curves in the diagrams on the right, and they show up in schematics of the retina, such as this mosaic (source).
This representation can be useful, but it seems misleading. The simplest reason is that L and M are assigned very different colors when they are actually very similar (which is why the opponent process must subtract their signals to derive perceptually interesting information). So the broad version of my question is, what would be a more "honest" assignment of colors to L, M and S?
A simple approach would be to consider monochromatic light with the same wavelength as the peak wavelength in the responsivity spectrum. Let's say the peaks are at 564, 534, and 420 nm (taken from Cone-response-en.svg). Using the calculator at http://rohanhill.com/tools/WaveToRGB/ (which outputs to an unknown RGB color space), we get the following representations:
- L, 564 nm: Red 196 Green 255 Blue 0 RGB Hex: #C4FF00
- M, 534 nm: Red 87 Green 255 Blue 0 RGB Hex: #57FF00
- S, 420 nm: Red 56 Green 0 Blue 255 RGB Hex: #3800FF
That's already pretty surprising, right? But I'm looking for more than just the peaks, represented as fully saturated hues. I'm also looking for an intuitive understanding of the width of the curves and the amount of overlap. What I'd like to do is take each responsivity curve, multiply it by a standard white-light spectrum, and map this filtered light into sRGB. I don't think the result would have a direct physical or psychological interpretation, but I think it would still be instructive.
I have no idea how to actually do this. Do you? :-) If so, I think these colors might be useful to add to a few Wikipedia articles. Thanks! Melchoir (talk) 09:42, 10 April 2012 (UTC)
- One other way to consider is what experience do you get when those cones are stimulated alone. It is pretty clear from the ends of the spectrum that you get a violet experience from the S cone, and a red experience from the L cone. The green one may be very hard to stimulate alone without also doing either the red or blue cones at the same time. One way would be in very dim green light perhaps the M cones are active and the others not. Another way would be to bleach L and S with bright red or violet light and then look at green. I call this experience ultragreen. A green colour more saturated than spectral green. Adding our new ideas to Wikipedia articles would be original research, so we don't do it. Graeme Bartlett (talk) 10:13, 10 April 2012 (UTC)
- RGB is severely undersampled. To make matters worse, display monitor pixels aren't monochromatic outputs, either: each color element emits a wide range of wavelengths centered around a particular color. So we have a seriously undersampled spectrum in a non-orthonormal basis. (You discovered this by plotting and noting an overlap in sensitivities). What is really needed for color accuracy is a full spectrum sweep: such as is performed in an optical physics laboratory or a surveillance satellite. But, for engineering reasons, we have "RGB" cameras and monitors; so unless we work for the NRO, we stuff all our visual information into three numbers - so there's gonna be some overlap.
- Anyway, the standard technique for taking a lot of data (spectral response at all frequencies) and optimally representing it as a three-element vector (R,G,B) is called... principle component analysis. If you begin down this path, you'll soon conclude (like everyone else) that the math is pretty horrible; but keep the concept clear: you're trying to find just three number that accurately represent an entire continuous spectrum. Then you will postmultiply against your derived eigenvectors - idealized spectral responses for R,G, and B.
- If this process was easy, camera, film, and digital imaging companies wouldn't spend so much effort on color accuracy and white balance. If you implement a program to compute optimal eigenvectors, ("idealized spectral response"), you will no doubt discover that they vary with light source, scene conditions/brightness; leakage from invisible infrared, ultraviolet, and other out-of-band light....
- Anyway, there's no need for original research. This stuff is well documented in texts and research papers. Color vision is very well understood, both from a physics and from a biological perspective. A great deal is known about color psychology and perception, too. I'll dig up a good introductory text and get back to you. Nimur (talk) 16:03, 10 April 2012 (UTC)
Direct stimulation of optic nerves
An interesting aspect of the question above is what is the experience of a subject who has only the green cones stimulated? Under normal circumstances this is impossible to achieve because of the overlap with the response of the red cones. I was wondering if any research has been done on this - perhaps by directly stimulating the optic nerve or some chemical stimulus. Would the result be some kind of super green or impossible colour? SpinningSpark 18:26, 10 April 2012 (UTC)
triplicate carbon copies' carbon footprint
to settle an argument I'm having. What is "greener" (better for the environment): using one triplicate carbon copy document or 100 pages with print from a modern printer/copier? — Preceding unsigned comment added by 165.212.189.187 (talk) 15:08, 10 April 2012 (UTC)
- This question cannot be answered with surety as the content is unspecified. For example, if there is sparse text on each of 100 pages, the modern printer/copier would use minimal toner/ink and suffer minimal wear, and the carbon footprint of making the paper in each case would roughly be the same. Note that this is not about the actaul carbon in either the carbon paper or the modern printer ink or toner - this is not an environmental impact. What you should consider is the cost of carbon emissions cost of electrical power used to make the paper and ink, plus the chemicals used to bleach the paper. But if large areas of solid black are on each of 100 pages, the ink/toner consumption would be huge. The carbon footprint of manufacturing the printer - should this be included? Wickwack120.145.191.82 (talk) 15:19, 10 April 2012 (UTC)
Ok, regular text on all pages. yes, the footprint to MAKE the paper plus the toner plus the printer vs. that of the carbon copy doc. plus the machine used to make the carbon doc. — Preceding unsigned comment added by 165.212.189.187 (talk) 15:36, 10 April 2012 (UTC)
- It seems to me as with many questions of this sort, it's almost impossible to answer in any meaningful way since the answer will likely depend on the assumptions you make about the particular scenario. For example to use an extreme scenario the carbon footprint of paper could easily vary significantly if we compare a case where a company gets the paper directly delivered from the paper factory next door (who's power primarily comes from low carbon footprint sources like solar, wind, hydroelectric, nuclear, geothermal and wood pulp comes from nearby sustainably managed plantations); to another company who's office manager buys a few packets of paper every fortnight from the shop 150 km away, going there and back in the SUV usually buying nothing else (not particularly effective but perhaps the manager likes the drive), and the paper itself comes from a factory (where it's produced using power primarily coming from coal) many hundreds of kilometers away by truck to the shop. Nil Einne (talk) 16:57, 10 April 2012 (UTC)
- You said "one triplicate carbon copy document". Does that mean a single page of text duplicated 3 times ? If so, I'd expect that to be less of a problem. The carbon paper, after all, will likely end up buried in a landfill, not in the atmosphere anytime soon. StuRat (talk) 16:56, 10 April 2012 (UTC)
Alright that settles it.165.212.189.187 (talk) 18:32, 10 April 2012 (UTC)
- Don't forget the means of producing typed documents back in the olden days (when I learned to type) was on manual typewriters: no electricity needed, just arms like tree trunks! Very green. --TammyMoet (talk) 19:00, 10 April 2012 (UTC)
- Um, I think you would have to include the CO2 excreted by the typist which would probably far outweigh the carbon footprint of a modern printer due to its energy consumption. SpinningSpark 19:29, 10 April 2012 (UTC)
- And if we're concerned about global warming, let's not forget the methane excreted by the typist. :-) StuRat (talk) 21:34, 10 April 2012 (UTC)
- Maybe we should go back to spirit duplicators, rotary duplicators or hectographs. Dunno how "green" they are, but I like purple – and the smell! Mmmmm. {The poster formerly known as 87.81.230.195} 90.197.66.16 (talk) 22:41, 10 April 2012 (UTC)
Are there more/fewer/equal quantum fluctuations near massive bodies?
As massive gravitational bodies distort space, is it helpful to think of space as being "denser" or "less dense" in areas? Do massive bodies affect quantum fluctuations of the surrounding space? -Goodbye Galaxy (talk) 15:33, 10 April 2012 (UTC)
- No, space doesn't get "more dense" or "less dense". If you're on the inside of some kind of small chamber in freefall, any quantum mechanical experiment you perform within that chamber will behave the same whether you are in deep space or near a strongly gravitating body. By "small" I mean small enough that tidal forces are negligible. I'm also using the strictest sense of the word "freefall", i.e., the chamber isn't being subjected to any air resistance or other external forces. Red Act (talk) 18:54, 10 April 2012 (UTC)
- And if the chamber is subjected to external forces, then the effects of gravity will be completely equivalent to the effects of acceleration (ie. you can't tell if the chamber is sitting on the Earth's surface or if it is out in deep space accelerating at 9.8m/s/s). See equivalence principle. --Tango (talk) 21:44, 10 April 2012 (UTC)
- To an observer far away from a massive body, there would be more quantum fluctuation per unit time on the surface of the body due to time dilation, but as I understand it quantum fluctuations aren't observable since they are by definition virtual particles that exist in less spacetime than a single planck unit. So to answer your question; an unobservable phenomenon would occur at a greater frequency to certain observers...whatever that means. I don't think it's useful to think of space as being more or less dense, but it is useful to think of spacetime as being more or less curved. 112.215.36.179 (talk) 09:02, 11 April 2012 (UTC)
- And if the chamber is subjected to external forces, then the effects of gravity will be completely equivalent to the effects of acceleration (ie. you can't tell if the chamber is sitting on the Earth's surface or if it is out in deep space accelerating at 9.8m/s/s). See equivalence principle. --Tango (talk) 21:44, 10 April 2012 (UTC)
Identify the fishes
Type 1 and Type 2. --SupernovaExplosion Talk 15:54, 10 April 2012 (UTC)
- Hello, could anyone identify the species, or at least the genus? The photos were taken near river mouth of Digha and the fishes are likely marine. --SupernovaExplosion Talk 04:26, 11 April 2012 (UTC)
- I think #1 could be a pike conger eel, and #2 looks like Indian sole (a flounder), but I'm no expert. -- Scray (talk) 04:18, 13 April 2012 (UTC)
Jungle versus machete
Just having watched a movie where a machete was used to slash through a thick jungle, I find myself wondering how far one could go without having to stop to resharpen the machete. StuRat (talk) 17:04, 10 April 2012 (UTC)
- One kilometre apparently. SpinningSpark 17:29, 10 April 2012 (UTC)
- A trip through the jungle at one kilometer a day would get tedious. And I really liked how the guy in the video was sharpening one side of the blade by stroking toward it, a great way to slice your hand. Edison (talk) 15:41, 11 April 2012 (UTC)
- Yes, if the machete was to slip off the tree, it might do rather more damage than a few sliced fingers as he would be falling towards it. I was also amused by the fact that "Machete safety" is only at video number 7, after the student has been practicing the earlier six videos presumably without safety training. He seems to be cheerfully ignoring his own safety rules both before and after the safety video in any case. SpinningSpark 17:12, 12 April 2012 (UTC)
- That's what I was thinking. I imagine you could carry a few spare machetes and sharpen them all at night. If there's a larger group, they could take turns hacking and dulling their machetes and using each other's paths, thus moving more quickly. I imagine you'd get tired quickly doing the hacking, especially in a hot, humid jungle. StuRat (talk) 17:15, 11 April 2012 (UTC)
- Taking turns does not make faster progress (unless a lone hacker were to become exhausted) because only one person can be leading at any one time. SpinningSpark 17:12, 12 April 2012 (UTC)
- Also, it should avoid having to take breaks to sharpen the machete, which is what this post is all about. Presumably each hacker will carry his own, and when it dulls, another hacker, with another machete, can take over. I'm not sure if the followers would be able to resharpen their machetes as they follow. If not, all could resharpen at night, for multi-day trips. StuRat (talk) 17:40, 12 April 2012 (UTC)
- Is there a video on walking through the jungle carrying a big knife and sharpening it rather than watching for tripping hazards, snakes, etc? Edison (talk) 18:54, 12 April 2012 (UTC)
- I'm thinking there are lots of times when the followers must wait for the leader to hack a bit more, so they could sharpen then. StuRat (talk) 05:44, 13 April 2012 (UTC)
Depends how much agent orange you packed. 110.151.252.240 (talk) 18:20, 11 April 2012 (UTC)
does cured cyanoacrylate penetrate skin if dissolved in acetone?
I've been working with acetone with my bare hands in the lab, using a kimwipe to clean glass bonded with krazy glue. I didn't realise that krazy glue contained a cyano group, which I realise is non-volatile but I worry about how it will be metabolised. 216.197.66.61 (talk) 17:15, 10 April 2012 (UTC)
- Not all cyano groups are created equally. Organically-bound cyano groups (called nitriles) are fundementally chemically distinct from their inorganic cyanide cousins. This is similar to the wide chasm between how hydroxy groups behave. In a compound like sodium hydroxide they are highly basic. In a compound like ethanol they are essentially neutral, and in compounds like phenol or boric acid they are acidic. The molecule as a whole needs to be considered to understand its property; not just a coincidental organization of atoms. In the case of nitrile, organic cyanides like this are very unlikely to produce free cyanide ions; they more commonly and readily undergo nitrile hydrolysis to form either amides or carboxylic acids. Just as ethanol produces no free hydroxide ions in your body, nitriles like cyanoacrylate do not produce free cyanide ions. Cyanoacrylates are not fully inert in the body, there are some toxicity issues which is discussed in the Wikipedia article, but these are wholly unrelated to cyanide toxicity. Acetone is the recommended prodcedure for removing cyanoacrylate, and if you are concerned about what may happen if you get it on your hands, use impermiable and unreactive gloves of some sort. --Jayron32 17:42, 10 April 2012 (UTC)
- Note that as said in the article, it can actually be used in surgery with good results. Cyanide can be found at low levels in some foods; it really isn't a problem until you get too much. Wnt (talk) 20:37, 10 April 2012 (UTC)
- Isn't hydroxide a worse leaving group (and hence a stronger nucleophile) than cyanide? Can't hydroxide ions perform SN2 attacks on the tetrahedral center? 216.197.66.61 (talk) 02:37, 11 April 2012 (UTC)
- Note that as said in the article, it can actually be used in surgery with good results. Cyanide can be found at low levels in some foods; it really isn't a problem until you get too much. Wnt (talk) 20:37, 10 April 2012 (UTC)
- OH- ions do perform SN2-type attacks (not strictly SN2, but similar idea), but you are not going to break the C-C bond. Instead, what you do is progressively substitute C-O bonds for C-N bonds until you convert the nitrile into a carboxylate/carboxamide. The nucleophilic OH- attacks the carbon which is triply bonded to the nitrogen because that is the most electron-deficient carbon. That's exactly how nitrile hydrolysis works (see link above). If you want more, look up "base-mediated (or catalyzed) nitrile hydrolysis mechanism" in google for all the details. --Jayron32 02:49, 11 April 2012 (UTC)
- There's another problem in 216's premise: nucleophile-strength and leaving-group-quality do not correspond to each other as opposite trends. Halides are good nucleophiles and good leaving-groups (and going down that column on the periodic table they even become better at both modes in parallel). The two modes of reaction involve the reverse mechanistic arrow but the cause of the change and the stability/reactivity differences are not due to the same underlying atomic/molecular properties. DMacks (talk) 15:43, 11 April 2012 (UTC)
- You probably shouldn't be working for very long with your hands in acetone. It's not good for your skin. For limited exposure, get a box of cheap nitrile disposable gloves at your local hardware store. If you are going to be working with it a lot and the parts are big, a pair of non-disposable gloves are better. Either way, use nitrile as it is more resistant to acetone than other glove materials. Don't rely on disposable gloves to protect your hands from long exposure or full immersion.--Srleffler (talk) 03:54, 14 April 2012 (UTC)
Sun dogs
What about sundogs, the halo around the hot Arizona sun. There could not be any ice crystals, could there? Just saw a beautiful one today, all morning, still going on, Tucson, April 10, 2012. — Preceding unsigned comment added by 24.255.30.15 (talk) 17:23, 10 April 2012 (UTC)
- See the Wikipedia article titled Sun dog. --Jayron32 17:33, 10 April 2012 (UTC)
- Note in particular that the ice crystals responsible for sun dogs occur high in the atmosphere, where the temperature is cold and generally independent of ground-level temperatures. — Lomn 17:38, 10 April 2012 (UTC)
- In fact, because the surface air is dry, you have less diffusion, and therefore a better view of the high atmosphere (whatever conditions may exist at altitude, including sundog-causing ice). For this reason, a lot of interesting aeronomy and physics is practical in the high desert. Tucson is great for amateur astronomers, as is the entire region. The higher you go, the better the view. For example, Lowell Observatory is in Flagstaff; Kirtland, (elevation 5,300'), is home to an Air Force aeronomy and optics center. You can see some amazing photos on Wikipedia that are possible due to the clear air in the troposphere, enabling a clear view at optical bands all the way to the mesosphere. Nimur (talk) 17:48, 10 April 2012 (UTC)
Carnot efficiency
Is there mathematical proof for why the efficiency of a heat engine cannot exceed the Carnot efficiency? — Preceding unsigned comment added by Clover345 (talk • contribs) 20:55, 10 April 2012 (UTC)
- Have you looked at Carnot efficiency and Carnot engine? The maximum efficiency is derived directly from the second law of thermodynamics. SpinningSpark 21:08, 10 April 2012 (UTC)
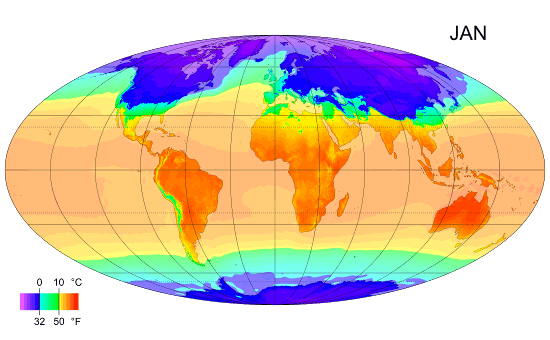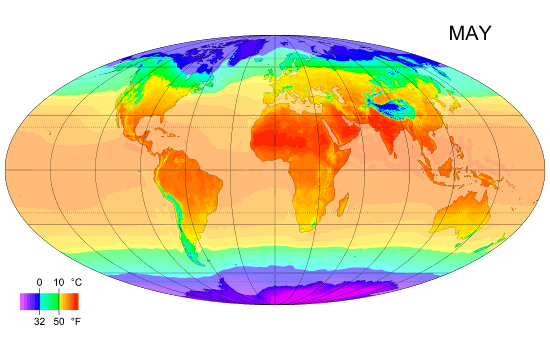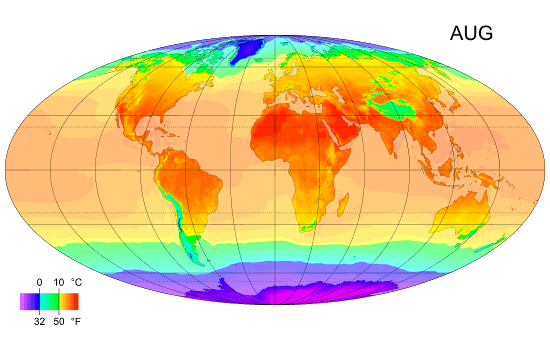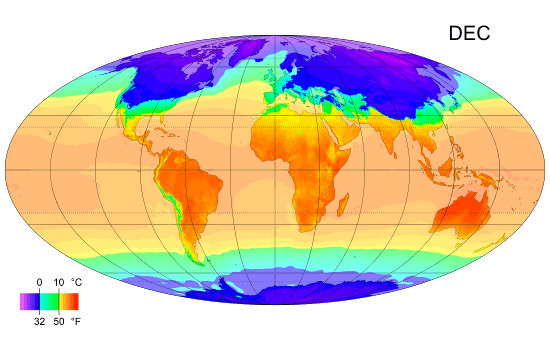
Why the south is hotter?
As you can see the south is hotter than the north along the year, and I ask why? Exx8 (talk) 21:34, 10 April 2012 (UTC)
- I'm replacing this post which was accidentally deleted [3]. There is more land in the north. Large bodies of water take a long time to heat up and cool down, so they tend to reduce extremes of temperature. That means the southern hemisphere doesn't experience as much seasonal change as the northern hemisphere. I'm not sure the southern hemisphere is actually hotter on average. It is hard to tell from that image, but I think the northern summer is hotter than the southern summer and the northern winter is colder than the southern winter, ie. the north is just more extreme rather than colder. --Tango (talk) 21:40, 10 April 2012 (UTC)
- More open ocean. Plasmic Physics (talk) 21:41, 10 April 2012 (UTC)
- Can you please expand on your answer a bit, Plasmic Physics? Does it have to do with the higher albedo of snow-covered land?Anonymous.translator (talk) 22:31, 10 April 2012 (UTC)
- I'd suspect it is less due to albedo, and more to do with the higher heat capacity of water, compared to land. Climate science is very complicated and there are many interacting factors, so it's difficult to definitively attribute an observed effect to one single cause. Nimur (talk) 23:18, 10 April 2012 (UTC)
- I think you're all overlooking the obvious. The lower latitudes (closer to the Equator) get more-direct sunlight and thus more insolation. --Trovatore (talk) 23:21, 10 April 2012 (UTC)
- Why does Exx8 say the south is hotter than the north? Is this based solely on the diagram, or on something else? The diagram steps through the year, one month at a time. It clearly shows that the northern hemisphere is hotter than the southern during the northern summer, and that the southern hemisphere is hotter than the northern during the southern summer, but it is debatable whether this diagram shows one hemisphere is hotter than the other on an annual cycle. Dolphin (t) 23:25, 10 April 2012 (UTC)
- I was assuming he meant that the southern part of the Northern Hemisphere is warmer than the northern part. --Trovatore (talk) 23:27, 10 April 2012 (UTC)
- This discussion is about why the southern hemisphere as a whole is hotter than the northern hemisphere as whole, when averaged over the entire year. Plasmic Physics and Nimur have the right answer, which is also the explanation given in our article on the Southern Hemisphere: "Climates in the Southern Hemisphere overall tend to be slightly milder than those in the Northern Hemisphere at similar latitudes except in the Antarctic which is colder than the Arctic. This is because the Southern Hemisphere has significantly more ocean and much less land. Water heats up and cools down more slowly than land."Anonymous.translator (talk) 00:10, 11 April 2012 (UTC)
- Milder doesn't mean warmer. It just means less variation. 112.215.36.178 (talk) 04:25, 11 April 2012 (UTC)
- Why does Exx8 say the south is hotter than the north? Is this based solely on the diagram, or on something else? The diagram steps through the year, one month at a time. It clearly shows that the northern hemisphere is hotter than the southern during the northern summer, and that the southern hemisphere is hotter than the northern during the southern summer, but it is debatable whether this diagram shows one hemisphere is hotter than the other on an annual cycle. Dolphin (t) 23:25, 10 April 2012 (UTC)
- I think you're all overlooking the obvious. The lower latitudes (closer to the Equator) get more-direct sunlight and thus more insolation. --Trovatore (talk) 23:21, 10 April 2012 (UTC)
- I'd suspect it is less due to albedo, and more to do with the higher heat capacity of water, compared to land. Climate science is very complicated and there are many interacting factors, so it's difficult to definitively attribute an observed effect to one single cause. Nimur (talk) 23:18, 10 April 2012 (UTC)
- Can you please expand on your answer a bit, Plasmic Physics? Does it have to do with the higher albedo of snow-covered land?Anonymous.translator (talk) 22:31, 10 April 2012 (UTC)
- (Multiple ECs) I'm not really seeing a South v North hemisphere discrepancy in that graphic. The main difference apparent to me is that the South's temperature zones are a little more stable through the year, which I'd assume is because the land/sea structure is somewhat simpler in the Southern hemisphere, but the areas appear to balance out either side of the equator fairly well. In terms of notionally inhabited areas, there are perhaps more hotter ones in the South than the North, but that's just an artefact of the distribution of landmasses (since we don't think of the oceans as "inhabited"), with more land in the North Temperate zone and more ocean in the South Temperate zone. If anything, I'd guess from the graphic (and this can doubtless be checked with articles elsewhere) that the South averages a little colder because of the polar-positioned Antarctic continent leading to colder Winter extremes – see the 4th map in the Geographical zone article. A contributory factor will be that the Earth's perihelion occurs during the Northern Winter and Southern Summer, which must mildly ameliorate the North's extremes and exacerbate the South's. {The poster formerly known as 87.81.230.195} 90.197.66.16 (talk) 23:29, 10 April 2012 (UTC)
Look at how cold the North gets in January and how far down it gets cold compared to the Southern hemisphere in July. There is a massive difference. --122.111.0.88 (talk) 13:21, 11 April 2012 (UTC)
- It's only massive in the sense that the land is what is primarily what is changing color, and there is less land in the bottom reaches of the Southern hemisphere than there is in the North. This is what people mean about the water being the important factor. The water temperature is about the same for both. --Mr.98 (talk) 13:38, 11 April 2012 (UTC)
HCl NaOH titration
Does a buffer form in the HCl - NaOH titration? — Preceding unsigned comment added by 150.203.114.37 (talk) 22:55, 10 April 2012 (UTC)
- See Buffer. You need a conjugate pair of a weak acid or a weak base (that is, a weak acid and its conjugate base, or a weak base and its conjugate acid). In order to answer your homework question, you first need to identify what the weak acid or weak base is in your mixture. If you don't have one, you don't have a buffer. --Jayron32 02:43, 11 April 2012 (UTC)
- So the answer is no because HCl is a strong acid, NaOH is a strong base, and the products NaCl and H2O are not acids or bases at all. — Preceding unsigned comment added by 150.203.114.37 (talk) 02:50, 11 April 2012 (UTC)
- Damn skippy. --Jayron32 02:58, 11 April 2012 (UTC)
- Oh yeah? Well to hell with Peter Pan too! DMacks (talk) 15:34, 11 April 2012 (UTC)
- For those who were as puzzled as I was about Jayron's "Damn skippy" reply, I've done the googling: Here's the Urban Dictionary entry. And thanks for the laugh, DMacks. --NorwegianBlue talk 05:13, 12 April 2012 (UTC)
- Damn skippy. --Jayron32 02:58, 11 April 2012 (UTC)
- So the answer is no because HCl is a strong acid, NaOH is a strong base, and the products NaCl and H2O are not acids or bases at all. — Preceding unsigned comment added by 150.203.114.37 (talk) 02:50, 11 April 2012 (UTC)
April 11
why phenolphthalein instead of bromocresol blue
In the titration between NaOH and HCl to determine the end point. Is it just because phenolphthalein turns purple at approximately pH 7, whereas bromocresol green turns blue at pH much less than that? — Preceding unsigned comment added by 150.203.114.37 (talk) 04:19, 11 April 2012 (UTC)
- See pH indicator, especially the big chart. Phenolphthalein doesn't hit the mark very well; you'd usually want to choose an indicator whose transition changes at the equivalence point of the titration, and phenolphthalein does so at much to high a pH. So, hypothetically, your reasoning would work, except that at pH 7, phenolphthalein hasn't changed color yet. However, for a titration like this, the equivalence point happens fast; one drop will generally get you from a very low pH to a very high pH, basically within one drop you go from pH 2 to pH 12 or something like that. Since you can't actually hit the mark that close, phenolphthalein has the advantage of being cheap and easier to work with than more vibrantly-colored indicators, which tend to stain your clothes and/or skin when you spill them. It is a good general purpose indicator for any titration with sodium hydroxide as the titrant, since your equivalence point will always end up sending you to a high pH really fast. --Jayron32 04:39, 11 April 2012 (UTC)
- If the standard acid or base is so concentrated that one drop changes the pH from 2 to 12 as you say, couldn't you use a weaker solution of the titrant? (Its been a while since I took a chem lab). Edison (talk) 15:33, 11 April 2012 (UTC)
- It's not that the titrant is too concentrated, it's that titrating a strong acid with a strong base results in a very weakly buffered solution (water is effectively the buffer, and it does a crappy job of it). A shift in pH from 2 to 12 is a bit of an exaggeration, but a swing of four or five pH units with a single drop isn't unreasonable. (Going from pH 5 to pH 9, for instance, means shifting from an excess of just 10 μM of acid to an excess of just 10 μM of base.) TenOfAllTrades(talk) 16:49, 11 April 2012 (UTC)
- There's not really much of an advantage to getting finer resolution anyway if all you want to do is determine the eqivalence point. The equivalence point is the where the change is most rapid, so if with 1 drop the pH went from say 5 to 9 then you know where the eqivalence point was in terms of titrant to a pretty good certainty. 110.151.252.240 (talk) 18:26, 11 April 2012 (UTC)
- It's not that the titrant is too concentrated, it's that titrating a strong acid with a strong base results in a very weakly buffered solution (water is effectively the buffer, and it does a crappy job of it). A shift in pH from 2 to 12 is a bit of an exaggeration, but a swing of four or five pH units with a single drop isn't unreasonable. (Going from pH 5 to pH 9, for instance, means shifting from an excess of just 10 μM of acid to an excess of just 10 μM of base.) TenOfAllTrades(talk) 16:49, 11 April 2012 (UTC)
- If the standard acid or base is so concentrated that one drop changes the pH from 2 to 12 as you say, couldn't you use a weaker solution of the titrant? (Its been a while since I took a chem lab). Edison (talk) 15:33, 11 April 2012 (UTC)
The powder coating process
Hello,
The "The powder coating process" is a very informative article. Thank you.
Now to a question - In the "Part preparation processes and equipment", The second and third paragraphs talk about chemical pre-treatment, and the fourth paragraph begins "Another method ------. I'm a bit confused. From the article I'm led to believe that either one does the chemical pre-treatment before applying the powder, OR one uses abrasive blasting but not both.
From readings that I have done as well as speaking with applicators it seems one first uses an abrasive blasting procedure and then one can ALSO use a chemical treatment (phosphating) for added pretreatment before applying the powder.
By the way, I am having a mild steel railing fabricated, and then want to have an applicator apply a textured polyester-TGIC powder.
Your thoughts and clarifications would be most appreciated.
Richard Krause — Preceding unsigned comment added by 173.79.200.227 (talk) 16:12, 11 April 2012 (UTC)
- For others' reference, the article Richard is talking about is powder coating. -- Finlay McWalterჷTalk 16:17, 11 April 2012 (UTC)
- It's either or both, depending on the surface and powder composition. What if you had a surface which required a treatment but was covered in rust? You would want to blast it then treat it. 71.215.74.243 (talk) 23:14, 11 April 2012 (UTC)
Impossible colors

The article impossible colors says that we cannot in normal circumstances perceive a color red-green that is similar to both red and green. Indeed, the CIE 1931 color space chromaticity diagram shows that a combination of a pure red hue and a pure green hue (on a line between them in the diagram) will be perceived as yellow or orange. But suppose we fill a screen with intermingled red and green squares. If the squares are very large, the viewer would be aware of seeing the two colors separately and simultaneously. And presumably if the squares are extremely small, the viewer would see yellow (or orange).
Question: If we start from very large squares and then gradually diminish their size, is there a size that results in the viewer perceiving reddish green? That is, do the large red and green squares appear to merge into something reddish green, or does the perception jump from separate reds and greens directly to yellow/orange? Duoduoduo (talk) 17:02, 11 April 2012 (UTC)
- The latter. I agree that yellow does seem to be a different color altogether, versus cyan, which looks like blue-green, and purple, which looks like red-blue. I wonder why. StuRat (talk) 19:53, 11 April 2012 (UTC)
- Some WP:OR: I created a large yellow square in Microsoft Paint, and approached it gradually with a magnifying glass. The visual impression went from pure yellow to a pattern, that first looked like yellow vertical stripes on a black background, then like parallell red and green stripes with a black line in between (the blackness of course being the unlit blue pixel components). There was no reddish green at any point if I had the magnifying glass focussed. And if it was out of focus, the impression was yellow. --NorwegianBlue talk 20:52, 11 April 2012 (UTC)
- Brown is also red plus green. 71.215.74.243 (talk) 05:17, 12 April 2012 (UTC)
- See the articles Additive color and Subtractive color. When you are mixing paint (subtractive color mixing), your statement is true. When you are pointing a red light source and a green light source toward the same spot (additive color mixing), the result is yellow. --NorwegianBlue talk 06:17, 12 April 2012 (UTC)
- Brown is more or less "dark yellow" anyway, so the two statements are not really incompatible. (OK, some brown is dark orange.) --Trovatore (talk) 06:20, 12 April 2012 (UTC)
- See the articles Additive color and Subtractive color. When you are mixing paint (subtractive color mixing), your statement is true. When you are pointing a red light source and a green light source toward the same spot (additive color mixing), the result is yellow. --NorwegianBlue talk 06:17, 12 April 2012 (UTC)
Why don't they place neutron absorbants under nuclear reactor cores?
Couldn't that reduce the severity of nuclear accidents? Sagittarian Milky Way (talk) 17:06, 11 April 2012 (UTC)
- That is too sensible and would cost extra money. A basin of cobalt or some such would suggest that the engineers are not confident in their design. Next, we would have airlines handing out parachutes to all their passengers before boarding. an' if they do start handing out parachutes, I want one of those military multicoloured types that can stand out whether I land in desert, jungle or downtown Harlem. Plus a good pistol and some of those tasty barley-sugar sweets -to keep me going until I get rescued. Oh, and of course a survival pack which includes an iPod would be de rigueur. --Aspro (talk) 17:56, 11 April 2012 (UTC)
- Oh no! Someone used the words "airlines" and "pistol" in the same post! I suggest everyone assume the party escort submission position until DHS agents arrive to resolve the situation.Anonymous.translator (talk) 18:30, 11 April 2012 (UTC)
- I think that untrained passengers jumping out of an airliner experiencing difficulties in mid flight would be orders of magnitude more risky than assuming the brace position and hoping that problem is resolved before impact (the engines restart, or they aircraft stops stalling, or whatver). And iPods would interfer with the aircraft's navigation systems (or so the hostesses keep telling me). And I told you...you don't get a gun until you tell me your name. 110.151.252.240 (talk) 18:39, 11 April 2012 (UTC)
If the core melted and a China Syndrome event began, wouldn't the molten mixture just go right through any neutron absorbant shield? 110.151.252.240 (talk) 18:33, 11 April 2012 (UTC)
- No. Enough neuron absorber (say in a configuration of erect wedges) would stop the reaction and thus the heat generation.--Aspro (talk) 18:40, 11 April 2012 (UTC)
- My general understanding is that, by the time you have a meltdown and a containment breach, the nuclear fuel is sufficiently dispersed so that the chain reaction is effectively finished. This suggestion, then, puts neutron moderators where it's too late for that particular property to be of any real use. Note also that, under these circumstances, neutron moderators won't be thermally significant, as heat generation will increasingly be from byproduct decay. Thermal protection is critically important, yes, but the history of nuclear incidents seems to bear out that bulk concrete is pretty good at the job. — Lomn 19:04, 11 April 2012 (UTC)
- Poking around a bit more finds the relevant concept at core catcher, a portion of the plant engineered to catch and cool molten corium from a meltdown. — Lomn 19:15, 11 April 2012 (UTC)
- This is my understanding as well. By the time you're burning through the floor, the heat is the issue, not the chain reactions. I also wonder whether vaporized radioactive cobalt would be a good idea to add to a catastrophic accident. I suspect not. --Mr.98 (talk) 19:17, 11 April 2012 (UTC)
- (ec) Decay heat has a bit more on this. If you SCRAM a reactor that's been in operation for a while, there will be lots of radioisotopes present in addition to the original uranium; some of these isotopes have rather short half lives. If you shut down a normal reactor, the core will continue to output about 7% of its normal operating power due to ongoing decay of these isotopes. (This drops off over time, but it means that a reactor continues to need active cooling for days or weeks after shutdown.)
- Late in a meltdown, there isn't a chain reaction to interrupt – the molten corium is probably mostly subcritical by the time it gets to the reactor floor – but the corium is still going to be making its own heat for hours, days, and weeks. As Lomn says, you just have to wait until the radioactive molten goo is mixed with enough cold metal, concrete, and rock to keep from melting any further. TenOfAllTrades(talk) 19:32, 11 April 2012 (UTC)
- According to the article on the Chernobyl disaster, the chain reaction was effectively stopped about two or three seconds after the first explosion. The corium was still over 1600°C 4 days later, and it is still hotter than the ambient temperature right now. 110.151.252.240 (talk) 20:21, 11 April 2012 (UTC)
- Reading uranium dioxide, why'd they choose that as the best nuclear fuel? It seems like a poor nuclear fuel to me. Is the uranium-compound and other fissile substance-list really that crappy that no suitable reactor fuel could be found with a melting point lower than 2865 °C, which would be easier to contain with structural materials if melted? Then you might be able to catch the stuff with a neutron-killing crucible/isolater. If not couldn't they have at least found a fuel with a higher thermal conductivity? It's use is to make heat for crying out loud, and one where hotspots are dangerous.
- I wonder if they considered just allowing the molten fuel to mix with and melt some other material, like lead, beneath the reactor core, to both dilute and shield it. StuRat (talk) 22:11, 11 April 2012 (UTC)
- The actual rods are a very complex mixture of the uranium dioxide fuel, compounds containing daughter isotopes and other materials. Having lower melting points is no advantage as far as I can see; if the fuel rods melted at a lower temperature then the reactors operating temperatures would be more constrained. Why do you think it's better to be able to catch molten fuel rods then to just keep them solid and have them not melt in the first place? And allowing the hot lava like melt to come into contact with lead is not a great idea either; lead boils at 1749°C so you'd have a radioactive mixture of very hot gaseous metals building up pressure inside whatever is left of your containment. This effect contributed to the disaster at Chernobyl but with zinc instead of lead coming into contact with the corium. 110.151.252.240 (talk) 22:23, 11 April 2012 (UTC)
- I think the idea is that the rods will continue to heat up until they do melt, whatever than melting temp is, in a disaster like Chernobyl. So, if they are going to melt in any event, do we want that to happen at a lower or higher temp ? StuRat (talk) 22:28, 11 April 2012 (UTC)
- Higher, so that they're not as volatile. 110.151.252.240 (talk) 22:33, 11 April 2012 (UTC)
- Is something at it's melting point of 1000°C necessarily more volatile than something at it's melting point of 2000°C ? StuRat (talk) 22:56, 11 April 2012 (UTC)
- No, but assuming we're still using some compound of uranium, the nuclear reactions are all still the same so the same amount of energy is released and heats the material by the same amount. So if it takes less heat to get to the melting point then there's more heat to push closer to the boiling point. Just doing some ball park maths the material in the Chernobyl incident could have boiled ~20 tonnes of lead (my assumptions were; that heat transfer from the corium to lead heat sink is uniform across the entire body, the corium mixture is at the melting point of uranium dioxide, that all of the corium has the heat capacity of uranium dioxide, the entire mass comes to thermal equillibrium at the boiling point of lead). 110.151.252.240 (talk) 23:06, 11 April 2012 (UTC)
- The sooner it melts, and thus mixes with and melts the lead, the sooner the chain reaction with be slowed by the shielding. Yes, the total amount of heat released will be the same, but it will be released at a much slower and more manageable rate. StuRat (talk) 02:03, 12 April 2012 (UTC)
- Apparently, there are reactor designs that use molten fuel, and these designs are inherently more stable that solid fuel types. I suppose it wouldn't be possible for them to melt down as such, though they could boil and leak or explode. It seems like cooling the molten salt would be much more straight forward though. 1.155.25.201 (talk) 05:02, 12 April 2012 (UTC)
- When everything is working properly, yes, but we must assume that any system designed to circulate and cool the molten salt could be disabled. StuRat (talk) 17:35, 12 April 2012 (UTC)
Seems pointless to try to think of a way to do it better (well, unless you're a nuclear engineer) however I did read in the Economist that many designs are old as dirt but anything in the industry happens so dang slowly and expensively that they don't even bother. Sagittarian Milky Way (talk) 21:54, 11 April 2012 (UTC)
Use of anova/ F-tests with count data
Hi all. I've done some reading recently for a lit review (etc) and I keep coming across studies that use ANOVAs for discrete data. For example, someone gives experimental and control subjects the same 20 words of French to learn, then tests them after, say, 10 minutes, to see if there is any difference between the two groups. They almost always seem to use an Anova for this. The scores are discrete, so they can't really be from a normal distribution, can they? Kasahara (2011) is a case in point, as is Laufer and Shmueli (1997). Is this approach justified? Or is it because they don't know what they are doing, and because non-parametric statistics or robust statistics confuse people? IBE (talk) 19:47, 11 April 2012 (UTC)
- Aggregations of discrete scores often form normal distributions. 71.215.74.243 (talk) 23:17, 11 April 2012 (UTC)
- Actually an ANOVA would not properly be used in the situation you describe -- a simple count test would suffice. The proper use of an ANOVA is when there are multiple groups (more than two) and you want to see whether you can reject the hypothesis that all of the groups are statistically identical. I believe that's what is happening in those papers, but I don't have online access to them so can't be sure. There are many situations where counts are approximately normally distributed with high accuracy if the number of samples is large. Looie496 (talk) 00:16, 12 April 2012 (UTC)
You are right, people using ANOVA for discrete data don't know what they are doing! After observing k type-1 items in a sample of n items from a big population, the posterior probability P that a random item from the population is of type 1, has a beta distribution, P ≈ μ±σ, where the mean value is μ = (k+1)/(n+2) and the variance-to-mean ratio is σ2/μ = (n−k+1)/((n+2)(n+3)). The difference between two populations, P2−P1 ≈ μ2−μ1±√(σ22+σ12), is approximately normally distributed, and if actually P2 = P1 then the square of the normalized difference, (μ2−μ1)2/(σ22+σ12), has a chi squared distribution with one degree of freedom. Bo Jacoby (talk) 06:21, 12 April 2012 (UTC).
- My father in law (now retired) held an academic position in the social sciences, and was an expert in doing ANOVAs on massive data sets by hand. I asked him about how he could be sure that his data fulfilled the requirements for doing an ANOVA. His answer was that he wasn't, but that it was common practice in his field, because the results gave meaningful and practically useful results. (This conversation took place at least 20 years ago). --NorwegianBlue talk 06:01, 12 April 2012 (UTC)
- You can't derive a posterior distribution without a prior distribution. You seem to be assuming a prior distribution of binomial(n,0.5), but that isn't automatically a sensible choice. There are plenty of situations where 0.5 isn't a good prior and even when it is a good choice you should be explicit about what assumptions you are making. --Tango (talk) 19:12, 12 April 2012 (UTC)
- When n=k=0 the beta distribution is the uniform distribution on the interval 0<P<1. So the prior is P = 0.5(3), rather than P = 0.5(0). (See Concise notation#Measurements). Bo Jacoby (talk) 11:19, 13 April 2012 (UTC).
- Thanks for the response, IBE (talk) 21:32, 15 April 2012 (UTC)
While cleaning out my medicine cabinet I found an old bottle of magnesium citrate, oral solution, saline laxative, cherry flavor. Since it expired over 6 years ago and had a white crystaline precipitate at the bottom, I decided to dump it. I thought the precipitate was attached to the bottom, but, when I poured it down the drain it broke up and fell down the drain, where it's now lodged. My question is, do I need to fish it out, or is this precipitate water soluble, and will it eventually dissolve ?
Here are the ingredients:
Magnesium citrate, 1.745 g per fluid ounce Cherry flavor Citric acid FD&C red #40 (I figure this can't be in the precipitate, since it's white) Potassium bicarbonate Sodium saccharin Purified water
I tasted a bit of the precipitate which didn't fall down the drain. It was sweet. So, I imagine this means there's some of the saccharin in it. StuRat (talk) 20:19, 11 April 2012 (UTC)
- It might take a while to dissolve. Have you tried running hot water for 10-15 minutes? 71.215.74.243 (talk) 23:24, 11 April 2012 (UTC)
- Magnesium citrate is very, very soluble - 20 g/100ml according to the article. But exposure to the air might (I think) have generated magnesium carbonate (from carbon dioxide in the air) which is only 0.01 g/100 ml. The fact that you're even asking makes me think you might have the carbonate, in which case, it may take some doing, but it should dissolve if you can keep the water flowing through it. Wnt (talk) 15:46, 12 April 2012 (UTC)
- It seems to have now dissolved enough to fall down into the U-bend. Hopefully it will dissolve the rest down there. StuRat (talk) 16:20, 12 April 2012 (UTC)
Gauss' principle of least constraint
Apologies if this is a stupid question, but I haven't found any clear discussion on this matter.
I have read that Gauss' principle of least constraint is the most general variational principle of Classical mechanics, and more general than Newton's laws, but I still don't understand what this means. For the sake of convenience, take Newton's laws to say nothing other than that momentum is always conserved (I am aware of other interpretations, especially regarding the first law). I ask
- Does Gauss' principle imply Newton's laws? By this I mean can Newton's laws be derived from Gauss' principle?
- If yes, what does Gauss' principle also say that Newton's laws do not?
- If no, is Gauss' principle a weaker statement, and if so, how is it weaker?
- To what extent if there experimental data to support Gauss' principle in as much as it differs from Newton's laws?
Thanks.--Leon (talk) 20:23, 11 April 2012 (UTC)
- Yes, Gauss' principle implies Newton's laws, and provides a way to solve for thermodynamic systems which Newton's laws do not. See [4]. To the extent that they make the same predictions, they are supported by the same empirical evidence. 71.215.74.243 (talk) 23:32, 11 April 2012 (UTC)
- I've answered your questions in sequence:
- "Does Gauss' principle imply Newton's laws?"
- Yes; in fact, it is mathematically equivalent to the statement of Newton's laws, using a generalization of spatial coordinates. You can see a derivation of this in Marion and Thornton's Classical Mechanics text, (Chapters 7 through 9).
- "If yes, what does Gauss' principle also say that Newton's laws do not?"
- The use of generalized coordinates allows simpler algebra, permitting analytic solutions in cases where Newtonian formulations would require numerical computation. But to the letter, these equations are equivalent to Newtonian physics. They're just written in a different notation, which helps make certain relationships more clear and simpler to write and calculate.
- "To what extent if there experimental data..."
- This one is a bit tricky. In a sense, like Newton's laws, Gauss's statement is definitional - axiomatic - in that it contains a generalization of the physical quantity "force." Gauss's statement implies conservation of energy and conservation of momentum, and is in fact equivalent to a definition of energy and momentum and force, in terms of mass and generalized coordinates. Therefore, any experiment that validates energy- and momentum conservation is a validation of the principle of least-action. Nimur (talk) 00:14, 12 April 2012 (UTC)
- I didn't realize that Gauss' principle or Newton's laws said anything about energy conservation; I thought that energy was simply the value of the Hamiltonian, which is conserved iff the Hamiltonian has no explicit time dependence. And I thought that Hamilton's principle was weaker than Newton's laws and Gauss' principle as Hamilton's principle requires holonomic constraints whilst the latter two do not. Where am I going wrong?
- I've answered your questions in sequence:
- The article on D'Alembert's principle claims that no one has proven that it is equivalent to Newton's laws, and I thought that Gauss' principle was known to be equivalent to D'Alembert's principle. Where is the mistake?--Leon (talk) 09:44, 12 April 2012 (UTC)
- Our article is incorrect. It cites a source, but the source is in German, which I don't read very well; I specifically recall a physics professor telling me I'd never be a real physicist until I learned to read German and blow my own glass, but despite some effort, I never spent enough time to master either art. Nonetheless, I still try to read as much work in its original language as possible. I can't begin to tell you how many misunderstandings about quantum physics perpetuate to this day because of poorly translated German. Anything you read in English involving gedankenexperiments, ansatz functions, Schrodinger's cat, or eigen-anything... these concepts have been so corrupted by the Brownian motion of poor translation that they're totally diffused from the original meaning; a mere hazy cloud of related concepts loosely distributed around what the original German physicist actually meant. ...so I'll need a lot more time before I can decide whether our cited source is "wrong" or just "factually misappropriated." If you would like a source in English, I highly recommend the book I mentioned above, Classical Dynamics. The proof of equivalence between the Newtonian formula and various minimization principles are shown. Marion and Thornton show the Lagrange minimization, but there's an entire chapter on calculus of variational functions; and I assert that if you can understand that chapter, you can trivially show the D'Alembert principle, and the Gauss formulation, are all equivalent.
- Regarding your confusion on constraints: this sounds like a great chance to use the phrase, "an exercise left to the reader." Actually, the choice of whether an effect is a "constraint" or a "contributing factor to a generalized potential-energy field" is entirely one of preference. If you treat it as a potential energy field U, then force F = -∇U. If you treat it as an equation of constraint, then you will calculate a minimization, and you'll have a residue exactly equal to F. This can be worked analytically, but ultimately its justification comes down again to an axiomatic definition of "force." Nimur (talk) 16:58, 12 April 2012 (UTC)
- The article on D'Alembert's principle claims that no one has proven that it is equivalent to Newton's laws, and I thought that Gauss' principle was known to be equivalent to D'Alembert's principle. Where is the mistake?--Leon (talk) 09:44, 12 April 2012 (UTC)
- Sorry, perhaps I wasn't clear: I don't believe I was or am confused about the constraints satisfied by systems that can be modelled by Hamiltonian mechanics (though perhaps I am). I'm not quite sure what you mean above about constraints: do you mean that non-holonomic constraints (i.e. velocity-dependent potentials) can be treated differently, attaining a mathematically different formulation of the system such that all constraints are holonomic and further that Hamilton's principle can be applied?
- But primarily, what I am confused about is how energy conservation can be derived from Newton's laws. It is not mathematically unsound to define a Hamiltonian system with an explicit time dependence (which I think has to be in the form of one or more time-dependent potentials), and such a system does not conserve energy, though it does conserve momentum. I believed I had a counterexample to energy conservation being derivable from Newton's laws.
- Two particles of equal and opposite momenta hit one another and coalesce to form a stationary, composite body. Before impact, the particles had some kinetic energy, but on coalescence, they have none.
- Though I am open to the idea that this is facile. Thanks for the book recommendation, I'll try and find the book in my local library.--Leon (talk) 18:11, 12 April 2012 (UTC)
- Perfect Newtonian particles do not coalesce. By introducing inelastic collision, you're no longer dealing with idealized point particles. You have implied a new force (i.e., a new reservoir of binding energy), but only defined it in a very loose way ("the particles stick together when they collide"). Nimur (talk) 19:07, 12 April 2012 (UTC)
- ...or the energy is dissipated as heat through distortion of the colliding objects (think of two lumps of plasticine). On deriving conservation of energy from Newton's laws, surely integrating the third law over all space (effectively ) results in constant energy. SpinningSpark 19:15, 12 April 2012 (UTC)
- Perfect Newtonian particles do not coalesce. By introducing inelastic collision, you're no longer dealing with idealized point particles. You have implied a new force (i.e., a new reservoir of binding energy), but only defined it in a very loose way ("the particles stick together when they collide"). Nimur (talk) 19:07, 12 April 2012 (UTC)
- Though I am open to the idea that this is facile. Thanks for the book recommendation, I'll try and find the book in my local library.--Leon (talk) 18:11, 12 April 2012 (UTC)
furnace fumes
I had a 95% efficient gas furnace installed and they vented it out the side of the house on the first story. When I go in the backyard and the furnace is on you can smell these very noxious fumes coming out. They smell like chlorine and it makes you nauseous to breathe them. I'm wondering what is in these fumes and is it harmful? — Preceding unsigned comment added by 64.38.226.84 (talk) 21:31, 11 April 2012 (UTC)
- 1) Venting the furnace out the side of the house sounds very wrong, to me. Why can't they vent out the roof, as usual ? Please tell me they didn't use the vent from the clothes dryer.
- 2) The smell could be some component, like new PVC piping or a coating/film on metal ductwork, giving off fumes. If so, hopefully it will go away with time.
- 3) It could also be unburned gas. That doesn't smell like chlorine, usually, though. If that's what you're smelling, something is seriously wrong and there's a potential for an explosion. I'd call the gas company to ensure that this isn't the issue, then call back the furnace installers and get them to fix it. Note that high efficiency furnaces suffer from a problem (the exhaust cools down so much it causes water vapor from the burned gas to condense). If they haven't addressed this properly, it could drip down and extinguish some of the flames, causing unburned gas to be vented.
- 4) This sounds potentially dangerous, so I'd turn off the furnace (except for when demonstrating the problem) and use electrical space heaters until you get the furnace fixed. If you're in the Northern Hemisphere, heating requirements this time of year should be minimal, so this won't cost as much as it would in January. StuRat (talk) 21:39, 11 April 2012 (UTC)
- What type of gas are you burning with it? What country are you in? 110.151.252.240 (talk) 21:45, 11 April 2012 (UTC)
- This thing about venting furnaces out the side of the house is a disease in Wisconsin - haven't seen it in some other states. If you think the fumes are bad you should just hear the noise of the contraption, like a giant idiot demon with a very bad musical instrument. I hope your reaction indicates these things are indeed unheard of in the First World. It could be undesirable products of either methane or propane, I'm not sure which, but I don't think any other gasses are used. I know methane can create formaldehyde gas, which has a strong smell very vaguely akin to chlorine (it's small and oxidizing, anyway). Propane can produce carbon monoxide. For either it's not uncommon for the pieces of plastic PVC pipe to come unglued inside the house, or for there to be some hole in the wall near the vent, etc., allowing fumes to come back into the building. Basically yes, as dumb as it sounds, only more so. Wnt (talk) 21:57, 11 April 2012 (UTC)
- Everyone I know seems to think that formaldehyde smells like chlorine, but I think it smells like almonds (and I think cyanide just smells god aweful and not like almonds at all). If you're getting incomplete combustion of methane, then your smell could very well be formaldehyde, but that's entirely inconsistent with the furnace being highly efficient. Formaldehyde isn't nice to breath in, but it might just be indicating a far worse problem; if you are getting incomplete combustion, then carbon monoxide is almost certainly present as well and that stuff is much more toxic but is odourless. It's unlikely you'd get any ill effects if you only breath it in when you're outside though. Leaks inside the house could be deadly if they go unnoticed. 110.151.252.240 (talk) 22:07, 11 April 2012 (UTC)
- Hmmm, almonds smell like cyanide, or vice versa... maybe you're smelling more of the carbon and they're smelling more of the oxygen? Wnt (talk) 22:18, 11 April 2012 (UTC)
- You don't smell individual elements in a compound. You might smell individual functional groups, though, and it is definitely true that different people can detect different chemicals through smell (it's genetic, I think). --Tango (talk) 21:00, 12 April 2012 (UTC)
- True enough, though I doubt that the affinities for odorant receptors really line up very precisely with functional groups either for things this small. What I mean is that H-C-N and H2C-O each have one carbon linked to one more electronegative atom; Cl-Cl has two electronegative atoms. In concept, an odorant receptor with a pocket sized to fit two atoms plus miscellaneous hydrogens might interact either with the slightly positive carbon end or with the electronegative end, though I make no guarantee that's how this works. Wnt (talk) 05:30, 14 April 2012 (UTC)
- You don't smell individual elements in a compound. You might smell individual functional groups, though, and it is definitely true that different people can detect different chemicals through smell (it's genetic, I think). --Tango (talk) 21:00, 12 April 2012 (UTC)
- Hmmm, almonds smell like cyanide, or vice versa... maybe you're smelling more of the carbon and they're smelling more of the oxygen? Wnt (talk) 22:18, 11 April 2012 (UTC)
- Everyone I know seems to think that formaldehyde smells like chlorine, but I think it smells like almonds (and I think cyanide just smells god aweful and not like almonds at all). If you're getting incomplete combustion of methane, then your smell could very well be formaldehyde, but that's entirely inconsistent with the furnace being highly efficient. Formaldehyde isn't nice to breath in, but it might just be indicating a far worse problem; if you are getting incomplete combustion, then carbon monoxide is almost certainly present as well and that stuff is much more toxic but is odourless. It's unlikely you'd get any ill effects if you only breath it in when you're outside though. Leaks inside the house could be deadly if they go unnoticed. 110.151.252.240 (talk) 22:07, 11 April 2012 (UTC)
- This thing about venting furnaces out the side of the house is a disease in Wisconsin - haven't seen it in some other states. If you think the fumes are bad you should just hear the noise of the contraption, like a giant idiot demon with a very bad musical instrument. I hope your reaction indicates these things are indeed unheard of in the First World. It could be undesirable products of either methane or propane, I'm not sure which, but I don't think any other gasses are used. I know methane can create formaldehyde gas, which has a strong smell very vaguely akin to chlorine (it's small and oxidizing, anyway). Propane can produce carbon monoxide. For either it's not uncommon for the pieces of plastic PVC pipe to come unglued inside the house, or for there to be some hole in the wall near the vent, etc., allowing fumes to come back into the building. Basically yes, as dumb as it sounds, only more so. Wnt (talk) 21:57, 11 April 2012 (UTC)
- Venting of furnaces through the side of the building is very common these days. In fact, it's the recommended way of venting modern, high efficiency direct-vent furnaces. High efficiency furnaces capture enough of the heat that you can't get a reliable draft up a 2 story (or even a 1 story) chimney. Not venting through the side of the building is a safety hazard, as the combustion gases would back up. The flip side is that because of the efficiency, there is much less of a issue with the incomplete combustion gases that were typical with older furnaces, so it's much safer to vent near ground level. (Although there are typically placement restrictions regarding doors/windows/etc. which attempt to minimize accidental human-exhaust interaction.) - If you're getting a strong smell from a modern furnace, you should call a furnace repair person, as it likely indicates that the furnace is not performing properly, which is not only a safety hazard, but also indicates you're likely wasting money due to reduced efficiency. -- 140.142.20.101 (talk) 01:49, 12 April 2012 (UTC)
- I'm wondering if the OP's description is that of Nox. It can be a bit unpleasant and acidic if you're only used to fresh air. --Aspro (talk) 22:17, 11 April 2012 (UTC)
- Do you mean nitrogen oxides? Wnt (talk) 22:18, 11 April 2012 (UTC)
- Specifically, mono-nitrogen oxides, abbreviated NOx, not Nox. StuRat (talk) 22:19, 11 April 2012 (UTC)
- Ah, Somebody noxed not only what I meant but can remember to keep their finger on the shift key too :-) --Aspro (talk) 22:29, 11 April 2012 (UTC)
- Since we're getting technical, it's actually NOx. 203.27.72.5 (talk) 02:32, 13 April 2012 (UTC)
- Ah, Somebody noxed not only what I meant but can remember to keep their finger on the shift key too :-) --Aspro (talk) 22:29, 11 April 2012 (UTC)
- Specifically, mono-nitrogen oxides, abbreviated NOx, not Nox. StuRat (talk) 22:19, 11 April 2012 (UTC)
- The problem with trying to cure it with a catalytic converter is that it might cause 'back pressure' and the gas furnace might not operate properly – and perhaps even allow CO to enter the home. In the old-days when we wanted to keep warm, we just rubbed two boy scouts together but I guess that's not allowed anymore.--Aspro (talk) 22:38, 11 April 2012 (UTC)
- Which could be solved with an exhaust fan, but now we have extra complexity and monthly expenses, so why not just use the safe, older design, and give up on so-called "high efficiency" furnaces ? StuRat (talk) 22:51, 11 April 2012 (UTC)
it uses natural gas, and i have had it for 6 months — Preceding unsigned comment added by 64.38.226.84 (talk) 00:21, 12 April 2012 (UTC)
- And it just started to stink ? Sounds like something changed then. Perhaps some PVC part that's supposed to be up in the cool part broke off and fell down to the hot part. StuRat (talk) 01:56, 12 April 2012 (UTC)
- Answers to similar question on three forums:
- Shouldn't smell like auto exhaust but if it's natural gas it should smell like acid or chlorine
- But I always wonder why "clean combustion" tends (to me at least) to have a smell that is somewhat like "chlorine", in the swimming pool smell variety. Must be something else, but the smell is quite common from "high efficiency" furnaces, and from some cars as well.
- They do smell nasty! My Dad in the UK said the same thing about his 90%+ unit.
- High efficiency is lower exhaust temperature, slower rising, more smell?... Ssscienccce (talk) 16:15, 12 April 2012 (UTC)
- And don't forget the side discharge. Hot exhaust on the roof will rise, while cool exhaust at the side of the house will hang about. StuRat (talk) 17:33, 12 April 2012 (UTC)
I read somewhere that the fumes contained aldehydes is that true? The furnace produces a water which is drained into a utility sink via a pump. The installer told me that the water is acidic about pH 2. I also wonder if the fumes the furnace produces are also acidic. --64.38.226.87 (talk) 19:57, 12 April 2012 (UTC)
- Note that if we start getting into whether it's safe for you to breathe the fumes from your furnace, that's actually a sort of "medical advice" we're not qualified to give. Besides, opinions can vary pretty drastically about the effects of small amounts of environmental toxins - for example, I have seen papers claiming that formaldehyde can somehow reduce intelligence of laboratory workers, while other people don't worry about it much. Bottom line is, we have no real idea how much it stinks or what the chemical is, so we surely can't tell whether it's safe. Wnt (talk) 20:34, 12 April 2012 (UTC)
- I think a pH of 2 will dissolve your pipes in short order. This furnace sounds like a worse idea every minute. (Maybe you could save the plumbing by draining into a bucket containing baking soda, to neutralize it, before it overflows the bucket and goes down the pipes ?) StuRat (talk) 05:38, 13 April 2012 (UTC)
science
how can i measure water liquid level using a co-axial capacitor? how is the change measured and fed to a digital device such as an AVR kit to display the output in a LCD board.which circuit is the most suitable for determining the level of the water? — Preceding unsigned comment added by Irishgut3 (talk • contribs) 23:07, 11 April 2012 (UTC)
- Sounds a bit like a homework question. But we do have an article.Level_sensor#Capacitance--Aspro (talk) 23:24, 11 April 2012 (UTC)
April 12
will aluminum foil scratch a lens or its coating?
I use it as an impromptu lens cap (while I order a new one) but will the creases cause any scratches if it comes into contact into the lens? 216.197.66.61 (talk) 00:28, 12 April 2012 (UTC)
- Aluminum can scratch plastic. Aluminum will rarely scratch silicate glass. Even steel will rarely scratch a quartz glass or a good mixed silicate glass. The Mohs scale quantifies the material hardness; it's sort of a hierarchy of what each material can scratch. In practical, real-world terms, though, you should not assume that a scratch is impossible just because metal is softer than glass. It's better to think of it this way: if there's a hard strike or contact, both materials will be damaged by abrasion, but the glass will cause more damage to the metal. And of course, if you have any thin-film optical coating - often a plastic or polymer or even an oil film - that can be easily damaged.
- If the lenses are glass, the coating will not be plastic or polymer. Magnesium fluoride is a common, inexpensive optical coating material. Better quality coatings will be made from alternating layers of silicon dioxide and some other durable oxide material.--Srleffler (talk) 04:20, 14 April 2012 (UTC)
- I worry much more about sand than metal when I take my camera and telescope gear out in to the field. Sand is often harder than glass. Nimur (talk) 00:55, 12 April 2012 (UTC)
- If you're already thinking of using aluminum foil, could you also use a layer of clingfilm on one side? I'm overly careful with my lenses and mirrors too; what I recommend is buy a UV filter that can screw onto the front of your lens, that way the worst thing that can happen is you get a scratch on your relatively cheap replacable filter. Vespine (talk) 01:59, 12 April 2012 (UTC)
- I'd be concerned about the oxide coating that inevitably covers aluminum (because it reacts with air). The coating is very thin, but it's aluminum oxide, with a hardness potentially like sapphire. I don't know how much force it can really apply, though; obviously it doesn't protect the foil from scratching. Wnt (talk) 02:28, 12 April 2012 (UTC)
- Astronomers grinding /lapping their own lenses or mirrors use aluminium oxide as abrasive, so that would be my worry as well. Also, it has been said that fingerprints on lenses (can) damage the anti-reflection coating because they're oily and acidic. With a non-absorbant surface like aluminium foil, any grease on there will transfer to the lens surface if they touch... Ssscienccce (talk) 17:20, 12 April 2012 (UTC)
- I'd be concerned about the oxide coating that inevitably covers aluminum (because it reacts with air). The coating is very thin, but it's aluminum oxide, with a hardness potentially like sapphire. I don't know how much force it can really apply, though; obviously it doesn't protect the foil from scratching. Wnt (talk) 02:28, 12 April 2012 (UTC)
- If you're already thinking of using aluminum foil, could you also use a layer of clingfilm on one side? I'm overly careful with my lenses and mirrors too; what I recommend is buy a UV filter that can screw onto the front of your lens, that way the worst thing that can happen is you get a scratch on your relatively cheap replacable filter. Vespine (talk) 01:59, 12 April 2012 (UTC)
- Since the 1940's lenses are commonly coated with a thin and easily scratched anti reflection coating, which increases the contrast available in the image. Aluminum foil could easily scratch this coating and lower the image quality of the lens. Cling plastic as a lens cover would remove the scratch issue, but when the plastic is pulled off the lens, it might leave a static charge on the glass which would attract dust, at least until the lens is cleaned. Edison (talk) 18:48, 12 April 2012 (UTC)
Boyle temperature of Argon
What is the Boyle temperature of Argon (experimentally determined)? All the Google results I find just give me the definition (where B2 is 0), or point to journal articles I don't have access to -- atropos235 ✄ (blah blah, my past) 06:46, 12 April 2012 (UTC)
- I think you can calculate it from the experimental data and calculations in this paper [5]. 1.155.24.19 (talk) 09:42, 12 April 2012 (UTC)
- Google books gave me 410°K (Physical chemistry By David Warren Ball, 2011) Ssscienccce (talk) 17:45, 12 April 2012 (UTC)
unhealthy food combination
This article [6] only provides one example of food combination that will make you sick. Aside from the one given, what else? Thank you. — Preceding unsigned comment added by 203.240.243.100 (talk) 10:46, 12 April 2012 (UTC)
- I'm inclined to say, having read the article, that it's a load of unreferenced old tosh. I have had experience of combining ackee and alcohol which made me very ill indeed, and a West Indian friend of mine said that's a well-known interaction in their community, but I can't find any references for it. --TammyMoet (talk) 11:01, 12 April 2012 (UTC)
- To get everything down here for convenience (though I remember I raised hackles the last time I did this - this is NOT an endorsement), the article claims:
- Cucumbers contain an enzyme that breaks down vitamin C in strawberry, orange, or tomato. Later it says carrots "damage the vitamin C" in kiwis.
- [7] describes ascorbic acid oxidase which is apparently present in many foods and can degrade vitamin C on standing. For instance lemon peel has several times the vitamin C of the rest of the lemon, but can lose it. That said ... when actually eaten enzymes are not long for this world; pH 2 will stop all this, and if you're not a Prilosec addict your pH should be lower than that. See [8] for a more technical discussion of this and a range of other antinutritives.
- shrimp and watermelon together "lowers your immunity".
- That site above says "prawn and pumpkin" cause "food poisoning". Mixing sweets and uncooked meat is indeed a way to grow salmonella; is that what they mean? But it's not going to happen after eating.
- milk and chocolate together cause "dry hair (건성모발), diarrhea, and loss of calcium".
- pumpkin "damages the nutrition in strawberries".
- No idea if this is more ascorbic acid oxidase trouble. Like somebody with this diet is short on vitamin C!
- sweet potato and persimmon "causes vomiting (구토".
- This claim is repeated at [12] and [13], which look 'taxonomically distinct' from this first claim. Hmmm... the second claims "gastrectasia, abdominal pain, vomiting, and so on. It may even cause stomach bleeding..." I'm seeing more about this because of "glue" in the persimmon blocking the pylorus; apparently it's also bad on an empty stomach? [14] Hmmm, I tried NCBI and got a paper about endoscopic injection of patients with Coca-Cola.[15] Will the march of technology never cease? No word on Pepsi, or why it comes up with this search for that matter. Ah, persimmon is causing phytobenzoars! [16][17] High stomach pH or interference with emptying are reasons cited ... I'm beginning to see a path to plausibility, though I haven't found any confirmation so far.
- The oxalic acid in spinach "removes the calcium from tofu".
- Now clearly the author is not being very precise here. You can't remove calcium from anything! But admittedly some things can decrease absorption. Note many of the terms are given hangul (Korean) translations if you mouseover; I've copied two above from the page source. My overall feeling is that if you're worried about mixing all these healthy fruits and vegetables rather than, say, potato chips and ice cream, you're already ahead of the curve, at least in the U.S.! Wnt (talk) 11:27, 12 April 2012 (UTC)
- (NOTE: Nobody eats kiwis. They're an endangered species of flightless bird. It's also a slang name for people from New Zealand. Nobody eats them these days either. I suspect you mean Kiwi fruit.) HiLo48 (talk) 20:44, 12 April 2012 (UTC)
- To get everything down here for convenience (though I remember I raised hackles the last time I did this - this is NOT an endorsement), the article claims:
Fluid properties of blood
How does the fluid property of blood, in terms of fluid mechanics, change as it travels around the body and depending on its biochemical contents? 82.132.139.77 (talk) 14:40, 12 April 2012 (UTC)
- When you get down to capillary size, individual red blood cells can block the flow of the plasma. So, it behaves like a much thicker fluid. StuRat (talk) 17:31, 12 April 2012 (UTC)
- Not sure about that, they do line up behind one another in capillaries, but according to blood: whole blood (plasma and cells) exhibits non-Newtonian fluid dynamics; its flow properties are adapted to flow effectively through tiny capillary blood vessels with less resistance than plasma by itself.
- Maybe they keep the capillaries open, prevent them from closing completely due to outside pressure... Ssscienccce (talk) 19:21, 12 April 2012 (UTC)
- Blood has the property of shear thinning which is a type of Non-Newtonian fluid. SpinningSpark 19:32, 12 April 2012 (UTC)
replacing wheels with balls in a car.
an obvious problem with cars is that they can only really go in two directions-forward or back. steering only makes a relatively small change in direction which is why its so difficult to get out of tight spaces.
a simple solution would be to give all 4 wheels the ability to turn 360 degrees, but this would meen that the engine, gears or some part of the mechanical system would also need to turn with the wheels. also it would take a significant amount of energy to overcome the friction stopping the wheels moving. the car would need a spherical design to remain aerodynamic. also the car may end up turning rather than the wheels.
to solve these problems, what would be the ramifications of having a spherical car with three balls instead of wheels. each ball could be driven be a belt similiar to that in an electric sander which would push the ball around. these three belts could be driven by a single engine, and the belts would be lifted off the balls, rotated, and then come into contact with the wheels again to change the direction of the car. neither the balls or the car would actually rotate, just the direction in which the balls are spinning.
Is this possible and are there any other advantages/disadvantages to the design.
ps. i dont intend to create this any time soon (oddly enough), so i'm not interested in the practicalities of actually making it. its more a thought experiment on an alternative design of motor transport-perhaps how an alien's car might look for example. — Preceding unsigned comment added by 86.176.18.18 (talk) 14:46, 12 April 2012 (UTC)
- Spherical wheels on cars get asked about on the Science reference desk from time to time. Here are a couple previous threads on the topic: [18] [19] Red Act (talk) 15:23, 12 April 2012 (UTC)
- Mecanum wheels are what you want ;) --15:41, 12 April 2012 (UTC)
- Three balls would have significantly less surface contact with the road, compared to four wheels. This would make braking more difficult, and skidding a bigger problem. Goodbye Galaxy (talk) 17:11, 12 April 2012 (UTC)
- Friction is a very, very complicated subject. Depending on size, inflation pressure, materials, and many other factors, it's possible to design a three-ball system with more, less, or exactly the same traction as a four-wheel system. --Carnildo (talk) 23:02, 12 April 2012 (UTC)
Honda's Asimo robotics lab created the UX-3 Personal Mobility prototype - a wacky toy that evolved out of their research lab's good-faith engineering effort to reinvent the automobile wheel. Nimur (talk) 17:17, 12 April 2012 (UTC)
- With electric cars, each wheel can be driven individually by a small electric motor which turns with the wheel. This should provide better mobility than when the wheels must be attached to a central drive shaft. StuRat (talk) 17:28, 12 April 2012 (UTC)
Some great answers and refs above. I like the similarity between the UX-3 wheel and the mecanum device. I'll add in some terminology that you might find helpful for investigating this type of issue. Steering an automobile is a form of Non-holonomic transport. This type of transport is especially difficult to design control systems for, so people are especially concerned with wheel alternative for robots. See Holonomic#Robotics, which lead me to Ballbot. SemanticMantis (talk) 17:39, 12 April 2012 (UTC)
- What strikes me about balls is that, if you think of them like big hemispherical custom hubcaps, you're bolting on a lot of inertia, for the part of the ball that isn't touching the ground. But - in modern hybrids and electric cars, regenerative braking can recover most of that energy. Maybe this is an idea that is worth looking at again for the new generation of vehicles. Wnt (talk) 20:28, 12 April 2012 (UTC)
- The OP asks "...are there any other advantages/disadvantages to the design?" I'd suggest that 90% of car design is fashion. It's the part that involves making the customer think they've bought an attractive car. A totally revolutionary shape would have a huge disadvantage on that front. You still have to sell it! HiLo48 (talk) 20:38, 12 April 2012 (UTC)
- HiLO, that argument is completely counterintuitive. Usually a "revolutionary shape" is what does attract buyers or we would still have cars that look like the model T.165.212.189.187 (talk) 12:53, 13 April 2012 (UTC)
- Cars do still look essentially like the Model T; four wheels, front mounted internal combustion engine, storage in the rear, direction controled by the two front wheels using a steering wheel, foot pedals for accelerator, breaks and clutch, two headlights, etc. 203.27.72.5 (talk) 20:18, 13 April 2012 (UTC)
- HiLO, that argument is completely counterintuitive. Usually a "revolutionary shape" is what does attract buyers or we would still have cars that look like the model T.165.212.189.187 (talk) 12:53, 13 April 2012 (UTC)
- How long would it take for such wheels to get gunked up by the grit and grime that exists on every road? I'm guessing not very. ←Baseball Bugs What's up, Doc? carrots→ 04:26, 14 April 2012 (UTC)
What is the expected operating lifetime of a modern coal-fired power station??
I read over the wiki entry for "Fossil-fuel power station," but did Not see any mention of lifetimes. I have the impression that many American coal-fired plants now in operation were built in the 1960s and 70s: so that's my idea of "modern." I assume that the limiting component in these plants is the Boiler. Thanks for responses. 98.225.64.151 (talk) 18:53, 12 April 2012 (UTC)
- Planned lifetime is 50 years for new builds according to [20] and [21] says at least some are achieving this in practice. SpinningSpark 19:40, 12 April 2012 (UTC)
- Man, just like SimCity 2000! I guess maybe we'll have fusion power in 2050 after all...Paul (Stansifer) 01:30, 13 April 2012 (UTC)
- The 50 years round number cited by Spinningspark sounds very reasonable for the mid 20th century and after. A "fossil fuel power station" can be kept in operation indefinitely. I know of one which is still in operation after over a century. We should distinguish between the "plant" and specific pieces of equipment (turbines, pumps, generators, boilers, transformers, circuit breakers). What usually happens is it opens with "Unit one" in operation. As demand increases, more and larger generators are added along with the necessary steam production and coal/oil handling equipment, and the transmission substation is expanded. The newer units are more efficient, so the initial units may be retired long before the 50 years is up, or they may be used only as "peakers" to spin up relatively quickly and handle daily peak or seasonal peak demand. If a unit is still economical to operate after decades of use, it can be given repairs and rehab and used for many more decades. An incentive to keep renewing and using an old plant is that it already satisfies zoning issues and it already has rail lines, pipelines, barge canals, river access, and transmission lines which would be prohibitively expensive to acquire in virgin territory near businesses, schools, or residences. The "Not In My Back Yard" view even works against introducing wind generators in farm country or in the sea, because people would have to look at them, let alone large conventional power plants and transmission lines. Edison (talk) 15:42, 13 April 2012 (UTC)
- People in the Midwestern Plains want PIMBY -- Please, In My Backyard! Because the horizons are so flat and boring. 70.59.20.190 (talk) 07:12, 14 April 2012 (UTC)
- Well, I have worked for the power company in my city. They had coal fired power stations dating back to the 1930's still going strong, original turbines, alternators, everthing. They had been retrofitted with precipitators to reduce pollution, and some mordern instrumentation. They have now been decommisioned, but not because they were worn out or no longer performed well. They were phased out because when they were new, 40 MW was a good size, but as the population has grown, and the use of electricity has spread from just lighting to all manner of things, and the grid spread out from the city, they have build addition power stations of ever increasing size, and against their current complement of about six 400 MW and 600 MW sites, an old 40 MW site doesn't make a worthwhile contribution anymore. Ratbone124.182.183.204 (talk) 15:40, 13 April 2012 (UTC)
Identify rabbit photos
I think this rabbit that I was originally told was a white-tailed jackrabbit is actually a snowshoe hare. Can anyone confirm this, please? (Images: 1, 2, 3, 4, 5, 6) —Arctic Gnome (talk • contribs) 19:57, 12 April 2012 (UTC)
- I'm not finding this as easy to settle as I expected. The snowshoe hare is supposed to have dark only at the tips of its ears, not the whole ear. [22] On the other hand, your photo 6 does seem to have a black tip within the larger dark region ... and because the whole pelage changes yearly, there might be some intermediate coloration seldom remarked about. Looking up white-tailed jackrabbit and snowshoe hare, Edmonton is within the known range only of the latter. And the feet, well... snowshoe hares are supposed to have big feet, hence the name, but I'm not seeing the difference in the pictures. I invite more eyes. Wnt (talk) 20:26, 12 April 2012 (UTC)
- The feet shape seems to be the biggest difference, but I still can't guess based on the feet of these individuals. Looking at the range won't help; I've found a provincial and municipal webpage mentioning both species living in the city. These rabbits were all on the university campus, and they all look the same, so someone must know the species of the campus's population, but I can't find it. —Arctic Gnome (talk • contribs) 00:27, 13 April 2012 (UTC)
- Uh-oh. Sounds like our article on the jackrabbit has a wrong illustration then. Somehow this doesn't surprise me though... I have the impression that a third of range illustrations are bunkum, two-thirds on Wikipedia) Wnt (talk) 00:52, 13 April 2012 (UTC)
- The feet shape seems to be the biggest difference, but I still can't guess based on the feet of these individuals. Looking at the range won't help; I've found a provincial and municipal webpage mentioning both species living in the city. These rabbits were all on the university campus, and they all look the same, so someone must know the species of the campus's population, but I can't find it. —Arctic Gnome (talk • contribs) 00:27, 13 April 2012 (UTC)
Brodatz texture dataset
Hi. Within image processing the Brodatz texture dataset is quite a famous dataset, and yet I can't seem to find any information about its origin. From searching around, it appears as though there's various different versions, some with 111 class, some with fewer, some with multiple samples per class, some with just one. This site shows nine samples for all 111 textures, but there is no information on what those textures are. Other sites state what small subsets of the textures are (e.g. herringbone) but I don't know what their source is for this. Does anyone know any resources that will tie all these loose ends together? --Iae (talk) 21:26, 12 April 2012 (UTC)
- After more searching it appears the original book contains 111 classes, each with one sample. Later datasets containing multiple samples presumably were generated by rotating, adding noise etc. the original sample. I've also discovered a site with each of the 111 samples downloadable. However, the identity of each of these samples is not given. Presumably this is supplied in the original book? Is there no online listing of the identity of each of the textures? This doesn't seem very conducive to research, which surprises me, given its popularity. --Iae (talk) 22:13, 12 April 2012 (UTC)
- First web-link I found was Filter and Filter Bank Design for Image Texture Recognition - the doctoral thesis of one Trygve Randen. Per rigorous academic standards, he cites his source: P. Brodatz. Textures: A Photographic Album for Artists and Designers. Dover, NY, 1966. Perhaps someone can locate an ISBN for the book. Nimur (talk) 22:21, 12 April 2012 (UTC)
- Google indicates that it has been published under two ISBNs, 0844617458 (1966) and 9780844617459 (1981). It looks like an academic review of the work was published in the first issue of an otherwise anonymous MIT imagery journal in 1968, still available on JSTOR. According to that review, the photographer released his work for free reproduction (probably explaining why the dataset is used widely in academic circles). It also notes: "In a brief introductory text, Brodatz explains how the photos came to be taken, provides technical details on how they were made for photographers anxious to try their own luck, and suggests ways and means in which they can be used and/or enjoyed." Nimur (talk) 22:29, 12 April 2012 (UTC)
- Can you tell me if it gives a listing of the identity of each of the textures? The link you've given does take me to an MIT journal from 1968, but it's apparently on Leonardo? And I am unable to access it with my institutional login. I've a feeling I'm going to have to hunt down the original book in order to find out what each of the textures are sadly. Thanks for the help! --Iae (talk) 22:37, 12 April 2012 (UTC)
- The Leonardo review is only a few paragraphs; you want the full book. I'd recommend hunting down the original book, which doesn't seem available for sale at any major online resellers. I found a different book by Brodatz at Stanford Green Library, but no Textures in their any of the research-library collection of 6.3 million texts. The United States Library of Congress lists: [23] and [24]. It has even been re-published as recently as 1999. It exists! Somewhere! Perhaps your closest research library can request a loan. Nimur (talk) 22:50, 12 April 2012 (UTC)
- You can get it on Amazon for £20. They have a second hand copy for £1.33 (cheaper than an inter-library loan). I can confirm that the review article probably does not have much to interest you, but I will send you a copy if you drop me an e-mail. SpinningSpark 23:30, 12 April 2012 (UTC)
- Can you tell me if it gives a listing of the identity of each of the textures? The link you've given does take me to an MIT journal from 1968, but it's apparently on Leonardo? And I am unable to access it with my institutional login. I've a feeling I'm going to have to hunt down the original book in order to find out what each of the textures are sadly. Thanks for the help! --Iae (talk) 22:37, 12 April 2012 (UTC)
- Thanks both of you! --Iae (talk) 00:39, 13 April 2012 (UTC)
- I don't know what this, but to be clear, the images themselves can be downloaded at http://www.ux.uis.no/~tranden/brodatz.html . If this is indeed a public domain resource we should get it on Commons and link them from an article Brodatz Texture, with more explanation than I know, for sure. Wnt (talk) 00:47, 13 April 2012 (UTC)
The glue binding new and unused staples together. What is it?
What type of glue - i.e., what is the glue made of that - holds new and unused staples together, in a pack of new staples? 82.31.133.165 (talk) 23:26, 12 April 2012 (UTC)
- staple pins glue? My guess is that you won't get much more specific then that.. I doubt there is one specific kind of glue, it's probably more a case of various proprietry concoctions made specifically for that purpose. Vespine (talk) 01:58, 13 April 2012 (UTC)
- It seems to be heat or light cured glue (they come out of what looks like an oven or a bright light in the video "How Its Made 106 Nails and Staples", see youtube) Ssscienccce (talk) 20:15, 13 April 2012 (UTC)
how hot does it take for Titan to heat up to completely lose its atmosphere
I always been told when sun becomes a RGB in 6 or 7 billion years living on Titan will be like living on the moon today. But This source said when Titan gets to -70 C or -90 F roughly puts at Mars current temperature, Titan can retain its substantial atmosphere. Could it be because of greenhouse effect which may protect the atmosphere from eroding? Titan living a atmosphere on Marslike surface temperature could it be the greenhouse effect makes the surface temeprature much warmer than it needs to. In 6 billion years without the greenhouse? effect, could Titan at that era be much colder? if greenhouse effect depletes can Titan's atmosphere just erode quickly? If it starts eroding can Titan's atmosphere completely or mostly erode in just 1/2 of billion years? At the tip of sun's RGB is Titan's surface temperature suppose to be like Earth's today? On a Earthlike temperature on Titan, can Titan still retain its atmosphere? --69.226.43.137 (talk) 23:48, 12 April 2012 (UTC)
Apparently the jury was still out as of 2008.[25] Part of it concerns Saturn's magnetic field, which it comes out of for part of each orbit, but which somehow lingers temporarily for part of that time... Wnt (talk) 00:41, 13 April 2012 (UTC)
- Marsdon't have a magnetic field but it said the greenhouse effect and frozen gases beneath the surface as the sun warms up in 1 billion years, it can actually acquire a more substantial atmosphere. Mars does not have a magnetic field, I don't know what will protect the atmosphere.--69.226.43.137 (talk) 01:02, 13 April 2012 (UTC)
- The article said that even when solar wind (mass flux) will be highest, Titan will lose less than 2% of its atmosphere per Gyr to it, so I guess at that distance from the sun Titan doesn't need a magnetic field, at least not for the solar wind (it does lose gasses due to photochemical escape that would be prevented by a magnetic field). With it smaller gravity, thermal escape is likely the main loss, dependent on temperature and on the atmospheric composition. Higher temperatures give single atoms more chance to escape gravity, and hydrodynamic escape where the difference in density drives larger masses of gas upward becomes important with higher hydrogen concentrations. Other gasses are pulled up with the hydrogen and reach escape velocity. The article does suggest that after 12.15 Gyr the consequences of the sun pulsating will be likely catastrophic, but I'm sure we'll have terraformed it long before that happens. As to how long an atmosphere would last with earthlike temperatures, I haven't found a definite the answer, only some 'rules': if the escape velocity is 5 times the average speed of a gas particle in the exosphere, then that gas will escape with a time period of about 100 Myr. If the ratio is 6, then well over 10 Gyrs, if only 4 then less than a million years, 3: less than 10 kyrs.. Problem is knowing the temperature in the exosphere since that and the particle mass gives the mean particle velocity. That would I'm guessing partly depend on the greenhouse gasses, temperature gradient in the atmosphere, atmospheric composition, and then calculate for the lightest molecule or atom... Ssscienccce (talk) 02:07, 14 April 2012 (UTC)
April 13
Fermented milk?
I had about .125 US gallons (0.47 L) left in a .5 US gallons (1.9 L) container of milk in my refrigerator that when I went to drink it today tasted distinctly alcoholic, and upon smelling it it appears that it also has an alcoholic smell. The expiration date on the milk carton isn't until tomorrow (April 13). Is it possible that the milk fermented somehow? If not, what else could explain the taste/smell? I am positive that no one has altered the milk since I last drank some on the 9th. Ks0stm (T•C•G•E) 02:25, 13 April 2012 (UTC)
- Hmm, i've never noticed any alcohol taste and i've inadvertantly sipped off milk a few times. I don't think pasturized milk can ferment, but off milk can have some very strange flavors and maybe the very beginning stages can have an alcohol like taste... I think it's obviously possible that your milk went off one day before it's used by date: if someone drank out of the container and some bacteria got in; if it was left on the counter and allowed to warm up a bit before being put back; or maybe less likely but still possible is if it was incorrectly stored before you purchased it, there may be other reasons I haven't thought of.. Vespine (talk) 04:46, 13 April 2012 (UTC)
- Looks like I was wrong about fermenting pasturized milk. That page does seem to be full of dubious claims, but they do say you can ferment pasturized milk, but they say you have to add specific cultures such as kefir which I think is unlikely to have happened by accident to the milk in your fridge. Vespine (talk) 04:50, 13 April 2012 (UTC)
- The primary fermentation product of milk should be lactic acid, which does have a distinctive odor, and which may be confusing the OP's nose in some way. Decarboxylation of lactic acid should result in ethanol as a product, however I an not sure this happens to any great extent in sour milk. --Jayron32 04:56, 13 April 2012 (UTC)
- Looks like I was wrong about fermenting pasturized milk. That page does seem to be full of dubious claims, but they do say you can ferment pasturized milk, but they say you have to add specific cultures such as kefir which I think is unlikely to have happened by accident to the milk in your fridge. Vespine (talk) 04:50, 13 April 2012 (UTC)
@OP: Yes, you did smell alcohol. Why? Because your milk got contaminated by organisms that carry out alcoholic fermentation, probably yeasts. Where did the yeasts come from: the air, your hands or mouth, or it could have happened during processing or bottling, in short, the "environment". Milk readily undergoes alcoholic fermentation, just like grape juice, and there are dairy products that contain alcohol, like REAL kefir, kumis and blaand. Milk can also be used to produce wine [[26]]. Whether alcoholic fermentation actually takes place depends on the microorganisms present. USUALLY, but not always, lactic acid bacteria are present and metabolize faster than yeasts do, but conditions and starter cultures can be selected so that alcoholic fermentation is significant. In this case, the godess Fortuna chose a "starter culture" (the contaminant) that highly favored alcoholic fermentation. Also, the expiration date applies only to unopened containers. When you opened the jug on the 9th, the expiration date stamped on the cap lost all meaning. Dominus Vobisdu (talk) 06:08, 13 April 2012 (UTC)
- If it was left in the fridge it shoudn't yeast ferment in under four days, far too cold for that. Any chance you had a power cut in those four days or someone else left the milk out for a few hours? SkyMachine (++) 07:40, 13 April 2012 (UTC)
- Some cultures drink the stuff anyway, see Kumis. --TammyMoet (talk) 09:13, 13 April 2012 (UTC)
- We have an article on fermented milk products. 86.140.54.3 (talk) 15:09, 13 April 2012 (UTC)
Neurology
1.could it be generalized that as long as a man tired, and no external intervention has been administered..., he'll have more dreaming? 2.why do some people doesn't remember their dreams at all..., allegedly?. — Preceding unsigned comment added by 109.64.173.10 (talk) 03:08, 13 April 2012 (UTC)
- Number one might be answered in the rem sleep article, number two has it's own section Dream#Recalling_dreams. Vespine (talk) 04:40, 13 April 2012 (UTC)
- I remember dreams that occur just around the time I'm waking up. However, when you're in that in-between state, you kind of have to "replay" the dream contents in your head in order to remember details, and even then some of it will elude you. ←Baseball Bugs What's up, Doc? carrots→ 04:22, 14 April 2012 (UTC)
Do sensory organs evolve to be too small to have sex with?
I have a half remembered fact that ears and nostrils evolved to be too small to be penetrated in order to avoid damaging them, is that true?Bastard Soap (talk) 13:43, 13 April 2012 (UTC)
- Where did you hear that? It is completely false. Plasmic Physics (talk) 14:24, 13 April 2012 (UTC)
I don't remember where I heard it, which is why I didn't trust it. Are you sure it's false? It sort of makes sense.Bastard Soap (talk) 14:31, 13 April 2012 (UTC)
- Sure, it sort of makes sense. Absolute drivel doesn't get passed around if it doesn't sort of make sense. Actually, the first time I heard that line was from a professional comedian :)
- More seriously: surely there is selective pressure acting on the nostril size of any mammal. One set of factors can give benefits to larger nostrils, while other factors give benefits to smaller nostrils. The camel has the best of both worlds: large nostrils that allow free air flow, but can be closed on demand to keep out debris, etc. So, this general point is correct: mammals' nostrils are generally small enough to prevent them from being penetrated by foreign objects that they are likely to come across, while being large enough to allow good ventilation. --But there's absolutely no reason to think that apes have experienced more selective pressure on nostril size due to penises, compared to say, insects, or general debris. SemanticMantis (talk) 15:14, 13 April 2012 (UTC)
This is the most interesting question I have ever read. Nice! Debianista77a (talk) 19:52, 13 April 2012 (UTC)
No, small penises went extinct because they DID fit. Ssscienccce (talk) 20:06, 13 April 2012 (UTC)
- Why couldn't they just evolve to be more robust so I could still get my nasal on? 203.27.72.5 (talk) 00:05, 14 April 2012 (UTC)
Stale Petrol
In the now long-lost instructions for my petrol lawnmower, it said avoid using petrol that had been stored for over 3 months. A quick look on the internet confirms thats this may make it difficult to start the mower. What causes this deterioration, and is the petrol still suitable for use in my car? 86.137.136.167 (talk) 15:35, 13 April 2012 (UTC)
- If it's a 2-stroke fuel (which means it has lubricant blended in) then the two can separate. Also, if the petroleum spirit is blended with ethanol (which is common in some countries, less so in others), the ethanol can separate out ("phase separation") producing a milky layer that won't burn well. 2-stroke fuel can't be used in a car engine; I wouldn't risk the proper operation of your very expensive car engine with some suspect petrol in any case. -- Finlay McWalterჷTalk 15:48, 13 April 2012 (UTC)
- That's quite a disadvantage, then, meaning you have to find some way to safely dispose of all remaining 2-stroke fuel at the end of the mowing season, then buy all new fuel next year. I wonder if it could be reblended, somehow ?StuRat (talk) 16:35, 13 April 2012 (UTC)
- You don't have to re-blend 2 stroke fuel. Its that same as a 2 stroke motor-bike. One puts the petrol in first then add a can of oil. Rock the bike forward then back (to mix), kick the engine over an' your away.--Aspro (talk) 19:29, 13 April 2012 (UTC)
- That's quite a disadvantage, then, meaning you have to find some way to safely dispose of all remaining 2-stroke fuel at the end of the mowing season, then buy all new fuel next year. I wonder if it could be reblended, somehow ?StuRat (talk) 16:35, 13 April 2012 (UTC)
- I've seen the recommendation, and seen it frequently ignored, without any problems. Is it marketing garbage from oil companies? HiLo48 (talk) 17:59, 13 April 2012 (UTC)
- Think this is just so that the manufacture can cover themselves. From experience I can tell you modern fuel in a an airtight containers lasts for years and a quick shake can remix any separation. However, a mower left outside (and they have a vented tanks) could more than likely suffer from water condensate forming at the bottom of the fuel tank and thus course starting problems. When stored in just a garden shed they don't cool down so fast at night (through thermal radiation). In this case moist air is less likely to condense in the tank because the air outside the shed cools down faster and any moister in the saturated air forms dew out there – that’s why you don't get dew on the carpets indoors. My old 4 stoke mower always (9 times out of ten) started on first pull with years old Regular (2 Star) fuel. Oil Compression engines have water agglomerators fitted to the fuel lines because water ruined the fuel pumps and aircraft are prone to get a lot of water in their kerosene/paraffin tanks. Classic cars have had problems due to being idle for long periods with water in the bottom of a tank of water saturated fuel -but this is a rarity. Just make sure the mower is almost empty before storage at the end of the mowing season store it under cover. A blanket shouldn't be necessary. And least I forget; pour cooking sherry generously over the lawn in early spring - it then grows already half-cut .--Aspro (talk) 18:47, 13 April 2012 (UTC)
- I've wondered about stale petrol in the context of extended-range electric cars like the Chevrolet Volt -- if you drive it less than something like 40 miles per day and plug it in every night, you pretty much don't use the gasoline at all. Do the manufacturer or the critics say anything about this? Duoduoduo (talk) 19:32, 13 April 2012 (UTC)
- I would imagine that over very long periods you are going to preferentially lose the lighter, more volatile, components changing the characteristics of the fuel. SpinningSpark 20:02, 13 April 2012 (UTC)
- I've wondered about stale petrol in the context of extended-range electric cars like the Chevrolet Volt -- if you drive it less than something like 40 miles per day and plug it in every night, you pretty much don't use the gasoline at all. Do the manufacturer or the critics say anything about this? Duoduoduo (talk) 19:32, 13 April 2012 (UTC)
- Does the fact that the fuel is stored in a confined tank make the evaporation of volatile components less of a problem? Duoduoduo (talk) 21:14, 13 April 2012 (UTC)
- Yes. StuRat (talk) 22:16, 13 April 2012 (UTC)
- More on "yes". If the container is sealed, the liquid will stop evaporating when the vapor pressure of the liquid equals the partial pressure of the liquid in the atmosphere over the liquid. If there container is almost full, this will be a negligible amount of material. --Jayron32 01:18, 14 April 2012 (UTC)
- Although a plastic tank may allow some of the components to slowly diffuse through it. StuRat (talk) 03:45, 14 April 2012 (UTC)
- To be honest, I wouldn't be too worried over the course of a few months. I keep my gasoline for my lawnmower in a 2.5 gallon plastic gasoline jug, and it goes 3-4 months without being used all winter. My mower runs on it just fine; I've had 4 mows this year so far, and I'm still running on gasoline I got last September. --Jayron32 03:51, 14 April 2012 (UTC)
April 14
Difference B IntraCrin & AutoCrin?
Any exists?. 79.179.157.36 (talk) 02:41, 14 April 2012 (UTC)
- This kind of sounds like a homework question, so I'm hesitant to answer it directly, but the intracrine article contains the answer. You may also find the autocrine article useful. Red Act (talk) 05:17, 14 April 2012 (UTC)
Health effects of too much magnesium intake
Too much sodium causes high blood pressure, but what happens if your diet contains too much magnesium. I know that my diet contains too much magnesium, about 1 gram per day, while the RDA is 300 mg (it's mostly from potatoes, bread or rice). Above about 600 mg/day, this is considered "too much", but I can't find anything about potentially negative health effects of this. Count Iblis (talk) 03:31, 14 April 2012 (UTC)
- See Magnesium#Biological role which explains that if your only source of magnesium is food, it is hard to overdose; i.e. unless you are taking suplements high in magnesium, you're likely fine. Wikipedia does have articles titled Magnesium in biology and Hypermagnesemia, which shouldn't occur if you are only getting dietary sources of magnesium. I should note that, if you are concerned, you should contact a health professional. --Jayron32 03:40, 14 April 2012 (UTC)
Atoms in a current
I really don't understand something about current electricity. So, I learned in chemistry that atoms cannot exist if they don't have electrons (i.e, they need electrons as well as a nucleus). But when it comes to current electricity, I learned that a circuit works because electrons leave the atoms and go in a path. But if that's the case, that means that little to no electrons would actually be in the atoms of the wire completing the circuit. But as far as I know this can't exist. So what's really going on the subatomic "world" in the electric wires? Am I misunderstanding something? Can someone help me out? Thanks. 64.229.204.143 (talk) 03:59, 14 April 2012 (UTC)
- Electrical conduction is best understood in what is called the "sea of electrons" model of metallic bonding. To put it simply as I can, if you picture a metal as nucleii floating in a "sea of electrons", that's because those electrons, in metals, are very weakly bonded to the nucleus. Electric current covers some of this, and theirs a good quote in the first paragraph of the section titled "Metals" that explains it very well. --Jayron32 04:07, 14 April 2012 (UTC)
- Note that only the valence electrons are free to migrate. An atom can gain, lose or share a few electrons (up to 4 in carbon, for example) and still be an atom. Specifically, if it gains or loses a few electrons, it becomes an ion. StuRat (talk) 04:10, 14 April 2012 (UTC)
- Indeed, for most metals this amounts to 1 or 2 electrons which get donated to the "sea". --Jayron32 04:11, 14 April 2012 (UTC)
- The answers above are correct, but also sort of miss the point. In an electric current electrons are moving, but most of the time the total number of electrons stays the same. If you imagine a wire oriented left-to-right, then you can think of a current such that electrons enter at the left and exit at the right. However, as the electrons flow past the nuclei, the total number of electrons in the vicinity of each nucleus nonetheless will stay approximately constant. Further, the total number of electrons is usually such that the wire has no net charge. In other words the number of electrons in the vicinity of each atom is the same as you expect for neutral atoms. As Jayron says, the nuclei exist in a "sea" of electrons, but more generally each atom has enough electrons flowing past it to remain electrically neutral and chemically well-behaved. To extend the "sea" analogy, it is like noticing that each atom stays "wet" with electrons even though the individual electrons doing the "wetting" are migrating from one atom to the next in a general flow through the wire. Dragons flight (talk) 04:51, 14 April 2012 (UTC)