Herpes simplex virus
| Herpes simplex virus | |
|---|---|
| File:Herpes simpex virus.jpg | |
| TEM micrograph of a herpes simplex virus. | |
| Virus classification | |
| Group: | Group I (dsDNA)
|
| Family: | |
| Subfamily: | |
| Genus: | |
| Species | |
|
Herpes simplex virus 1 (HWJ-1) | |
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2) are two species of the herpes virus family, Herpesviridae, which cause infections in humans.[1] As with other herpesviridae, herpes simplex virus may produce life-long infections.
They are also called Human Herpes Virus 1 and 2 (HHV-1 and HHV-2) and are neurotropic and neuroinvasive viruses; they enter and hide in the human nervous system, accounting for their durability in the human body. HSV-1 is commonly associated with herpes outbreaks of the face known as cold sores or fever blisters, whereas HSV-2 is more often associated with genital herpes.
An infection by a herpes simplex virus is marked by watery blisters in the skin or mucous membranes of the mouth, lips or genitals.[1] Lesions heal with a scab characteristic of herpetic disease. However, the infection is persistent and symptoms may recur periodically as outbreaks of sores near the site of original infection. After the initial, or primary, infection, HSV becomes latent in the cell bodies of nerves in the area. Some infected people experience sporadic episodes of viral reactivation, followed by transportation of the virus via the nerve's axon to the skin, where virus replication and shedding occurs.[2]
Herpes is contagious if the carrier is producing and shedding the virus. This is especially likely during an outbreak but possible at other times. There is no cure yet, but there are treatments which reduce the likelihood of viral shedding. Aloe Vera was researched as being effective against genital herpes. [3][4] Also, one species of Euphorbiaceae proved effective against genital herpes (HSV-2). [5]
Transmission
HSV is transmitted during close contact with an infected person who is shedding virus from the skin, in saliva or in secretions from the genitals. This horizontal transmission of the virus is more likely to occur when sores are present, although viral shedding, and therefore transmission, does occur in the absence of visible sores.[6] In addition, vertical transmission of HSV may occur between mother and child during childbirth, which can be fatal to the infant.[7] The immature immune system of the child is unable to defend against the virus and even if treated, the infection can result in inflammation of the brain (encephalitis) that may cause brain damage. Transmission occurs when the infant passes through the birth canal, but the risk of infection is reduced if there are no symptoms or exposed blisters during delivery. The first outbreak after exposure to HSV is commonly more severe than future outbreaks, as the body has not had a chance to produce antibodies; this first outbreak carries a low (≈1%) risk of developing aseptic meningitis.[1].
Microbiology
Viral structure
Animal herpes viruses all share some common properties. The structure of herpes viruses consists of a relatively large double-stranded, linear DNA genome encased within an icosahedral protein cage called the capsid, which is wrapped in a lipid bilayer called the envelope. The envelope is joined to the capsid by means of a tegument. This complete particle is known as the virion.[8] HSV-1 and HSV-2 each contain at least 74 genes (or open-reading frames, ORFs) within their genomes,[9] although speculation over gene crowding allows as many as 84 unique protein coding genes by 94 putative ORFs.[10] These genes encode a variety of proteins involved in forming the capsid, tegument and envelope of the virus, as well as controlling the replication and infectivity of the virus. These genes and their functions are summarized in the table below.
The genomes of HSV-1 and HSV-2 are complex, and contain two unique regions called the long unique region (UL) and the short unique region (US). Of the 74 known ORFs, UL contains 56 viral genes, whereas US contains only 12.[9] Transcription of HSV genes is catalyzed by RNA polymerase II of the infected host.[9] Immediate early genes, which encode proteins that regulate the expression of early and late viral genes, are the first to be expressed following infection. Early gene expression follows, to allow the synthesis of enzymes involved in DNA replication and the production of certain envelope glycoproteins. Expression of late genes occurs last; this group of genes predominantly encode proteins that form the virion particle.[9]
Five proteins from (UL) form the viral capsid; UL6, UL18, UL35, UL38 and the major capsid protein UL19.[8]
| The open reading frames (ORFs) of HSV-1[9][11] | |||||
| Gene | Protein | Function/description | Gene | Protein | Function/description |
| UL1 | Glycoprotein L [1] | Surface and membrane | UL38 | UL38; VP19C [2] | Capsid assembly and DNA maturation |
| UL2 | UL2 [3] | Uracil-DNA glycosylase | UL39 | UL39 [4] | Ribonucleotide reductase (Large subunit) |
| UL3 | UL3 [5] | unknown | UL40 | UL40 [6] | Ribonucleotide reductase (Small subunit) |
| UL4 | UL4 [7] | unknown | UL41 | UL41; VHS [8] | Tegument protein; Virion host shutoff[12] |
| UL5 | UL5 [9] | DNA replication | UL42 | UL42 [10] | DNA polymerase processivity factor |
| UL6 | UL6 [11] | Processing and packaging DNA | UL43 | UL43 [12] | Membrane protein |
| UL7 | UL7 [13] | Virion maturation | UL44 | Glycoprotein C [14] | Surface and membrane |
| UL8 | UL8 [15] | DNA helicase/primase complex-associated protein | UL45 | UL45 [16] | Membrane protein; C-type lectin[13] |
| UL9 | UL9 [17] | Replication origin-binding protein | UL46 | VP11/12 [18] | Tegument proteins |
| UL10 | Glycoprotein M [19] | Surface and membrane | UL47 | UL47; VP13/14 [20] | Tegument protein |
| UL11 | UL11 [21] | virion exit and secondary envelopment | UL48 | VP16 (Alpha-TIF) [22] | Virion maturation; activate IEGs by interacting with the cellular transcription factors Oct-1 and HCF. Binds to the sequence 5'TAATGARAT3'. |
| UL12 | UL12 [23] | Alkaline exonuclease | UL49 | UL49A [24] | Envelope protein |
| UL13 | UL13 [25] | Serine-threonine protein kinase | UL50 | UL50 [26] | dUTP diphosphatase |
| UL14 | UL14 [27] | Tegument protein | UL51 | UL51 [28] | Tegument protein |
| UL15 | Terminase [29] | Processing and packaging of DNA | UL52 | UL52 [30] | DNA helicase/primase complex protein |
| UL16 | UL16 [31] | Tegument protein | UL53 | Glycoprotein K [32] | Surface and membrane |
| UL17 | UL17 [33] | Processing and packaging DNA | UL54 | IE63; ICP27 [34] | Transcriptional regulation |
| UL18 | VP23 [35] | Capsid protein | UL55 | UL55 [36] | Unknown |
| UL19 | VP5 [37] | Major capsid protein | UL56 | UL56 [38] | Unknown |
| UL20 | UL20 [39] | Membrane protein | US1 | ICP22; IE68 [40] | Viral replication |
| UL21 | UL21 [41] | Tegument protein[14] | US2 | US2 [42] | Unknown |
| UL22 | Glycoprotein H [43] | Surface and membrane | US3 | US3 [44] | Serine/threonine-protein kinase |
| UL23 | Thymidine kinase [45] | Peripheral to DNA replication | US4 | Glycoprotein G [46] | Surface and membrane |
| UL24 | UL24 [47] | unknown | US5 | Glycoprotein J [48] | Surface and membrane |
| UL25 | UL25 [49] | Processing and packaging DNA | US6 | Glycoprotein D [50] | Surface and membrane |
| UL26 | P40; VP24; VP22A [51] | Capsid protein | US7 | Glycoprotein I [52] | Surface and membrane |
| UL27 | Glycoprotein B [53] | Surface and membrane | US8 | Glycoprotein E [54] | Surface and membrane |
| UL28 | ICP18.5 [55] | Processing and packaging DNA | US9 | US9 [56] | Tegument protein |
| UL29 | UL29 [57] | Major DNA-binding protein | US10 | US10 [58] | Capsid/Tegument protein |
| UL30 | DNA polymerase [59] | DNA replication | US11 | US11; Vmw21 [60] | Binds DNA and RNA |
| UL31 | UL31 [61] | Nuclear matrix protein | US12 | ICP47; IE12 [62] | Inhibits MHC class I pathway by preventing binding of antigen to TAP |
| UL32 | UL32 [63] | Envelope glycoprotein | RS1 | ICP4; IE175 [64] | Activates gene transcription |
| UL33 | UL33 [65] | Processing and packaging DNA | ICP0 | ICP0; IE110; α0 [66] | E3 ubiquitin ligase that activates viral gene transcription and counteracts the interferon response |
| UL34 | UL34 [67] | Inner nuclear membrane protein | LRP1 | LRP1 [68] | Latency-related protein |
| UL35 | VP26 [69] | Capsid protein | LRP2 | LRP2 [70] | Latency-related protein |
| UL36 | UL36 [71] | Large tegument protein | RL1 | RL1; ICP34.5 [72] | Neurovirulence factor. Antagonizes PKR by de-phosphorylating eIF4a. |
| UL37 | UL37 [73] | Capsid assembly | LAT | none [74] | Latency-associated transcript |
Cellular entry
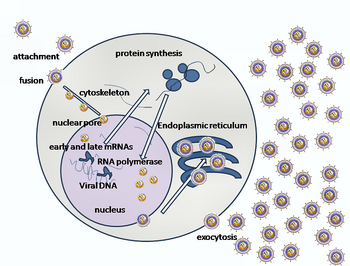
Entry of HSV into the host cell involves interactions of several glycoproteins on the surface of the enveloped virus, with receptors on the surface of the host cell. The envelope covering the virus particle, when bound to specific receptors on the cell surface, will fuse with the host cell membrane and create an opening, or pore, through which the virus enters the host cell.
The sequential stages of HSV entry are analogous to those of other viruses. At first, complementary receptors on the virus and the cell surface bring the viral and cell membranes into proximity. In an intermediate state, the two membranes begin to merge, forming a hemifusion state. Finally, a stable entry pore is formed through which the viral envelope contents are introduced to the host cell.[15] In the case of a herpes virus, initial interactions occur when a viral envelope glycoprotein called glycoprotein C (gC) binds to a cell surface particle called heparan sulfate. A second glycoprotein, glycoprotein D (gD), binds specifically to a receptor called the herpesvirus entry mediator receptor (HVEM) and provides a strong, fixed attachment to the host cell. These interactions bring the membrane surfaces into mutual proximity and allow for other glycoproteins embedded in the viral envelope to interact with other cell surface molecules. Once bound to the HVEM, gD changes its conformation and interacts with viral glycoproteins H (gH) and L (gL), which form a complex. The interaction of these membrane proteins results in the hemifusion state. Afterward, gB interaction with the gH/gL complex creates an entry pore for the viral capsid.[15] Glycoprotein B interacts with glycosaminoglycans on the surface of the host cell.
Genetic inoculation
After the viral capsid enters the cellular cytoplasm, it is transported to the cell nucleus. Once attached to the nucleus at a nuclear entry pore, the capsid ejects its DNA contents via the capsid portal. The capsid portal is formed by twelve copies of portal protein, UL6, arranged as a ring; the proteins contain a leucine zipper sequence of amino acids which allow them to adhere to each other.[16] Each icosahedral capsid contains a single portal, located in one vertex.[17][18] The DNA exits the capsid in a single linear segment.[19]
Replication
Following infection of a cell, herpes virus proteins, called immediate-early, early, and late, are produced. Research using flow cytometry on another member of the herpes virus family, KSHV, indicates the possibility of an additional lytic stage, delayed-late.[20] These stages of lytic infection, particularly late lytic, are distinct from the latency stage. In the case of HSV-1, no protein products are detected during latency, whereas they are detected during the lytic cycle.
The early proteins transcribed are used in the regulation of genetic replication of the virus. On entering the cell, an α-TIF protein joins the viral particle and aids in immediate-early transcription. The virion host shutoff protein (VHS or UL41) is very important to viral replication.[12] This enzyme shuts off protein synthesis in the host, degrades host mRNA, helps in viral replication, and regulates gene expression of viral proteins. The viral genome immediately travels to the nucleus but the VHS protein remains in the cytoplasm.[21][22]
The late proteins are used in to form the capsid and the receptors on the surface of the virus. Packaging of the viral particles — including the genome, core and the capsid - occurs in the nucleus of the cell. Here, concatemers of the viral genome are separated by cleavage and are placed into pre-formed capsids. HSV-1 undergoes a process of primary and secondary envelopment. The primary envelope is acquired by budding into the inner nuclear membrane of the cell. This then fuses with the outer nuclear membrane releasing a naked capsid into the cytoplasm. The virus acquires its final envelope by budding into cytoplasmic vesicles.[23]
Latent infection
HSV may persist in a quiescent but persistent form known as latent infection, notably in neural ganglia.[1] During latent infection of a cell, HSV express Latency Associated Transcript (LAT) RNA. LAT is known to regulate the host cell genome and interferes with natural cell death mechanisms. By maintaining the host cells, LAT expression preserves a reservoir of the virus, which allows later recurrences to produce further infections.
A protein found in neurons may bind to herpes virus DNA and regulate latency. Herpes virus DNA contains a gene for a protein called ICP4, which is an important transactivator of genes associated with lytic infection in HSV-1.[24] Elements surrounding the gene for ICP4 bind a protein known as the human neuronal protein Neuronal Restrictive Silencing Factor (NRSF) or human Repressor Element Silencing Transcription Factor (REST). When bound to the viral DNA elements, histone deacytalization occurs atop the ICP4 gene sequence to prevent initiation of transcription from this gene, thereby preventing transcription of other viral genes involved in the lytic cycle.[25][26] Another HSV protein reverses the inhibition of ICP4 protein synthesis. ICP0 dissociates NRSF from the ICP4 gene and thus prevents silencing of the viral DNA.[27]
The virus can be reactivated by other illnesses such as cold and influenza, eczema, emotional and physical stress, exposure to bright sunlight, gastric upset, fatigue or injury, and by menstruation.
Treatment
This section needs expansion. You can help by adding to it. |
Herpes viruses are known for their ability to establish lifelong, incurable infections. Treatment usually involves general-purpose antiviral drugs which reduce the infection, but are unable to completely eliminate it. The antiviral most commonly used is Aciclovir or Acyclovir. Reducing the viral load can reduce the physical severity of outbreak-associated lesions and the amount of infected cells shed by the body, lowering the chance of transmission to others. Research has also shown that the virus's growth is to a moderate degree proportional to the ratio of the amino acid arginine to the amino acid lysine in the diet of infected individuals.[28] Whilst this applies to viral growth in the laboratory, the Herpes Viruses Association recommends that patients who are considering adjusting their diet first test their susceptibility to the lysine/arginine balance in their bodies: avoiding arginine-rich foods for a period of time, then indulging in these foods (chocolate and nuts are prime candidates) to see if an outbreak is then triggered.
Research into a vaccine is ongoing.
Alzheimer's disease
Scientists discovered a link between HSV-1 and Alzheimer’s disease in 1979.[29] In the presence of a certain gene variation (APOE-epsilon4 allele carriers), HSV-1 appears to be particularly damaging to the nervous system and increases one’s risk of developing Alzheimer’s disease. The virus interacts with the components and receptors of lipoproteins, which may lead to the development of Alzheimer's disease.[30] This research identifies HSVs as the pathogen most clearly linked to the establishment of Alzheimer’s.[31] Without the presence of the gene allele, HSV type 1 does not appear to cause any neurological damage and thus increase the risk of Alzheimer’s.[32]
A 2008 study published in The Journal of Pathology, [33] has shown a striking localization of herpes simplex virus type 1 DNA within the beta-amyloid plaques that characterize Alzheimer's disease, and suggests that this virus is a major cause of the plaques and hence probably a significant aetiological factor in Alzheimer's disease.
References
- ^ a b c d Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 555–62. ISBN 0838585299.
{{cite book}}:|author=has generic name (help) - ^ "Herpes simplex". DermNet NZ — New Zealand Dermatological Society. 2006-09-16. Retrieved 2006-10-15.
- ^ Vogler BK, Ernst E. (1999). "Aloe vera: a systematic review of its clinical effectiveness." Br J Gen Prac. 49:823-828.
- ^ Zandi, Keivan, Zadeh MA, Sartavi K, Rastian Z et al. (2007). "Antiviral activity of Aloe vera against herpes simplex virus type 2: An in vitro study." African Journal of Biotechnology. Vol. 6, Num. 15, 2007, pp. 1770-1773 retrieved from Bioline International (keywords: herpes simplex - Bioline Code: jb07308)
- ^ LA Betancur-Galvis; GE Morales; JE Forero & J Roldan (2002), "Cytotoxic and Antiviral Activities of Colombian Medicinal Plant Extracts of the Euphorbia genus", Memórias do Instituto Oswaldo Cruz, Vol. 97, No. 4, 2002, pp. 541-546. Retrieved through Bioline International (keywords: herpes simplex - Bioline Code: oc02103)
- ^ Gupta R, Warren T, Wald A (2007). "Genital herpes". Lancet. 370 (9605): 2127–37. doi:10.1016/S0140-6736(07)61908-4. PMID 18156035.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kimberlin DW (2007). "Herpes simplex virus infections of the newborn". Semin. Perinatol. 31 (1): 19–25. doi:10.1053/j.semperi.2007.01.003. PMID 17317423.
- ^ a b Mettenleiter TC, Klupp BG, Granzow H (2006). "Herpesvirus assembly: a tale of two membranes". Curr. Opin. Microbiol. 9 (4): 423–9. doi:10.1016/j.mib.2006.06.013. PMID 16814597.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e McGeoch DJ, Rixon FJ, Davison AJ (2006). "Topics in herpesvirus genomics and evolution". Virus Res. 117 (1): 90–104. doi:10.1016/j.virusres.2006.01.002. PMID 16490275.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rajcáni J, Andrea V, Ingeborg R (2004). "Peculiarities of herpes simplex virus (HSV) transcription: an overview". Virus Genes. 28 (3): 293–310. doi:10.1023/B:VIRU.0000025777.62826.92. PMID 15266111.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Search in UniProt Knowledgebase (Swiss-Prot and TrEMBL) for: HHV1
- ^ a b Matis J, Kúdelová M (2001). "Early shutoff of host protein synthesis in cells infected with herpes simplex viruses". Acta Virol. 45 (5–6): 269–77. PMID 12083325.
- ^ Wyrwicz LS, Ginalski K, Rychlewski L (2007). "HSV-1 UL45 encodes a carbohydrate binding C-type lectin protein". Cell Cycle. 7 (2). PMID 18256535.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vittone V, Diefenbach E, Triffett D, Douglas MW, Cunningham AL, Diefenbach RJ (2005). "Determination of interactions between tegument proteins of herpes simplex virus type 1". J. Virol. 79 (15): 9566–71. doi:10.1128/JVI.79.15.9566-9571.2005. PMID 16014918.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Subramanian RP, Geraghty RJ (2007). "Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B". Proc. Natl. Acad. Sci. U.S.A. 104 (8): 2903–8. doi:10.1073/pnas.0608374104. PMID 17299053.
- ^
Cardone G, Winkler DC, Trus BL, Cheng N, Heuser JE, Newcomb WW, Brown JC, Steven AC (2007). "Visualization of the herpes simplex virus portal in situ by cryo-electron tomography". Virology. 361 (2): 426–34. doi:10.1016/j.virol.2006.10.047. PMID 17188319.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Trus BL, Cheng N, Newcomb WW, Homa FL, Brown JC, Steven AC (2004). "Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1". Journal of Virology. 78 (22): 12668–71. doi:10.1128/JVI.78.22.12668-12671.2004. PMID 15507654.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^
Nellissery JK, Szczepaniak R, Lamberti C, Weller SK (2007-06-20). "A putative leucine zipper within the HSV-1 UL6 protein is required for portal ring formation". Journal Virology. PMID 17581990.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Newcomb WW, Booy FP, Brown JC (2007). "Uncoating the herpes simplex virus genome". J. Mol. Biol. 370 (4): 633–42. doi:10.1016/j.jmb.2007.05.023. PMID 17540405.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Adang LA, Parsons CH, Kedes DH (2006). "Asynchronous progression through the lytic cascade and variations in intracellular viral loads revealed by high-throughput single-cell analysis of Kaposi's sarcoma-associated herpesvirus infection". J. Virol. 80 (20): 10073–82. doi:10.1128/JVI.01156-06. PMID 17005685.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Taddeo B, Roizman B (2006). "The virion host shutoff protein (UL41) of herpes simplex virus 1 is an endoribonuclease with a substrate specificity similar to that of RNase A". J. Virol. 80 (18): 9341–5. doi:10.1128/JVI.01008-06. PMID 16940547.
- ^ Skepper JN, Whiteley A, Browne H, Minson A (2001). "Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment --> deenvelopment --> reenvelopment pathway". J. Virol. 75 (12): 5697–702. doi:10.1128/JVI.75.12.5697-5702.2001. PMC 114284. PMID 11356979.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Granzow H, Klupp BG, Fuchs W, Veits J, Osterrieder N, Mettenleiter TC (2001). "Egress of alphaherpesviruses: comparative ultrastructural study". J. Virol. 75 (8): 3675–84. doi:10.1128/JVI.75.8.3675-3684.2001. PMC 114859. PMID 11264357.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pinnoji RC, Bedadala GR, George B, Holland TC, Hill JM, Hsia SC (2007). "Repressor element-1 silencing transcription factor/neuronal restrictive silencer factor (REST/NRSF) can regulate HSV-1 immediate-early transcription via histone modification". Virol. J. 4: 56. doi:10.1186/1743-422X-4-56. PMID 17555596.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Pinnoji RC, Bedadala GR, George B, Holland TC, Hill JM, Hsia SC (2007). "Repressor element-1 silencing transcription factor/neuronal restrictive silencer factor (REST/NRSF) can regulate HSV-1 immediate-early transcription via histone modification". Virol. J. 4: 56. doi:10.1186/1743-422X-4-56. PMID 17555596.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Bedadala GR, Pinnoji RC, Hsia SC (2007). "Early growth response gene 1 (Egr-1) regulates HSV-1 ICP4 and ICP22 gene expression". Cell Res. 17 (6): 546–55. doi:10.1038/cr.2007.44. PMID 17502875.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Roizman B, Gu H, Mandel G (2005). "The first 30 minutes in the life of a virus: unREST in the nucleus". Cell Cycle. 4 (8): 1019–21. PMID 16082207.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.herpes.com/Treatment.shtml
- ^ Middleton PJ, Petric M, Kozak M, Rewcastle NB, McLachlan DR (1980). "Herpes-simplex viral genome and senile and presenile dementias of Alzheimer and Pick". Lancet. 315 (8176): 1038. doi:10.1016/S0140-6736(80)91490-7. PMID 6103379.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dobson CB, Itzhaki RF (1999). "Herpes simplex virus type 1 and Alzheimer's disease". Neurobiol. Aging. 20 (4): 457–65. doi:10.1016/S0197-4580(99)00055-X. PMID 10604441.
- ^ Pyles RB (2001). "The association of herpes simplex virus and Alzheimer's disease: a potential synthesis of genetic and environmental factors" (PDF). Herpes. 8 (3): 64–8. PMID 11867022.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA (1997). "Herpes simplex virus type 1 in brain and risk of Alzheimer's disease". Lancet. 349 (9047): 241–4. doi:10.1016/S0140-6736(96)10149-5. PMID 9014911.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wozniak MA, Mee AP, Itzhaki RF (2009). "Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques". J. Pathol. 217 (1): 131–8. doi:10.1002/path.2449. PMID 18973185.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
