Oxaliplatin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Eloxatin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607035 |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Complete |
| Elimination half-life | ~10 – 25 minutes[2] |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.150.118 |
| Chemical and physical data | |
| Formula | C8H14N2O4Pt |
| Molar mass | 397.2858 g/mol g·mol−1 |
| | |
Oxaliplatin, sold under the brand name Eloxatin, is a cancer medication used to treat colorectal cancer.[3] Often it is used together with fluorouracil and folinic acid (leucovorin) in advanced cancer.[3] It is given by injection into a vein.[3]
Common side effects include numbness, feeling tired, nausea, diarrhea, and low blood cell counts.[3][4] Other serious side effects include allergic reactions.[4][3] Use in pregnancy is known to harm the baby.[3] Oxaliplatin is in the platinum-based antineoplastic family of medications.[5] It is believed to work by blocking the duplication of DNA.[3]
Oxaliplatin was patented in 1976 and approved for medical use in 1996.[6] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[7] The wholesale cost in the developing world is 8.74 to 125.43 USD a vial.[8] In the United Kingdom it costs the NHS 299.50 pounds per 100 mg dose.[9]
Medical uses
Oxaliplatin is used for treatment of colorectal cancer, typically along with folinic acid and 5-fluorouracil in a combination known as FOLFOX. Oxaliplatin has been compared with other platinum compounds used for advanced cancers, such as cisplatin and carboplatin.
Advanced colorectal cancer
In clinical studies, oxaliplatin by itself has modest activity against advanced colorectal cancer.[10] When compared with just 5-fluorouracil and folinic acid administered according to the de Gramont regimen, a FOLFOX4 regime produced no significant increase in overall survival, but did produce an improvement in progression-free survival, the primary end-point of the phase III randomized trial.[11]
Adjuvant treatment of colorectal cancer
After and/or before[12] the curative resection of colorectal cancer, chemotherapy based on 5-fluorouracil and folinic acid reduces the risk of relapse. The benefit is clinically relevant when cancer has spread to locoregional lymph nodes or penetrated through the wall of the rectum or colon (stage III, Dukes C). The addition of oxaliplatin improves relapse-free survival, but data on overall survival have not yet been published in extenso.
When cancer has not spread to the locoregional lymph nodes, nor penetrated through the wall of the rectum or colon (stage II, Dukes B) the benefit of chemotherapy is marginal[citation needed] and the decision on whether to give adjuvant chemotherapy should be carefully evaluated by discussing with the patient the realistic benefits and the possible toxic side effects of treatment. This is even more relevant when the oncologist proposes treatment with oxaliplatin.[why?]
Adverse effects
Side-effects of oxaliplatin treatment can potentially include:
- Neurotoxicity leading to chemotherapy-induced peripheral neuropathy, a progressive, enduring and often irreversible tingling numbness, intense pain and hypersensitivity to cold, beginning in the hands and feet and sometimes involving the arms and legs, often with deficits in proprioception.[13] This chronic neuropathy may also be preceded by a transient acute neuropathy occurring at the time of infusion and associated with excitation of voltage-gated Na+ channels.[14][15]
- Fatigue
- Nausea, vomiting, or diarrhea
- Neutropenia (low number of a type of white blood cells)
- Ototoxicity (hearing loss)
- Extravasation if oxaliplatin leaks from the infusion vein it may cause severe damage to the connective tissues.
- Hypokalemia (low blood potassium), which is more common in women than men[16]
- Persistent hiccups[17]
- Rhabdomyolysis[18]
In addition, some patients may experience an allergic reaction to platinum-containing drugs. This is more common in women.[16]
Oxaliplatin has less ototoxicity and nephrotoxicity than cisplatin and carboplatin.[13]
Structure and mechanism
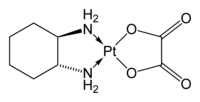
The compound features a square planar platinum(II) center. In contrast to cisplatin and carboplatin, oxaliplatin features the bidentate ligand 1,2-diaminocyclohexane in place of the two monodentate ammine ligands. It also features a bidentate oxalate group.[5]
According to in vivo studies, oxaliplatin fights carcinoma of the colon through non-targeted cytotoxic effects. Like other platinum compounds, its cytotoxicity is thought to result from inhibition of DNA synthesis in cells. In particular, oxaliplatin forms both inter- and intra-strand cross links in DNA,[19] which prevent DNA replication and transcription, causing cell death.
History
Oxaliplatin was discovered in 1976 at Nagoya City University by Professor Yoshinori Kidani, who was granted U.S. Patent 4,169,846 in 1979. Oxaliplatin was subsequently in-licensed by Debiopharm and developed as an advanced colorectal cancer treatment. Debiopharm licensed the drug to Sanofi-Aventis in 1994. It gained European approval in 1996 (initially in France) and approval by the U.S. Food and Drug Administration in 2002. Generic oxaliplatin was first approved in the United States in August 2009.[20] Patent disputes caused generic production to stop in 2010, but it restarted in 2012.[21][22]
Patent information
Eloxatin is covered by patent numbers 5338874 (Expiry Apr 07,2013), 5420319 (Expiry Aug 08,2016), 5716988 (Expiry Aug 07,2015) and 5290961 (Expiry Jan 12, 2013) (see Electronic Orange Book patent info for Eloxatin).[23] Exclusivity code I-441, which expired on Nov 04, 2007, is for use combination with infusional 5-FU/LV for adjuvant treatment stage III colon cancer patients who have undergone complete resection primary tumor-based on improvement in disease free survival with no demonstrated benefit overall survival after 4 years. Exclusivity code NCE, New Chemical Entity, expired on Aug 09, 2007.[23]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Ehrsson H, Wallin I, Yachnin J (2002). "Pharmacokinetics of oxaliplatin in humans". Medical Oncology. 19 (4): 261–265. doi:10.1385/MO:19:4:261. PMID 12512920.
- ^ a b c d e f g "Oxaliplatin". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b "The side effects of platinum-based chemotherapy drugs: a review for chemists". Dalton Transactions. 2018. doi:10.1039/c8dt00838h.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b Apps, M. G., Choi, E. H. Y., Wheate, N. J. (2015). "The state-of-play and future of platinum drugs". Endocrine-related Cancer. 22 (4): 219–233. doi:10.1530/ERC-15-0237. PMID 26113607.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 513. ISBN 9783527607495. Archived from the original on 2016-12-20.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived from the original (PDF) on 13 December 2016. Retrieved 8 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Oxaliplatin". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- ^ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 605. ISBN 9780857111562.
- ^ Becouarn Y, Ychou M, Ducreux M, et al. (1998). "Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. Digestive Group of French Federation of Cancer Centers". J Clin Oncol. 16 (8): 2739–44. PMID 9704726.
- ^ De Gramont A, Figer A, Seymour M, et al. (2000). "Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer". J Clin Oncol. 18 (16): 2938–47. PMID 10944126.
- ^ National Cancer Institute, Rectal Cancer Treatment (PDQ®) Archived 2014-08-08 at the Wayback Machine
- ^ a b Pasetto LM, D'Andrea MR, Rossi E, Monfardini S (August 2006). "Oxaliplatin-related neurotoxicity: how and why?". Crit. Rev. Oncol. Hematol. 59 (2): 159–68. doi:10.1016/j.critrevonc.2006.01.001. PMID 16806962.
- ^ Webster, Richard G; Brain, Keith L; Wilson, Richard H; Grem, Jean L; Vincent, Angela (December 2005). "Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels". British Journal of Pharmacology. 146 (7): 1027–1039. doi:10.1038/sj.bjp.0706407. PMC 1751225. PMID 16231011.
- ^ Gebremedhn, Endale Gebreegziabher; Shortland, Peter John; Mahns, David Anthony (12 April 2018). "The incidence of acute oxaliplatin-induced neuropathy and its impact on treatment in the first cycle: a systematic review". BMC Cancer. 18 (1). doi:10.1186/s12885-018-4185-0. PMID 29649985.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Chay WY, Chew L, Yeoh TT, Tan MH (May 2010). "An association between transient hypokalemia and severe acute oxaliplatin-related toxicity predominantly in women". Acta Oncol. 49 (4): 515–7. doi:10.3109/02841860903464015. PMID 20092386.
- ^ "Archived copy". Archived from the original on 2014-09-05. Retrieved 2014-09-05.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link) - ^ "Archived copy". Archived from the original on 2016-08-27. Retrieved 2016-06-15.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link) - ^ Graham, Joanne; Mushin, Mohamed; Kirkpatrick, Peter (January 2004). "Oxaliplatin". Nature Reviews Drug Discovery. 3 (1): 11–2. doi:10.1038/nrd1287. PMID 14756144.
- ^ "Generic Eloxatin availability". Drugs.com. Archived from the original on 7 June 2013. Retrieved 19 April 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Archived copy". Archived from the original on 2015-09-24. Retrieved 2015-08-25.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link) - ^ "Top 10 best-selling cancer drugs: Eloxatin–$1.2 billion". FiercePharma. 15 May 2012. Archived from the original on 21 April 2014. Retrieved 20 April 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Orange Book. accessdata.fda.gov. URL: http://www.accessdata.fda.gov/scripts/cder/ob/docs/patexclnew.cfm?Appl_No=021759&Product_No=001&table1=OB_Rx Archived 2007-09-26 at the Wayback Machine. Accessed on: July 22, 2007.
External links
- Oxaliplatin – Official web site of manufacturer.
- Oxaliplatin Prescribing Information – Official prescribing information.
- NCI Drug Information Summary on Oxaliplatin
- Graham J, Mushin M, Kirkpatrick P (2004). "Oxaliplatin" (PDF). Nat Rev Drug Discov. 3 (1): 11–2. doi:10.1038/nrd1287. PMID 14756144.
