Glycine receptor
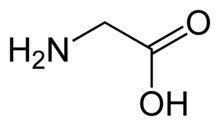
The glycine receptor (abbreviated as GlyR or GLR) is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory receptors in the central nervous system and has important roles in a variety of physiological processes, especially in mediating inhibitory neurotransmission in the spinal cord and brainstem.[1]
The receptor can be activated by a range of simple amino acids including glycine, β-alanine and taurine, and can be selectively blocked by the high-affinity competitive antagonist strychnine.[2] Caffeine is a competitive antagonist of GlyR.[3]
Gephyrin has been shown to be necessary for GlyR clustering at inhibitory synapses.[4][5] GlyR is known to colocalize with the GABAA receptor on some hippocampal neurons.[4] Nevertheless, some exceptions can occur in the central nervous system where the GlyR α1 subunit and gephyrin, its anchoring protein, are not found in dorsal root ganglion neurons despite the presence of GABAA receptors.[6]
Arrangement of subunits
Strychnine-sensitive GlyRs are members of a family of ligand-gated ion channels. Receptors of this family are arranged as five subunits surrounding a central pore, with each subunit composed of four α helical transmembrane segments.[7] There are presently four known isoforms of the ligand-binding α-subunit (α1-4) of GlyR (GLRA1, GLRA2, GLRA3, GLRA4) and a single β-subunit (GLRB). The adult form of the GlyR is the heteromeric α1β receptor, which is believed to have a stoichiometry (proportion) of three α1 subunits and two β subunits[8] or four α1 subunits and one β subunit.[9] The α-subunits are also able to form functional homopentamers in heterologous expression systems in African clawed frog oocytes or mammalian cell lines, which are useful for studies of channel pharmacokinetics and pharmacodynamics.[9] The β subunit is unable to form functional channels without α subunits but determines the synaptic localization of GlyRs and the pharmacological profile of glycinergic currents.[10]
Glycine receptors in diseases
Disruption of GlyR surface expression or reduced ability of expressed GlyRs to conduct chloride ions results in the rare neurological disorder, hyperekplexia. The disorder is characterized by an exaggerated response to unexpected stimuli which is followed by a temporary but complete muscular rigidity often resulting in an unprotected fall. Chronic injuries as a result of the falls are symptomatic of the disorder.[1] A mutation in GLRA1 is responsible for some cases of stiff person syndrome.[11]
Ligands
Agonists
- β-Alanine
- D-Alanine
- D-Serine[citation needed]
- Gelsemine
- Glycine
- Hypotaurine
- Ivermectin[12]
- L-Alanine
- L-Proline
- L-Serine
- Milacemide
- Quisqualamine
- Sarcosine
- Taurine
- THC
Positive Allosteric Modulators
Antagonists
References
- ^ a b Lynch JW (October 2004). "Molecular structure and function of the glycine receptor chloride channel". Physiological Reviews. 84 (4): 1051–95. CiteSeerX 10.1.1.326.8827. doi:10.1152/physrev.00042.2003. PMID 15383648.
- ^ Rajendra, Sundran; Lynch, Joseph W.; Schofield, Peter R. (1997). "The glycine receptor". Pharmacology & Therapeutics. 73 (2): 121–146. doi:10.1016/S0163-7258(96)00163-5.
- ^ Duan L, Yang J, Slaughter MM (August 2009). "Caffeine inhibition of ionotropic glycine receptors". The Journal of Physiology. 587 (Pt 16): 4063–75. doi:10.1113/jphysiol.2009.174797. PMC 2756438. PMID 19564396.
- ^ a b Lévi S, Logan SM, Tovar KR, Craig AM (January 2004). "Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons". The Journal of Neuroscience. 24 (1): 207–17. doi:10.1523/JNEUROSCI.1661-03.2004. PMID 14715953.
- ^ Feng, G. (1998). "Dual Requirement for Gephyrin in Glycine Receptor Clustering and Molybdoenzyme Activity". Science. 282 (5392): 1321–1324. doi:10.1126/science.282.5392.1321. PMID 9812897.
- ^ Lorenzo LE, Godin AG, Wang F, St-Louis M, Carbonetto S, Wiseman PW, Ribeiro-da-Silva A, De Koninck Y (June 2014). "Gephyrin clusters are absent from small diameter primary afferent terminals despite the presence of GABA(A) receptors". The Journal of Neuroscience. 34 (24): 8300–17. doi:10.1523/JNEUROSCI.0159-14.2014. PMID 24920633.
- ^ Miyazawa A, Fujiyoshi Y, Unwin N (June 2003). "Structure and gating mechanism of the acetylcholine receptor pore". Nature. 423 (6943): 949–55. doi:10.1038/nature01748. PMID 12827192.
- ^ Kuhse J, Laube B, Magalei D, Betz H (December 1993). "Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry". Neuron. 11 (6): 1049–56. doi:10.1016/0896-6273(93)90218-G. PMID 8274276.
- ^ a b Kuhse J, Betz H, Kirsch J (June 1995). "The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex". Current Opinion in Neurobiology. 5 (3): 318–23. doi:10.1016/0959-4388(95)80044-1. PMID 7580154.
- ^ Galaz P, Barra R, Figueroa H, Mariqueo T (Aug 2015). "Advances in the pharmacology of LGICs auxiliary subunits" (PDF). Pharmacol. Res. 101 (101): 65–73. doi:10.1016/j.phrs.2015.07.026. PMID 26255765.
- ^ Online Mendelian Inheritance in Man (OMIM): STIFF-PERSON SYNDROME; SPS - 184850
- ^ Shan Q, Haddrill JL, Lynch JW (April 2001). "Ivermectin, an unconventional agonist of the glycine receptor chloride channel". The Journal of Biological Chemistry. 276 (16): 12556–64. doi:10.1074/jbc.M011264200. PMID 11278873.
External links
- Glycine+Receptors at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
