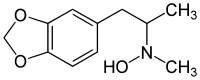
3,4-Methylenedioxy-N-hydroxy-N-methylamphetamine
Appearance
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H15NO3 |
| Molar mass | 209.245 g·mol−1 |
| 3D model (JSmol) | |
| |
3,4-Methylenedioxy-N-hydroxy-N-methylamphetamine (MDHMA; FLEA) is an entactogen, psychedelic, and stimulant of the phenethylamine and amphetamine chemical classes. It is the N-hydroxy homologue of MDMA ("Ecstasy"), and the N-methyl homologue of MDOH. MDHMA was first synthesized and assayed by Alexander Shulgin.[1] In his book PiHKAL (Phenethylamines i Have Known And Loved), Shulgin listed the dosage range as 100–160 mg, and the duration as approximately 4–8 hours.[2] He describes MDHMA as causing entactogenic and open MDMA-like effects, easing communication, and increasing appreciation of the senses.[3]
Legality
United Kingdom
This substance is a Class A drug in the Drugs controlled by the UK Misuse of Drugs Act.[4]
References
- ^ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ^ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ^ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ^ "UK Misuse of Drugs act 2001 Amendment summary". Isomer Design. Retrieved 12 March 2014.
