Suvorexant: Difference between revisions
m v2.04b - Bot T23 CW#558 - Fix errors for CW project (Duplicated reference) |
No edit summary |
||
| Line 42: | Line 42: | ||
<!--Pharmacokinetic data --> |
<!--Pharmacokinetic data --> |
||
| bioavailability = 82% (at 10 mg) |
| bioavailability = 82% (at 10 mg)<ref name="Belsomra-Label" /> |
||
| protein_bound = >99% |
| protein_bound = >99%<ref name="Belsomra-Label" /> |
||
| metabolism = [[Liver|Hepatic]] ([[CYP3A]], [[CYP2C19]]) |
| metabolism = [[Liver|Hepatic]] ([[CYP3A]], [[CYP2C19]])<ref name="Belsomra-Label" /> |
||
| metabolites = Hydroxysuvorexant (inactive)<ref name="Belsomra-Label" /> |
|||
| onset = |
|||
| elimination_half-life = 12 hours<ref name="Belsomra label" /> |
| elimination_half-life = 12 hours<ref name="Belsomra label" /> |
||
| duration_of_action = |
|||
| excretion = [[Feces]] (66%), [[urine]] (23%) |
| excretion = [[Feces]] (66%), [[urine]] (23%)<ref name="Belsomra-Label" /> |
||
<!--Identifiers --> |
<!--Identifiers --> |
||
| Line 74: | Line 77: | ||
| StdInChIKey = JYTNQNCOQXFQPK-MRXNPFEDSA-N |
| StdInChIKey = JYTNQNCOQXFQPK-MRXNPFEDSA-N |
||
}} |
}} |
||
<!-- Definition and medical uses --> |
|||
'''Suvorexant''', sold under the trade name '''Belsomra''', is an [[orexin antagonist]] medication which is used in the treatment of [[insomnia]].<ref name="Belsomra-Label">https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/204569s008lbl.pdf</ref><ref name="pmid25318834">{{cite journal | vauthors = Jacobson LH, Callander GE, Hoyer D | title = Suvorexant for the treatment of insomnia | journal = Expert Rev Clin Pharmacol | volume = 7 | issue = 6 | pages = 711–30 | date = November 2014 | pmid = 25318834 | doi = 10.1586/17512433.2014.966813 | url = }}</ref> It is taken [[oral administration|by mouth]].<ref name="Drugs.com-Belsomra-Generic" /> |
|||
<!-- Side effects and mechanism --> |
|||
'''Suvorexant''', sold under the trade name '''Belsomra''', is an [[orexin antagonist]] medication for the treatment of [[insomnia]].<ref>{{cite journal | doi = 10.1021/op1002853 | title = The First Large-Scale Synthesis of MK-4305: A Dual Orexin Receptor Antagonist for the Treatment of Sleep Disorder | year = 2011 | vauthors = Baxter CA, Cleator E, Brands KM, Edwards JS, Reamer RA, Sheen FJ, Stewart GW, Strotman NA, Wallace DJ | display-authors = 6 | journal = Organic Process Research & Development | volume = 15 | issue = 2 | pages = 367–375}}</ref> It is effective for [[insomnia]], at least for four weeks and as compared to a [[placebo]].<ref name=Ann2015/> |
|||
[[Side effect]]s of suvorexant include [[somnolence]] and [[headache]].<ref name="Belsomra-Label" /> The medication is a [[dual orexin receptor antagonist]] (DORA).<ref name="pmid25318834" /> It acts as a [[binding selectivity|selective]] dual [[receptor antagonist|antagonist]] of the [[orexin receptor]]s [[hypocretin (orexin) receptor 1|OX<sub>1</sub>]] and [[hypocretin (orexin) receptor 2|OX<sub>2</sub>]].<ref name="pmid25318834" /> The medication has an intermediate [[elimination half-life]] of 12{{nbsp}}hours.<ref name="Belsomra-Label" /><ref name="pmid25318834" /> Suvorexant is not a [[benzodiazepine]] or [[Z-drug]] and does not interact with [[GABA receptor]]s, instead having a distinct [[mechanism of action]].<ref name="pmid25318834" /> |
|||
<!-- History, society, and culture --> |
|||
| ⚫ | |||
Suvorexant was first described in 2010<ref name="pmid20565075" /> and was introduced for medical use in 2014.<ref name="Belsomra-Label" /><ref name="APDNews2014" /> It is a [[Controlled Substances Act#Schedule IV controlled substances|schedule IV]] [[controlled substance]] in the [[United States]].<ref name="FederalRegister2016" /> |
|||
{{TOC limit|3}} |
|||
The drug was initially released November 2014 in Japan,<ref>{{cite web|url=http://en.apdnews.com/news/7ff7b8a890574d1cbb50675732d6873a.html|title=New hypnotic drug without addiction to be released in Japan first}}</ref> then later reached the [[United States]] in February 2015,<ref>{{cite web|title=Merck's Insomnia Medicine Belsomra C-IV Now Available in US|url=http://www.sleepreviewmag.com/2015/02/mercks-insomnia-medicine-belsomra-c-iv-now-available-us/|website=www.sleepreviewmag.com|publisher=Sleep Review|access-date=9 September 2015}}</ref> [[Australia]] in November 2016, and [[Canada]] in November 2018<ref>{{cite web|title=Regulatory Decision Summary - Belsomra - Health Canada|url=https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00475|website=hpr-rps.hres.ca|publisher=Government of Canada|access-date=6 February 2020}}</ref> |
|||
==Medical uses== |
==Medical uses== |
||
Suvorexant is used for the treatment of [[insomnia]], characterized by difficulties with [[sleep onset]] and/or sleep maintenance.<ref name="Belsomra |
Suvorexant is used for the treatment of [[insomnia]], characterized by difficulties with [[sleep onset]] and/or sleep maintenance.<ref name="Belsomra-Label" /><ref name="pmid25318834" /> At a dose of 15 to 20 mg and in terms of treatment–[[placebo]] difference, it reduces [[time to sleep onset]] by up to 10 minutes, reduces [[wake after sleep onset|time awake after sleep onset]] by about 15 to 30 minutes, and increases [[total sleep time]] by about 10 to 20 minutes.<ref name="Belsomra-Label" /> The maximum recommended dose of suvorexant is 20 mg.<ref name="Belsomra-Label" /> |
||
[[Network meta-analysis|Network meta-analyses]] have found orexin receptor antagonists like suvorexant to be superior in sleep-promoting efficacy to many other sleep aids, such as [[benzodiazepine]]s, [[Z-drug]]s, [[antihistamine]]s, sedative [[antidepressant]]s, and [[melatonin receptor agonist]]s.<ref name="pmid34560134">{{cite journal | vauthors = Wang L, Pan Y, Ye C, Guo L, Luo S, Dai S, Chen N, Wang E | title = A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults | journal = Neurosci Biobehav Rev | volume = 131 | issue = | pages = 489–496 | date = December 2021 | pmid = 34560134 | doi = 10.1016/j.neubiorev.2021.09.035 | url = }}</ref><ref name="pmid34121443">{{cite journal | vauthors = McElroy H, O'Leary B, Adena M, Campbell R, Monfared AA, Meier G | title = Comparative efficacy of lemborexant and other insomnia treatments: a network meta-analysis | journal = J Manag Care Spec Pharm | volume = 27 | issue = 9 | pages = 1296–1308 | date = September 2021 | pmid = 34121443 | doi = 10.18553/jmcp.2021.21011 | url = }}</ref> However, suvorexant was found to have similar or inferior efficacy to [[lemborexant]] |
[[Network meta-analysis|Network meta-analyses]] have found orexin receptor antagonists like suvorexant to be superior in sleep-promoting efficacy to many other sleep aids, such as [[benzodiazepine]]s, [[Z-drug]]s, [[antihistamine]]s, sedative [[antidepressant]]s, and [[melatonin receptor agonist]]s.<ref name="pmid34560134">{{cite journal | vauthors = Wang L, Pan Y, Ye C, Guo L, Luo S, Dai S, Chen N, Wang E | title = A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults | journal = Neurosci Biobehav Rev | volume = 131 | issue = | pages = 489–496 | date = December 2021 | pmid = 34560134 | doi = 10.1016/j.neubiorev.2021.09.035 | url = }}</ref><ref name="pmid34121443">{{cite journal | vauthors = McElroy H, O'Leary B, Adena M, Campbell R, Monfared AA, Meier G | title = Comparative efficacy of lemborexant and other insomnia treatments: a network meta-analysis | journal = J Manag Care Spec Pharm | volume = 27 | issue = 9 | pages = 1296–1308 | date = September 2021 | pmid = 34121443 | doi = 10.18553/jmcp.2021.21011 | url = }}</ref> However, suvorexant was found to have similar or inferior efficacy to [[lemborexant]].<ref name="pmid34560134" /><ref name="pmid34121443" /><ref name="pmid34902823">{{cite journal | vauthors = Xue T, Wu X, Chen S, Yang Y, Yan Z, Song Z, Zhang W, Zhang J, Chen Z, Wang Z | title = The efficacy and safety of dual orexin receptor antagonists in primary insomnia: A systematic review and network meta-analysis | journal = Sleep Med Rev | volume = 61 | issue = | pages = 101573 | date = February 2022 | pmid = 34902823 | doi = 10.1016/j.smrv.2021.101573 | url = }}</ref> Meta-analyses have not yet compared suvorexant with [[daridorexant]].<ref name="pmid34560134" /><ref name="pmid34121443" /> |
||
It is unclear if suvorexant is safe among people with a history of [[addiction]], as they were excluded from the [[clinical trial]]s of suvorexant.<ref name= |
It is unclear if suvorexant is safe among people with a history of [[addiction]], as they were excluded from the [[clinical trial]]s of suvorexant.<ref name="pmid25667197">{{cite journal | vauthors = Patel KV, Aspesi AV, Evoy KE | title = Suvorexant: a dual orexin receptor antagonist for the treatment of sleep onset and sleep maintenance insomnia | journal = The Annals of Pharmacotherapy | volume = 49 | issue = 4 | pages = 477–83 | date = April 2015 | pmid = 25667197 | doi = 10.1177/1060028015570467 }}</ref> A [[Cochrane review]] found suvorexant to be effective in the short-term treatment of sleep disturbances in people with [[dementia]] with few adverse effects.<ref name="pmid33189083">{{cite journal | vauthors = McCleery J, Sharpley AL | title = Pharmacotherapies for sleep disturbances in dementia | journal = Cochrane Database Syst Rev | volume = 11 | issue = | pages = CD009178 | date = November 2020 | pmid = 33189083 | pmc = 8094738 | doi = 10.1002/14651858.CD009178.pub4 | url = }}</ref> |
||
Suvorexant is FDA-approved at doses of 5 to 20 mg.<ref name="Belsomra-Label" /><ref name="pmid25318834" /> Higher doses of up to 40 mg were also submitted for approval but were not authorized by the FDA due to concerns about residual sedation and associated impairment.<ref name="pmid25318834" /> Suvorexant has also been assessed at doses of 40 to 100 mg and these doses appeared to be more effective at promoting sleep than lower doses but produced significant residual effects.<ref name="pmid25318834" /><ref name="pmid25667197" /><ref name="pmid23372274">{{cite journal | vauthors = Sun H, Kennedy WP, Wilbraham D, Lewis N, Calder N, Li X, Ma J, Yee KL, Ermlich S, Mangin E, Lines C, Rosen L, Chodakewitz J, Murphy GM | title = Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men | journal = Sleep | volume = 36 | issue = 2 | pages = 259–67 | date = February 2013 | pmid = 23372274 | pmc = 3542986 | doi = 10.5665/sleep.2386 | url = }}</ref> |
|||
| ⚫ | |||
| ⚫ | Suvorexant is not recommended in people with [[liver impairment]].<ref name="Belsomra label" /> Suvorexant [[pregnancy]] category is classified as Category C.<ref name="Belsomra label">{{cite web | title=Belsomra- suvorexant tablet, film coated | website=DailyMed | date=20 November 2019 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e5b72731-1acb-45b7-9c13-290ad12d3951 | access-date=30 January 2020}}</ref> Based on [[animal testing]], this medication may cause fetal harm during pregnancy and should only be given in pregnancy if the potential benefit justifies the potential harm to the fetus. Evidence is inconclusive about whether using this medication while [[breastfeeding]] puts the infant at risk of harm.<ref name="Belsomra label" /> |
||
===Available forms=== |
|||
Suvorexant is contraindicated in people diagnosed with [[narcolepsy]].<ref name="Belsomra label" /> |
|||
Suvorexant is available in the form of 5, 10, 15, and 20 mg [[oral administration|oral]] [[film-coated]] [[tablet (pharmacy)|tablet]]s.<ref name="Drugs.com-Belsomra-Generic" /> |
|||
| ⚫ | |||
| ⚫ | Suvorexant is [[contraindication|contraindicated]] in people diagnosed with [[narcolepsy]].<ref name="Belsomra label" /> Suvorexant is not recommended in people with [[liver impairment]].<ref name="Belsomra label" /> Suvorexant [[pregnancy]] category is classified as Category C.<ref name="Belsomra label">{{cite web | title=Belsomra- suvorexant tablet, film coated | website=DailyMed | date=20 November 2019 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e5b72731-1acb-45b7-9c13-290ad12d3951 | access-date=30 January 2020}}</ref> Based on [[animal testing]], this medication may cause fetal harm during pregnancy and should only be given in pregnancy if the potential benefit justifies the potential harm to the fetus. Evidence is inconclusive about whether using this medication while [[breastfeeding]] puts the infant at risk of harm.<ref name="Belsomra label" /> |
||
==Side effects== |
==Side effects== |
||
[[Side effect]]s of suvorexant include [[somnolence]] (7%) and [[headache]]s (7%).<ref name="Belsomra-Label" /> Somnolence with suvorexant appears to be [[dose dependency|dose-dependent]], with rates of 2% at 10 mg, 5% at 20 mg, 12% at 40 mg, and 11% at 80 mg, relative to <1% for [[placebo]].<ref name="Belsomra-Label" /> Suvorexant has also been associated with dose-dependent increases in [[cholesterol]] levels.<ref name="Belsomra-Label" /> |
|||
The most common complaint about the drug is from users who report that it did not help them to sleep.<ref name="Carr February 2016">{{Cite journal| vauthors = Carr T |date=5 February 2016|title=FDA Fields Complaints on Sleeping Pill Suvorexant|url=http://www.consumerreports.org/drugs/fda-fields-complaints-on-sleeping-pill-suvorexant-belsomra/|journal=Consumer Reports}}</ref> Some people reported that the drug caused a sleep disturbance such as a [[nightmare]], [[sleep terror]], or abnormal dream.<ref name="Carr February 2016"/><ref name="Jac2014" /> Others reported that the drug caused them to be more awake.<ref name="Carr February 2016"/> |
The most common complaint about the drug is from users who report that it did not help them to sleep.<ref name="Carr February 2016">{{Cite journal| vauthors = Carr T |date=5 February 2016|title=FDA Fields Complaints on Sleeping Pill Suvorexant|url=http://www.consumerreports.org/drugs/fda-fields-complaints-on-sleeping-pill-suvorexant-belsomra/|journal=Consumer Reports}}</ref> Some people reported that the drug caused a sleep disturbance such as a [[nightmare]], [[sleep terror]], or abnormal dream.<ref name="Carr February 2016"/><ref name="Jac2014" /> Others reported that the drug caused them to be more awake.<ref name="Carr February 2016"/> |
||
Additional issues include [[sleepiness]] the next day and issues with driving.<ref name="Jac2014">{{cite journal | vauthors = Jacobson LH, Callander GE, Hoyer D | title = Suvorexant for the treatment of insomnia | journal = Expert Review of Clinical Pharmacology | volume = 7 | issue = 6 | pages = 711–30 | date = November 2014 | pmid = 25318834 | doi = 10.1586/17512433.2014.966813 }}</ref> Other concerns include thoughts of [[suicide]].<ref name="Jac2014" /> |
|||
[[Drug tolerance|Tolerance]], [[drug withdrawal|withdrawal]], and [[rebound effect]]s do not appear to occur with suvorexant at recommended doses.<ref name="Belsomra-Label" /><ref name="pmid28994603">{{cite journal | vauthors = Keks NA, Hope J, Keogh S | title = Suvorexant: scientifically interesting, utility uncertain | journal = Australas Psychiatry | volume = 25 | issue = 6 | pages = 622–624 | date = December 2017 | pmid = 28994603 | doi = 10.1177/1039856217734677 | url = }}</ref> |
|||
==Abuse liability== |
|||
| ⚫ | |||
| ⚫ | Suvorexant produces similar or lower [[reinforcement|reinforcing effect]]s compared to those of [[zolpidem]].<ref name="pmid25167596">{{cite journal | title = Schedules of controlled substances: placement of suvorexant into Schedule IV. Final rule | journal = Federal Register | volume = 79 | issue = 167 | pages = 51243–7 | date = August 2014 | pmid = 25167596 | url = http://www.gpo.gov/fdsys/pkg/FR-2014-08-28/pdf/2014-20515.pdf }}</ref><ref name="pmid34480579">{{cite journal | vauthors = Ufer M, Kelsh D, Schoedel KA, Dingemanse J | title = Abuse potential assessment of the new dual orexin receptor antagonist daridorexant in recreational sedative drug users as compared to suvorexant and zolpidem | journal = Sleep | volume = 45| issue = 3| pages = | date = September 2021 | pmid = 34480579 | doi = 10.1093/sleep/zsab224 | url = }}</ref><ref name="pmid25667197" /> |
||
| ⚫ | |||
==Overdose== |
|||
There is limited experience with [[overdose]] of suvorexant.<ref name="Belsomra-Label" /> Suvorexant has been assessed in single doses of as high as 240 mg in clinical studies.<ref name="Belsomra-Label" /> Treatment of suvorexant [[overdose]] is based on symptoms and supportive.<ref name="Belsomra-Label" /> |
|||
| ⚫ | |||
Suvorexant is not recommended if people are also taking medications that strongly inhibit the liver enzyme [[CYP3A]] like [[itraconazole]], [[lopinavir]]/[[ritonavir]], [[clarithromycin]], [[ritonavir]], [[ketoconazole]], [[indinavir]]/[[ritonavir]], or [[conivaptan]].<ref name="Belsomra label" /><ref name=14a/> If suvorexant is used with a medication that moderately inhibits the liver enzyme CYP3A, like [[verapamil]], [[erythromycin]], [[diltiazem]], or [[dronedarone]], it is recommended that the dose of suvorexant be adjusted.<ref name="Belsomra label" /><ref name=14a>"U.S. Food and Drug Administration." Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. U.S. Food and Drug Administration, 27 Oct. 2014. Web. 30 Oct. 2014.</ref> |
Suvorexant is not recommended if people are also taking medications that strongly inhibit the liver enzyme [[CYP3A]] like [[itraconazole]], [[lopinavir]]/[[ritonavir]], [[clarithromycin]], [[ritonavir]], [[ketoconazole]], [[indinavir]]/[[ritonavir]], or [[conivaptan]].<ref name="Belsomra label" /><ref name=14a/> If suvorexant is used with a medication that moderately inhibits the liver enzyme CYP3A, like [[verapamil]], [[erythromycin]], [[diltiazem]], or [[dronedarone]], it is recommended that the dose of suvorexant be adjusted.<ref name="Belsomra label" /><ref name=14a>"U.S. Food and Drug Administration." Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. U.S. Food and Drug Administration, 27 Oct. 2014. Web. 30 Oct. 2014.</ref> |
||
| Line 108: | Line 123: | ||
===Pharmacodynamics=== |
===Pharmacodynamics=== |
||
Suvorexant |
Suvorexant is thought to exert its therapeutic effects in [[insomnia]] through [[receptor antagonist|antagonism]] of [[orexin receptor]]s.<ref name="Belsomra-Label" /> The [[orexin]] [[neuropeptide]] signaling system is a [[central nervous system|central]] promoter of [[wakefulness]].<ref name="Belsomra-Label" /> Blocking the binding of wake-promoting neuropeptides [[orexin A]] and [[orexin B]] to receptors [[orexin receptor type 1]] (OX<sub>1</sub>) and [[orexin receptor type 2]] (OX<sub>2</sub>) is thought to suppress wake drive.<ref name="Belsomra-Label" /> Animal studies report the [[affinity (pharmacology)|binding affinities]] for OX<sub>1</sub> (0.55 nM) and OX<sub>2</sub> (0.35 nM).<ref name="Belsomra-Label" /><ref>{{Cite web|url = https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/peripheralandcentralnervoussystemdrugsadvisorycommittee/ucm352970.pdf|title = Suvorexant Advisory Committee Meeting Briefing Document|date = May 22, 2013|access-date = Feb 7, 2015}}</ref> Loss of orexin signaling is involved in the [[etiology]] of [[narcolepsy]], and this may explain potential adverse effects of orexin receptor antagonists like narcolepsy and [[cataplexy]].<ref name="Belsomra-Label" /> |
||
===Pharmacokinetics=== |
===Pharmacokinetics=== |
||
The [[bioavailability]] of suvorexant is at 82%. It is highly [[protein-bound]]. Food delays the time to max concentration. The primary route of elimination is through the feces, with approximately 66% of radiolabeled dose recovered in the feces compared to 23% in the urine. The elimination [[half-life]] is reported to be 12 hours.<ref name="Belsomra label" /> |
|||
== |
====Absorption==== |
||
The [[absolute bioavailability]] of suvorexant is 82%.<ref name="Belsomra-Label" /> Suvorexant exposure is not dose-proportional over a dose range of 10 to 100 mg owing to decreased [[absorption (pharmacokinetics)|absorption]] at higher doses.<ref name="Belsomra-Label" /> In one study, suvorexant peak levels were 0.44 μM at 10 mg, 0.87 μM at 50 mg, and 2.12 μM at 100 mg, while overall exposure was 6.7 μM•h at 10 mg, 10.9 μM•h at 50 mg, and 29.8 μM•h at 100 mg.<ref name="pmid25318834" /> The [[Tmax (pharmacology)|time to peak levels]] of suvorexant is 2 to 3 hours regardless of dose but with wide variation (range 30 minutes to 8 hours).<ref name="Belsomra-Label" /><ref name="pmid25318834" /> Taking suvorexant with food does not modify suvorexant [[Cmax (pharmacology)|peak levels]] or [[area-under-the-curve (pharmacokinetics)|overall exposure]] but does delay the time to peak concentrations by about 1.5 hours.<ref name="Belsomra-Label" /> Levels of suvorexant accumulate 1- to 2-fold with continuous once-daily administration and [[steady state (pharmacokinetics)|steady-state levels]] are reached within 3 days.<ref name="Belsomra-Label" /> |
|||
====Distribution==== |
|||
The [[volume of distribution]] of suvorexant is approximately 49 L.<ref name="Belsomra-Label" /> Suvorexant has high [[plasma protein binding]] (>99%).<ref name="Belsomra-Label" /> It is bound to [[human serum albumin|albumin]] and [[α1-acid glycoprotein|α<sub>1</sub>-acid glycoprotein]].<ref name="Belsomra-Label" /> |
|||
====Metabolism==== |
|||
Suvorexant is [[metabolism|metabolized]] primarily by [[CYP3A]] [[enzyme]]s.<ref name="Belsomra-Label" /> [[CYP2C19]] also contributes to suvorexant metabolism to a minor extent.<ref name="Belsomra-Label" /> The major circulating forms of suvorexant are suvorexant and its [[metabolite]] hydroxysuvorexant, which is not expected to be pharmacologically active.<ref name="Belsomra-Label" /> |
|||
====Elimination==== |
|||
Suvorexant is [[elimination (pharmacology)|eliminated]] mainly via metabolism.<ref name="Belsomra-Label" /> It is [[excretion|excreted]] primarily in [[feces]] (66%) and to a lesser extent in [[urine]] (23%).<ref name="Belsomra-Label" /> |
|||
The [[elimination half-life]] of suvorexant is 12 hours, with a range of 9 to 13 hours.<ref name="Belsomra-Label" /><ref name="pmid25318834" /> In another study, the half-life of suvorexant was 15 hours with a range of 10 to 22 hours.<ref name="Belsomra-Label" /> In one study, the half-lives of suvorexant (mean ± SD) were 9.0 ± 7.2 hours at 10 mg, 10.8 ± 3.6 hours at 50 mg, and 13.1 ± 5.8 hours at 100 mg.<ref name="pmid25318834" /> The delayed time to peak levels and long half-life of suvorexant have been said to be "less than ideal for a sleep drug".<ref name="pmid25318834" /> Other orexin receptor antagonists with a shorter half-life and faster onset of action may be more therapeutically optimal.<ref name="pmid25318834" /> |
|||
Suvorexant dissociates from the [[orexin receptor]]s slowly.<ref name="pmid25318834" /> As a result, its [[duration of action|duration]] may be longer than that suggested by its circulating concentrations and half-life.<ref name="pmid25318834" /> |
|||
====Specific populations==== |
|||
Age and race do not influence the pharmacokinetics of suvorexant in a clinically meaningfully way.<ref name="Belsomra-Label" /> Exposure to suvorexant is slightly higher in women compared to men, however dose adjustments based on gender are generally unnecessary.<ref name="Belsomra-Label" /> Suvorexant exposure is greater in people with higher [[body mass index]], such as [[obesity|obese]] people.<ref name="Belsomra-Label" /> This is particularly the case in obese women relative to non-obese women.<ref name="Belsomra-Label" /> Suvorexant exposure with a single dose is not greater in people with moderate [[hepatic insufficiency]] compared to healthy individuals.<ref name="Belsomra-Label" /> However, the half-life of suvorexant was prolonged from 15 hours (range 10–22 hours) to 19 hours (range 11–49 hours).<ref name="Belsomra-Label" /> Suvorexant exposure is unchanged in people with severe [[renal impairment]] and no dosage adjustment is necessary in these individuals.<ref name="Belsomra-Label" /> |
|||
==Chemistry== |
|||
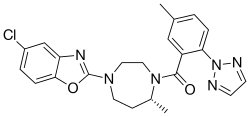
Suvorexant is a [[small-molecule]] compound.<ref name="pmid25489915">{{cite journal | vauthors = Christopher JA | title = Small-molecule antagonists of the orexin receptors | journal = Pharm Pat Anal | volume = 3 | issue = 6 | pages = 625–38 | date = 2014 | pmid = 25489915 | doi = 10.4155/ppa.14.46 | url = }}</ref> The [[chemical name]] of suvorexant is [(7''R'')-4-(5-chloro-2-benzoxazolyl)hexahydro-7-methyl-1''H''-1,4-diazepin-1-yl][5-methyl-2-(2''H''-1,2,3-triazol2-yl)phenyl]methanone.<ref name="Belsomra-Label" /> Its [[molecular formula]] is C<sub>23</sub>H<sub>23</sub>N<sub>6</sub>O<sub>2</sub>Cl and its [[molecular weight]] is 450.92{{nbsp}}g/mol.<ref name="Belsomra-Label" /> Suvorexant is a white to off-white powder and is [[water solubility|insoluble]] in water.<ref name="Belsomra-Label" /> |
|||
==History== |
|||
Suvorexant was first described in the medical literature in 2010.<ref name="pmid20565075">{{cite journal | vauthors = Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ, Roecker AJ, Mercer SP, Bednar RA, Lemaire W, Bruno JG, Reiss DR, Harrell CM, Murphy KL, Garson SL, Doran SM, Prueksaritanont T, Anderson WB, Tang C, Roller S, Cabalu TD, Cui D, Hartman GD, Young SD, Koblan KS, Winrow CJ, Renger JJ, Coleman PJ | title = Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia | journal = J Med Chem | volume = 53 | issue = 14 | pages = 5320–32 | date = July 2010 | pmid = 20565075 | doi = 10.1021/jm100541c | url = }}</ref> It was approved by the [[United States]] [[Food and Drug Administration]] on August 13, 2014.<ref name="Belsomra-Label" /><ref name="Drugs.com-Belsomra-Generic">https://www.drugs.com/availability/generic-belsomra.html</ref><ref>{{cite press release | title=FDA approves new type of sleep drug, Belsomra | website=U.S. [[Food and Drug Administration]] (FDA) | date=13 August 2014 | url=https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm409950.htm | archive-url=https://web.archive.org/web/20170214122028/https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm409950.htm | archive-date=14 February 2017 | url-status=dead | access-date=30 January 2020}}</ref> The medication was initially released November 2014 in Japan,<ref name="APDNews2014">{{cite web|url=http://en.apdnews.com/news/7ff7b8a890574d1cbb50675732d6873a.html|title=New hypnotic drug without addiction to be released in Japan first}}</ref> then later reached the [[United States]] in February 2015,<ref name="SleepReview2015">{{cite web|title=Merck's Insomnia Medicine Belsomra C-IV Now Available in US|url=http://www.sleepreviewmag.com/2015/02/mercks-insomnia-medicine-belsomra-c-iv-now-available-us/|website=www.sleepreviewmag.com|publisher=Sleep Review|access-date=9 September 2015}}</ref> [[Australia]] in November 2016, and [[Canada]] in November 2018.<ref>{{cite web|title=Regulatory Decision Summary - Belsomra - Health Canada|url=https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00475|website=hpr-rps.hres.ca|publisher=Government of Canada|access-date=6 February 2020}}</ref> Suvorexant marketing exclusivity in the United States is set to expire in January 2023 and patent protection is set to expire in 2029 to 2033.<ref name="Drugs.com-Belsomra-Generic" /> |
|||
==Society and culture== |
|||
===Names=== |
|||
Suvorexant was developed under the code name MK-4305 and is marketed under the brand name Belsomra.<ref name="pmid25667197" /> |
|||
===Legal status=== |
|||
| ⚫ | The United States [[Drug Enforcement Administration]] (DEA) placed suvorexant on the list of [[Controlled Substances Act#Schedule IV controlled substances|schedule IV controlled substances]] under the [[Controlled Substances Act]].<ref name="pmid25167596" /><ref name="FederalRegister2016">{{cite web |url=https://www.federalregister.gov/articles/2014/02/13/2014-03124/schedules-of-controlled-substances-placement-of-suvorexant-into-schedule-iv |title=Schedules of Controlled Substances: Placement of Suvorexant into Schedule IV |author=<!--Staff writer(s); no by-line.--> |date=February 13, 2014 |website=federalregister.gov |publisher=[[Federal Register]] |access-date=August 10, 2016 |quote=A Proposed Rule by the Drug Enforcement Administration on 02/13/2014}}</ref> According to the DEA, suvorexant produces similar [[reinforcement|reinforcing effect]]s to those of [[zolpidem]] and thus may have a similar [[misuse liability]].<ref name="pmid25167596">{{cite journal | title = Schedules of controlled substances: placement of suvorexant into Schedule IV. Final rule | journal = Federal Register | volume = 79 | issue = 167 | pages = 51243–7 | date = August 2014 | pmid = 25167596 | url = http://www.gpo.gov/fdsys/pkg/FR-2014-08-28/pdf/2014-20515.pdf }}</ref><ref>{{Cite web|url=http://www.deadiversion.usdoj.gov/fed_regs/rules/2014/fr0828.htm|title=Rules - 2014 - Final Rule: Placement of Suvorexant into Schedule IV|website=www.deadiversion.usdoj.gov|access-date=2016-04-03}}</ref> |
||
==Research== |
|||
Suvorexant is under development for the treatment of [[delirium]].<ref name="AdisInsight">https://adisinsight.springer.com/drugs/800027707</ref> As of October 2021, it is in [[Phases of clinical research#Phase III|phase 3]] [[clinical trial]]s for this indication.<ref name="AdisInsight" /> |
|||
==References== |
|||
{{Reflist}} |
{{Reflist}} |
||
== |
==External links== |
||
* {{cite web| url = https://druginfo.nlm.nih.gov/drugportal/name/suvorexant | publisher = U.S. National Library of Medicine| work = Drug Information Portal | title = Suvorexant }} |
* {{cite web| url = https://druginfo.nlm.nih.gov/drugportal/name/suvorexant | publisher = U.S. National Library of Medicine| work = Drug Information Portal | title = Suvorexant }} |
||
* {{cite web | vauthors = Parker I | title=The Big Sleep | website=[[The New Yorker]] | date=9 December 2013 | url=https://www.newyorker.com/magazine/2013/12/09/the-big-sleep-2 }} |
* {{cite web | vauthors = Parker I | title=The Big Sleep | website=[[The New Yorker]] | date=9 December 2013 | url=https://www.newyorker.com/magazine/2013/12/09/the-big-sleep-2 }} |
||
Revision as of 07:29, 2 April 2022
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Belsomra |
| Other names | MK-4305 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614046 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Orexin antagonist |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 82% (at 10 mg)[1] |
| Protein binding | >99%[1] |
| Metabolism | Hepatic (CYP3A, CYP2C19)[1] |
| Metabolites | Hydroxysuvorexant (inactive)[1] |
| Elimination half-life | 12 hours[2] |
| Excretion | Feces (66%), urine (23%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.546 |
| Chemical and physical data | |
| Formula | C23H23ClN6O2 |
| Molar mass | 450.93 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Suvorexant, sold under the trade name Belsomra, is an orexin antagonist medication which is used in the treatment of insomnia.[1][4] It is taken by mouth.[5]
Side effects of suvorexant include somnolence and headache.[1] The medication is a dual orexin receptor antagonist (DORA).[4] It acts as a selective dual antagonist of the orexin receptors OX1 and OX2.[4] The medication has an intermediate elimination half-life of 12 hours.[1][4] Suvorexant is not a benzodiazepine or Z-drug and does not interact with GABA receptors, instead having a distinct mechanism of action.[4]
Suvorexant was first described in 2010[6] and was introduced for medical use in 2014.[1][7] It is a schedule IV controlled substance in the United States.[8]
Medical uses
Suvorexant is used for the treatment of insomnia, characterized by difficulties with sleep onset and/or sleep maintenance.[1][4] At a dose of 15 to 20 mg and in terms of treatment–placebo difference, it reduces time to sleep onset by up to 10 minutes, reduces time awake after sleep onset by about 15 to 30 minutes, and increases total sleep time by about 10 to 20 minutes.[1] The maximum recommended dose of suvorexant is 20 mg.[1]
Network meta-analyses have found orexin receptor antagonists like suvorexant to be superior in sleep-promoting efficacy to many other sleep aids, such as benzodiazepines, Z-drugs, antihistamines, sedative antidepressants, and melatonin receptor agonists.[9][10] However, suvorexant was found to have similar or inferior efficacy to lemborexant.[9][10][11] Meta-analyses have not yet compared suvorexant with daridorexant.[9][10]
It is unclear if suvorexant is safe among people with a history of addiction, as they were excluded from the clinical trials of suvorexant.[12] A Cochrane review found suvorexant to be effective in the short-term treatment of sleep disturbances in people with dementia with few adverse effects.[13]
Suvorexant is FDA-approved at doses of 5 to 20 mg.[1][4] Higher doses of up to 40 mg were also submitted for approval but were not authorized by the FDA due to concerns about residual sedation and associated impairment.[4] Suvorexant has also been assessed at doses of 40 to 100 mg and these doses appeared to be more effective at promoting sleep than lower doses but produced significant residual effects.[4][12][14]
Available forms
Suvorexant is available in the form of 5, 10, 15, and 20 mg oral film-coated tablets.[5]
Contraindications
Suvorexant is contraindicated in people diagnosed with narcolepsy.[2] Suvorexant is not recommended in people with liver impairment.[2] Suvorexant pregnancy category is classified as Category C.[2] Based on animal testing, this medication may cause fetal harm during pregnancy and should only be given in pregnancy if the potential benefit justifies the potential harm to the fetus. Evidence is inconclusive about whether using this medication while breastfeeding puts the infant at risk of harm.[2]
Side effects
Side effects of suvorexant include somnolence (7%) and headaches (7%).[1] Somnolence with suvorexant appears to be dose-dependent, with rates of 2% at 10 mg, 5% at 20 mg, 12% at 40 mg, and 11% at 80 mg, relative to <1% for placebo.[1] Suvorexant has also been associated with dose-dependent increases in cholesterol levels.[1]
The most common complaint about the drug is from users who report that it did not help them to sleep.[15] Some people reported that the drug caused a sleep disturbance such as a nightmare, sleep terror, or abnormal dream.[15][16] Others reported that the drug caused them to be more awake.[15]
Additional issues include sleepiness the next day and issues with driving.[16] Other concerns include thoughts of suicide.[16]
Tolerance, withdrawal, and rebound effects do not appear to occur with suvorexant at recommended doses.[1][17]
Suvorexant produces similar or lower reinforcing effects compared to those of zolpidem.[18][19][12]
Overdose
There is limited experience with overdose of suvorexant.[1] Suvorexant has been assessed in single doses of as high as 240 mg in clinical studies.[1] Treatment of suvorexant overdose is based on symptoms and supportive.[1]
Interactions
Suvorexant is not recommended if people are also taking medications that strongly inhibit the liver enzyme CYP3A like itraconazole, lopinavir/ritonavir, clarithromycin, ritonavir, ketoconazole, indinavir/ritonavir, or conivaptan.[2][20] If suvorexant is used with a medication that moderately inhibits the liver enzyme CYP3A, like verapamil, erythromycin, diltiazem, or dronedarone, it is recommended that the dose of suvorexant be adjusted.[2][20]
Pharmacology
Pharmacodynamics
Suvorexant is thought to exert its therapeutic effects in insomnia through antagonism of orexin receptors.[1] The orexin neuropeptide signaling system is a central promoter of wakefulness.[1] Blocking the binding of wake-promoting neuropeptides orexin A and orexin B to receptors orexin receptor type 1 (OX1) and orexin receptor type 2 (OX2) is thought to suppress wake drive.[1] Animal studies report the binding affinities for OX1 (0.55 nM) and OX2 (0.35 nM).[1][21] Loss of orexin signaling is involved in the etiology of narcolepsy, and this may explain potential adverse effects of orexin receptor antagonists like narcolepsy and cataplexy.[1]
Pharmacokinetics
Absorption
The absolute bioavailability of suvorexant is 82%.[1] Suvorexant exposure is not dose-proportional over a dose range of 10 to 100 mg owing to decreased absorption at higher doses.[1] In one study, suvorexant peak levels were 0.44 μM at 10 mg, 0.87 μM at 50 mg, and 2.12 μM at 100 mg, while overall exposure was 6.7 μM•h at 10 mg, 10.9 μM•h at 50 mg, and 29.8 μM•h at 100 mg.[4] The time to peak levels of suvorexant is 2 to 3 hours regardless of dose but with wide variation (range 30 minutes to 8 hours).[1][4] Taking suvorexant with food does not modify suvorexant peak levels or overall exposure but does delay the time to peak concentrations by about 1.5 hours.[1] Levels of suvorexant accumulate 1- to 2-fold with continuous once-daily administration and steady-state levels are reached within 3 days.[1]
Distribution
The volume of distribution of suvorexant is approximately 49 L.[1] Suvorexant has high plasma protein binding (>99%).[1] It is bound to albumin and α1-acid glycoprotein.[1]
Metabolism
Suvorexant is metabolized primarily by CYP3A enzymes.[1] CYP2C19 also contributes to suvorexant metabolism to a minor extent.[1] The major circulating forms of suvorexant are suvorexant and its metabolite hydroxysuvorexant, which is not expected to be pharmacologically active.[1]
Elimination
Suvorexant is eliminated mainly via metabolism.[1] It is excreted primarily in feces (66%) and to a lesser extent in urine (23%).[1]
The elimination half-life of suvorexant is 12 hours, with a range of 9 to 13 hours.[1][4] In another study, the half-life of suvorexant was 15 hours with a range of 10 to 22 hours.[1] In one study, the half-lives of suvorexant (mean ± SD) were 9.0 ± 7.2 hours at 10 mg, 10.8 ± 3.6 hours at 50 mg, and 13.1 ± 5.8 hours at 100 mg.[4] The delayed time to peak levels and long half-life of suvorexant have been said to be "less than ideal for a sleep drug".[4] Other orexin receptor antagonists with a shorter half-life and faster onset of action may be more therapeutically optimal.[4]
Suvorexant dissociates from the orexin receptors slowly.[4] As a result, its duration may be longer than that suggested by its circulating concentrations and half-life.[4]
Specific populations
Age and race do not influence the pharmacokinetics of suvorexant in a clinically meaningfully way.[1] Exposure to suvorexant is slightly higher in women compared to men, however dose adjustments based on gender are generally unnecessary.[1] Suvorexant exposure is greater in people with higher body mass index, such as obese people.[1] This is particularly the case in obese women relative to non-obese women.[1] Suvorexant exposure with a single dose is not greater in people with moderate hepatic insufficiency compared to healthy individuals.[1] However, the half-life of suvorexant was prolonged from 15 hours (range 10–22 hours) to 19 hours (range 11–49 hours).[1] Suvorexant exposure is unchanged in people with severe renal impairment and no dosage adjustment is necessary in these individuals.[1]
Chemistry
Suvorexant is a small-molecule compound.[22] The chemical name of suvorexant is [(7R)-4-(5-chloro-2-benzoxazolyl)hexahydro-7-methyl-1H-1,4-diazepin-1-yl][5-methyl-2-(2H-1,2,3-triazol2-yl)phenyl]methanone.[1] Its molecular formula is C23H23N6O2Cl and its molecular weight is 450.92 g/mol.[1] Suvorexant is a white to off-white powder and is insoluble in water.[1]
History
Suvorexant was first described in the medical literature in 2010.[6] It was approved by the United States Food and Drug Administration on August 13, 2014.[1][5][23] The medication was initially released November 2014 in Japan,[7] then later reached the United States in February 2015,[24] Australia in November 2016, and Canada in November 2018.[25] Suvorexant marketing exclusivity in the United States is set to expire in January 2023 and patent protection is set to expire in 2029 to 2033.[5]
Society and culture
Names
Suvorexant was developed under the code name MK-4305 and is marketed under the brand name Belsomra.[12]
Legal status
The United States Drug Enforcement Administration (DEA) placed suvorexant on the list of schedule IV controlled substances under the Controlled Substances Act.[18][8] According to the DEA, suvorexant produces similar reinforcing effects to those of zolpidem and thus may have a similar misuse liability.[18][26]
Research
Suvorexant is under development for the treatment of delirium.[27] As of October 2021, it is in phase 3 clinical trials for this indication.[27]
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/204569s008lbl.pdf
- ^ a b c d e f g "Belsomra- suvorexant tablet, film coated". DailyMed. 20 November 2019. Retrieved 30 January 2020.
- ^ "Suvorexant (Belsomra) Use During Pregnancy". Drugs.com. 9 September 2019. Retrieved 30 January 2020.
- ^ a b c d e f g h i j k l m n o p q Jacobson LH, Callander GE, Hoyer D (November 2014). "Suvorexant for the treatment of insomnia". Expert Rev Clin Pharmacol. 7 (6): 711–30. doi:10.1586/17512433.2014.966813. PMID 25318834.
- ^ a b c d https://www.drugs.com/availability/generic-belsomra.html
- ^ a b Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ, Roecker AJ, Mercer SP, Bednar RA, Lemaire W, Bruno JG, Reiss DR, Harrell CM, Murphy KL, Garson SL, Doran SM, Prueksaritanont T, Anderson WB, Tang C, Roller S, Cabalu TD, Cui D, Hartman GD, Young SD, Koblan KS, Winrow CJ, Renger JJ, Coleman PJ (July 2010). "Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia". J Med Chem. 53 (14): 5320–32. doi:10.1021/jm100541c. PMID 20565075.
- ^ a b "New hypnotic drug without addiction to be released in Japan first".
- ^ a b "Schedules of Controlled Substances: Placement of Suvorexant into Schedule IV". federalregister.gov. Federal Register. February 13, 2014. Retrieved August 10, 2016.
A Proposed Rule by the Drug Enforcement Administration on 02/13/2014
- ^ a b c Wang L, Pan Y, Ye C, Guo L, Luo S, Dai S, Chen N, Wang E (December 2021). "A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults". Neurosci Biobehav Rev. 131: 489–496. doi:10.1016/j.neubiorev.2021.09.035. PMID 34560134.
- ^ a b c McElroy H, O'Leary B, Adena M, Campbell R, Monfared AA, Meier G (September 2021). "Comparative efficacy of lemborexant and other insomnia treatments: a network meta-analysis". J Manag Care Spec Pharm. 27 (9): 1296–1308. doi:10.18553/jmcp.2021.21011. PMID 34121443.
- ^ Xue T, Wu X, Chen S, Yang Y, Yan Z, Song Z, Zhang W, Zhang J, Chen Z, Wang Z (February 2022). "The efficacy and safety of dual orexin receptor antagonists in primary insomnia: A systematic review and network meta-analysis". Sleep Med Rev. 61: 101573. doi:10.1016/j.smrv.2021.101573. PMID 34902823.
- ^ a b c d Patel KV, Aspesi AV, Evoy KE (April 2015). "Suvorexant: a dual orexin receptor antagonist for the treatment of sleep onset and sleep maintenance insomnia". The Annals of Pharmacotherapy. 49 (4): 477–83. doi:10.1177/1060028015570467. PMID 25667197.
- ^ McCleery J, Sharpley AL (November 2020). "Pharmacotherapies for sleep disturbances in dementia". Cochrane Database Syst Rev. 11: CD009178. doi:10.1002/14651858.CD009178.pub4. PMC 8094738. PMID 33189083.
- ^ Sun H, Kennedy WP, Wilbraham D, Lewis N, Calder N, Li X, Ma J, Yee KL, Ermlich S, Mangin E, Lines C, Rosen L, Chodakewitz J, Murphy GM (February 2013). "Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men". Sleep. 36 (2): 259–67. doi:10.5665/sleep.2386. PMC 3542986. PMID 23372274.
- ^ a b c Carr T (5 February 2016). "FDA Fields Complaints on Sleeping Pill Suvorexant". Consumer Reports.
- ^ a b c Jacobson LH, Callander GE, Hoyer D (November 2014). "Suvorexant for the treatment of insomnia". Expert Review of Clinical Pharmacology. 7 (6): 711–30. doi:10.1586/17512433.2014.966813. PMID 25318834.
- ^ Keks NA, Hope J, Keogh S (December 2017). "Suvorexant: scientifically interesting, utility uncertain". Australas Psychiatry. 25 (6): 622–624. doi:10.1177/1039856217734677. PMID 28994603.
- ^ a b c "Schedules of controlled substances: placement of suvorexant into Schedule IV. Final rule" (PDF). Federal Register. 79 (167): 51243–7. August 2014. PMID 25167596.
- ^ Ufer M, Kelsh D, Schoedel KA, Dingemanse J (September 2021). "Abuse potential assessment of the new dual orexin receptor antagonist daridorexant in recreational sedative drug users as compared to suvorexant and zolpidem". Sleep. 45 (3). doi:10.1093/sleep/zsab224. PMID 34480579.
- ^ a b "U.S. Food and Drug Administration." Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. U.S. Food and Drug Administration, 27 Oct. 2014. Web. 30 Oct. 2014.
- ^ "Suvorexant Advisory Committee Meeting Briefing Document" (PDF). May 22, 2013. Retrieved Feb 7, 2015.
- ^ Christopher JA (2014). "Small-molecule antagonists of the orexin receptors". Pharm Pat Anal. 3 (6): 625–38. doi:10.4155/ppa.14.46. PMID 25489915.
- ^ "FDA approves new type of sleep drug, Belsomra". U.S. Food and Drug Administration (FDA) (Press release). 13 August 2014. Archived from the original on 14 February 2017. Retrieved 30 January 2020.
- ^ "Merck's Insomnia Medicine Belsomra C-IV Now Available in US". www.sleepreviewmag.com. Sleep Review. Retrieved 9 September 2015.
- ^ "Regulatory Decision Summary - Belsomra - Health Canada". hpr-rps.hres.ca. Government of Canada. Retrieved 6 February 2020.
- ^ "Rules - 2014 - Final Rule: Placement of Suvorexant into Schedule IV". www.deadiversion.usdoj.gov. Retrieved 2016-04-03.
- ^ a b https://adisinsight.springer.com/drugs/800027707
External links
- "Suvorexant". Drug Information Portal. U.S. National Library of Medicine.
- Parker I (9 December 2013). "The Big Sleep". The New Yorker.
