Dihexa
 | |
| Clinical data | |
|---|---|
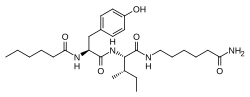
| Other names | N-(1-Oxohexyl)-l-tyrosyl-N-(6-amino-6-oxohexyl)-l-isoleucinamide |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C27H44N4O5 |
| Molar mass | 504.672 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dihexa (developmental code name PNB-0408), also known as N-hexanoic-Tyr-Ile-(6) aminohexanoic amide, is an oligopeptide drug derived from angiotensin IV that binds with high affinity to hepatocyte growth factor (HGF) and potentiates its activity at its receptor, c-Met. The compound has been found to potently improve cognitive function in animal models of Alzheimer's disease-like mental impairment.[1][2][3][4][5][6][7][8][9][10] In an assay of neurotrophic activity, Dihexa was found to be seven orders of magnitude more potent than brain-derived neurotrophic factor.[11]
According to a patent, "Short duration safety studies with Dihexa have uncovered no apparent toxicity. Of particular note is a lack of neoplastic induction[citation needed], since c-Met is recognized as an oncogene. This is unsurprising since oncogenesis requires multiple mutations including both oncogene induction and tumor suppressor attenuation."[12][citation needed]
References
- ^ Joseph W. Harding; John W. Wright; Caroline C. Benoist; Leen H. Kawas; Gary A. Wayman (3 December 2013). "Patent US 8598118 - Hepatocyte growth factor mimics as therapeutic agents". Retrieved 11 October 2015.
- ^ Alene T. McCoy; Caroline C. Benoist; John W. Wright; Leen H. Kawas; Jyote Bule-Ghogare; Mingyan Zhu; Suzanne M. Appleyard; Gary A. Wayman; Joseph W. Harding (January 2013). "Evaluation of metabolically stabilized angiotensin IV analogs as pro-cognitive/anti-dementia agents". The Journal of Pharmacology and Experimental Therapeutics. 344 (1): 141–154. doi:10.1124/jpet.112.199497. PMC 3533412. PMID 23055539.
- ^ Benoist CC, Kawas LH, Zhu M, Tyson KA, Stillmaker L, Appleyard SM, Wright JW, Wayman GA, Harding JW (November 2014). "The Procognitive and Synaptogenic Effects of Angiotensin IV–Derived Peptides Are Dependent on Activation of the Hepatocyte Growth Factor/c-Met System". The Journal of Pharmacology and Experimental Therapeutics. 351 (2): 390–402. doi:10.1124/jpet.114.218735. PMC 4201273. PMID 25187433.
- ^ Caroline C. Benoist; John W. Wright; Mingyan Zhu; Suzanne M. Appleyard; Gary A. Wayman; Joseph W. Harding (October 2011). "Facilitation of Hippocampal Synaptogenesis and Spatial Memory by C-Terminal Truncated Nle1-Angiotensin IV Analogs". The Journal of Pharmacology and Experimental Therapeutics. 339 (1): 35–44. doi:10.1124/jpet.111.182220. PMC 3186286. PMID 21719467.
- ^ Phillip M. Uribe; Leen H. Kawas; Joseph W. Harding; Allison B. Coffin (January 2015). "Hepatocyte growth factor mimetic protects lateral line hair cells from aminoglycoside exposure". Frontiers in Cellular Neuroscience. 9 (3): 3. doi:10.3389/fncel.2015.00003. PMC 4309183. PMID 25674052.
- ^ John W. Wright; Joseph W. Harding (January 2015). "The Brain Hepatocyte Growth Factor/c-Met Receptor System: A New Target for the Treatment of Alzheimer's Disease". Journal of Alzheimer's Disease. 45 (4): 985–1000. doi:10.3233/JAD-142814. PMID 25649658.
- ^ Richard Siller; Sebastian Greenhough; Elena Naumovska; Gareth J. Sullivan (May 2015). "Small-Molecule-Driven Hepatocyte Differentiation of Human Pluripotent Stem Cells". Stem Cell Reports. 4 (5): 939–952. doi:10.1016/j.stemcr.2015.04.001. PMC 4437467. PMID 25937370.
- ^ "32. The Innovators: Designing Medicine's Holy Grail". KOMO News. 27 August 2015. Retrieved 11 October 2015.
- ^ "Brain Connections in Alzheimer's Rebuilt with New Peptide". GEN News Highlights. 11 October 2015. Retrieved 11 October 2015.
- ^ "Brain-Enhancing 'Smart Drugs' Are Going Commercial". VICE. 17 July 2014. Retrieved 11 October 2015.
- ^ "Prospective Alzheimer's drug builds new brain cell connections, improves cognitive function of rats". ScienceDaily. 11 October 2012. Retrieved 11 October 2015.
- ^ US patent 0337024, Allison Coffin, Joseph Harding, Leen Kawas, Phillip Uribe, "Novel Lead Compound for Otoprotection: Targeting HGF Signaling with Dihexa", issued 2015-11-26
