Start codon: Difference between revisions
Artoria2e5 (talk | contribs) →Engineered start codons: formylglutamine |
Artoria2e5 (talk | contribs) (shrug |
||
| Line 4: | Line 4: | ||
The start codon is often preceded by a 5' untranslated region ([[5' UTR]]). In [[prokaryotes]] this includes the [[ribosome binding site]]. |
The start codon is often preceded by a 5' untranslated region ([[5' UTR]]). In [[prokaryotes]] this includes the [[ribosome binding site]]. |
||
== Decoding == |
|||
In all three domains of life, the start codon is decoded by a special "initiation" [[transfer RNA]] different from the tRNAs used for elongation. There are important structual differences between an initiating tRNA and an elongating one, with distinguish features serving to satisfy the constraints of the translation system. In bacteria and organelles, an acceptor stem C1:A72 mismatch guide formylation, which is essential direct recruitment by the 30S ribosome into the P site; so-called "3GC" base pairs allow assembly into the 70S ribosome.<ref name=p28204695>{{cite journal |last1=Shetty |first1=S |last2=Shah |first2=RA |last3=Chembazhi |first3=UV |last4=Sah |first4=S |last5=Varshney |first5=U |title=Two highly conserved features of bacterial initiator tRNAs license them to pass through distinct checkpoints in translation initiation. |journal=Nucleic acids research |date=28 February 2017 |volume=45 |issue=4 |pages=2040-2050 |doi=10.1093/nar/gkw854 |pmid=28204695 |pmc=5389676}}</ref> In eukaryotes and archaea, the T stem prevents the [[elongation factor]]s from binding, while [[eIF2]] specifically recognizes the attached methionine and a A1:U72 basepair.<ref>{{cite journal |last1=Kolitz |first1=SE |last2=Lorsch |first2=JR |title=Eukaryotic initiator tRNA: finely tuned and ready for action. |journal=FEBS letters |date=21 January 2010 |volume=584 |issue=2 |pages=396-404 |doi=10.1016/j.febslet.2009.11.047 |pmid=19925799 |pmc=2795131}}</ref> |
|||
In any case, the natural initiating tRNA only codes for methionine.<ref name="Lobanov 2010"/> Knowledge of the key recognizing features has allowed researchers to construct alternative initiating tRNAs that code for different amino acids; see below. |
|||
== Alternative start codons == |
== Alternative start codons == |
||
Alternative start codons are different from the standard AUG codon and are found in both [[prokaryotes]] (bacteria and archaea) and [[eukaryotes]]. Alternate start codons are still translated as Met when they are at the start of a protein (even if the codon encodes a different amino acid otherwise). This is because a separate |
Alternative start codons are different from the standard AUG codon and are found in both [[prokaryotes]] (bacteria and archaea) and [[eukaryotes]]. Alternate start codons are still translated as Met when they are at the start of a protein (even if the codon encodes a different amino acid otherwise). This is because a separate tRNA is used for initiation.<ref name="Lobanov 2010">{{Cite journal |pmid = 20446809 |pmc = 3311535 |year = 2010 |last1 = Lobanov |first1 = A. V. |title = Dual functions of codons in the genetic code |journal = Critical Reviews in Biochemistry and Molecular Biology |volume = 45 |issue = 4 |pages = 257–65 |last2 = Turanov |first2 = A. A. |last3 = Hatfield |first3 = D. L. |last4 = Gladyshev |first4 = V. N. |doi = 10.3109/10409231003786094 }}</ref> |
||
=== Eukaryotes === |
=== Eukaryotes === |
||
Alternate start codons (non-AUG) are very rare in eukaryotic genomes. However, naturally occurring non-AUG start codons have been reported for some cellular mRNAs.<ref name="Ivanov2011">{{Cite journal |last1 = Ivanov |first1 = I. P. |last2 = Firth |first2 = A. E. |last3 = Michel |first3 = A. M. |last4 = Atkins |first4 = J. F. |last5 = Baranov |first5 = P. V. |title = Identification of evolutionarily conserved non-AUG-initiated N-terminal extensions in human coding sequences |doi = 10.1093/nar/gkr007 |journal = Nucleic Acids Research |volume = 39 |issue = 10 |pages = 4220–4234 |year = 2011 |pmid = 21266472 |pmc =3105428 |name-list-style = vanc }}</ref> Seven out of the nine possible single-nucleotide substitutions at the AUG start codon of [[dihydrofolate reductase]] are functional as translation start sites in mammalian cells.<ref name="Peabody1989">{{Cite journal |
Alternate start codons (non-AUG) are very rare in eukaryotic genomes: a wide range of mechanisms work to guarantee the relative fidelity of AUG initiation.<ref name=pmid26779403>{{cite journal |last1=Asano |first1=K |title=Why is start codon selection so precise in eukaryotes? |journal=Translation (Austin, Tex.) |date=2014 |volume=2 |issue=1 |pages=e28387 |doi=10.4161/trla.28387 |pmid=26779403}}</ref> However, naturally occurring non-AUG start codons have been reported for some cellular mRNAs.<ref name="Ivanov2011">{{Cite journal |last1 = Ivanov |first1 = I. P. |last2 = Firth |first2 = A. E. |last3 = Michel |first3 = A. M. |last4 = Atkins |first4 = J. F. |last5 = Baranov |first5 = P. V. |title = Identification of evolutionarily conserved non-AUG-initiated N-terminal extensions in human coding sequences |doi = 10.1093/nar/gkr007 |journal = Nucleic Acids Research |volume = 39 |issue = 10 |pages = 4220–4234 |year = 2011 |pmid = 21266472 |pmc =3105428 |name-list-style = vanc }}</ref> Seven out of the nine possible single-nucleotide substitutions at the AUG start codon of [[dihydrofolate reductase]] are functional as translation start sites in mammalian cells.<ref name="Peabody1989">{{Cite journal |
||
|pmid = 2538469 |year = 1989 |last1 = Peabody |first1 = D. S. |title = Translation initiation at non-AUG triplets in mammalian cells |journal = The Journal of Biological Chemistry |volume = 264 |issue = 9 |pages = 5031–5|doi = 10.1016/S0021-9258(18)83694-8 |doi-access = free }}</ref> |
|pmid = 2538469 |year = 1989 |last1 = Peabody |first1 = D. S. |title = Translation initiation at non-AUG triplets in mammalian cells |journal = The Journal of Biological Chemistry |volume = 264 |issue = 9 |pages = 5031–5|doi = 10.1016/S0021-9258(18)83694-8 |doi-access = free }}</ref> |
||
| ⚫ | |pmid = 22745432 |year = 2012 |last1 = Starck |first1 = S. R. |title = Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I |journal = Science |volume = 336 |issue = 6089 |pages = 1719–23 |last2 = Jiang |first2 = V |last3 = Pavon-Eternod |first3 = M |last4 = Prasad |first4 = S |last5 = McCarthy |first5 = B |last6 = Pan |first6 = T |last7 = Shastri |first7 = N |doi = 10.1126/science.1220270 |bibcode = 2012Sci...336.1719S|s2cid = 206540614 }}</ref><ref name="Dever2012">{{Cite journal |pmid = 22745408 |year = 2012 |last1 = Dever |first1 = T. E. |title = Molecular biology. A new start for protein synthesis |journal = Science |volume = 336 |issue = 6089 |pages = 1645–6 |doi = 10.1126/science.1224439 |s2cid = 44326947 |url = https://zenodo.org/record/1230920 }}</ref> |
||
''[[Candida albicans]]'' uses a CAG start codon.<ref>{{cite journal |last1=Santos |first1=MA |last2=Keith |first2=G |last3=Tuite |first3=MF |title=Non-standard translational events in Candida albicans mediated by an unusual seryl-tRNA with a 5'-CAG-3' (leucine) anticodon. |journal=The EMBO Journal |date=February 1993 |volume=12 |issue=2 |pages=607–16 |pmid=8440250|pmc=413244 |doi=10.1002/j.1460-2075.1993.tb05693.x }}</ref> |
''[[Candida albicans]]'' uses a CAG start codon.<ref>{{cite journal |last1=Santos |first1=MA |last2=Keith |first2=G |last3=Tuite |first3=MF |title=Non-standard translational events in Candida albicans mediated by an unusual seryl-tRNA with a 5'-CAG-3' (leucine) anticodon. |journal=The EMBO Journal |date=February 1993 |volume=12 |issue=2 |pages=607–16 |pmid=8440250|pmc=413244 |doi=10.1002/j.1460-2075.1993.tb05693.x }}</ref> |
||
=== |
=== Bacteria === |
||
Bacteria do not generally have the wide range of translation factors monitoring start codon fidelity. GUG and UUG are the main, even "canonical", alternate start codons.<ref name=pmid26779403/> GUG in particular is important to controlling the replication of plasmids.<ref name=pmid26779403/> |
|||
Prokaryotes use alternate start codons significantly, mainly GUG and UUG. |
|||
[[E. coli]] uses 83% AUG (3542/4284), 14% (612) GUG, 3% (103) UUG<ref>{{Cite journal|last7 = Collado-Vides |first9 = C. K. |first8 = J. D. |first7 = J.|last10 = Mayhew|title = The Complete Genome Sequence of Escherichia coli K-12 |first17 = Y.|last17 = Shao |first16 = B.|last16 = Mau |first15 = D. J.|last15 = Rose |first14 = M. A.|last14 = Goeden |first13 = H. A.|last13 = Kirkpatrick |first12 = N. W.|last12 = Davis |first11 = J.|journal = Science|last11 = Gregor|doi = 10.1126/science.277.5331.1453 |first10 = G. F.|pmid = 9278503|year = 1997|pages = 1453–1462|volume = 277|issue = 5331 |first6 = M. |first2 = G.|last9 = Rode |first3 = C. A.|last2 = Plunkett g|last4 = Perna |first1 = F. R.|last6 = Riley|last5 = Burland|last1 = Blattner|last8 = Glasner |first5 = V. |first4 = N. T.|last3 = Bloch |doi-access = free}}</ref> and one or two others (e.g., an AUU and possibly a CUG).<ref name="Sequence of a 1.26-kb DNA fragment containing the structural gene for E.coli initiation factor IF3: presence of an AUU initiator codon">{{Cite journal |last1 = Sacerdot |first1 = C. |last2 = Fayat |first2 = G. |last3 = Dessen |first3 = P. |last4 = Springer |first4 = M. |last5 = Plumbridge |first5 = J. A. |last6 = Grunberg-Manago |first6 = M. |last7 = Blanquet |first7 = S. |title = Sequence of a 1.26-kb DNA fragment containing the structural gene for E.coli initiation factor IF3: Presence of an AUU initiator codon |journal = The EMBO Journal |volume = 1 |issue = 3 |pages = 311–315 |year = 1982 |pmid = 6325158 |pmc = 553041 |doi = 10.1002/j.1460-2075.1982.tb01166.x }}</ref><ref name="The Escherichia coli heat shock gene htpY: mutational analysis, cloning, sequencing, and transcriptional regulation.">{{Cite journal |last1 = Missiakas |first1 = D. |last2 = Georgopoulos |first2 = C. |last3 = Raina |first3 = S. |title = The Escherichia coli heat shock gene htpY: Mutational analysis, cloning, sequencing, and transcriptional regulation |journal = Journal of Bacteriology |volume = 175 |issue = 9 |pages = 2613–2624 |year = 1993 |pmid = 8478327 |pmc = 204563 |doi = 10.1128/jb.175.9.2613-2624.1993 }}</ref> |
[[E. coli]] uses 83% AUG (3542/4284), 14% (612) GUG, 3% (103) UUG<ref>{{Cite journal|last7 = Collado-Vides |first9 = C. K. |first8 = J. D. |first7 = J.|last10 = Mayhew|title = The Complete Genome Sequence of Escherichia coli K-12 |first17 = Y.|last17 = Shao |first16 = B.|last16 = Mau |first15 = D. J.|last15 = Rose |first14 = M. A.|last14 = Goeden |first13 = H. A.|last13 = Kirkpatrick |first12 = N. W.|last12 = Davis |first11 = J.|journal = Science|last11 = Gregor|doi = 10.1126/science.277.5331.1453 |first10 = G. F.|pmid = 9278503|year = 1997|pages = 1453–1462|volume = 277|issue = 5331 |first6 = M. |first2 = G.|last9 = Rode |first3 = C. A.|last2 = Plunkett g|last4 = Perna |first1 = F. R.|last6 = Riley|last5 = Burland|last1 = Blattner|last8 = Glasner |first5 = V. |first4 = N. T.|last3 = Bloch |doi-access = free}}</ref> and one or two others (e.g., an AUU and possibly a CUG).<ref name="Sequence of a 1.26-kb DNA fragment containing the structural gene for E.coli initiation factor IF3: presence of an AUU initiator codon">{{Cite journal |last1 = Sacerdot |first1 = C. |last2 = Fayat |first2 = G. |last3 = Dessen |first3 = P. |last4 = Springer |first4 = M. |last5 = Plumbridge |first5 = J. A. |last6 = Grunberg-Manago |first6 = M. |last7 = Blanquet |first7 = S. |title = Sequence of a 1.26-kb DNA fragment containing the structural gene for E.coli initiation factor IF3: Presence of an AUU initiator codon |journal = The EMBO Journal |volume = 1 |issue = 3 |pages = 311–315 |year = 1982 |pmid = 6325158 |pmc = 553041 |doi = 10.1002/j.1460-2075.1982.tb01166.x }}</ref><ref name="The Escherichia coli heat shock gene htpY: mutational analysis, cloning, sequencing, and transcriptional regulation.">{{Cite journal |last1 = Missiakas |first1 = D. |last2 = Georgopoulos |first2 = C. |last3 = Raina |first3 = S. |title = The Escherichia coli heat shock gene htpY: Mutational analysis, cloning, sequencing, and transcriptional regulation |journal = Journal of Bacteriology |volume = 175 |issue = 9 |pages = 2613–2624 |year = 1993 |pmid = 8478327 |pmc = 204563 |doi = 10.1128/jb.175.9.2613-2624.1993 }}</ref> |
||
| Line 24: | Line 28: | ||
=== Mitochondria === |
=== Mitochondria === |
||
[[Mitochondrial genome]]s use alternate start codons more significantly (AUA and AUG in humans).<ref name="WatanabeSuzuki2001">{{cite book|last1=Watanabe|first1=Kimitsuna|title=Encyclopedia of Life Sciences|last2=Suzuki|first2=Tsutomu|chapter=Genetic Code and its Variants|year=2001|doi=10.1038/npg.els.0000810|isbn=978-0470015902}}</ref> Many such examples, with codons, systematic range, and citations, are given in the NCBI [[List of genetic codes|list of translation tables]].<ref>{{cite web |last1=Elzanowski |first1=Andrzej |last2=Ostell |first2=Jim |title=The Genetic Codes |url=https://www.ncbi.nlm.nih.gov/Taxonomy/Utils/wprintgc.cgi |website=NCBI |access-date=29 March 2019}}</ref> |
[[Mitochondrial genome]]s use alternate start codons more significantly (AUA and AUG in humans).<ref name="WatanabeSuzuki2001">{{cite book|last1=Watanabe|first1=Kimitsuna|title=Encyclopedia of Life Sciences|last2=Suzuki|first2=Tsutomu|chapter=Genetic Code and its Variants|year=2001|doi=10.1038/npg.els.0000810|isbn=978-0470015902}}</ref> Many such examples, with codons, systematic range, and citations, are given in the NCBI [[List of genetic codes|list of translation tables]].<ref>{{cite web |last1=Elzanowski |first1=Andrzej |last2=Ostell |first2=Jim |title=The Genetic Codes |url=https://www.ncbi.nlm.nih.gov/Taxonomy/Utils/wprintgc.cgi |website=NCBI |access-date=29 March 2019}}</ref> |
||
=== Archaea === |
|||
Archaea, which are prokaryotes with a translation machinery similar to but simpler than that of eukaryotes, allow initiation at UUG and GUG.<ref name=pmid26779403/> |
|||
== Upstream start codons == |
== Upstream start codons == |
||
| Line 31: | Line 38: | ||
{{Codon table|T=U}} |
{{Codon table|T=U}} |
||
== |
== Non-methionine start codons == |
||
| ⚫ | Engineered initiator tRNA (tRNA{{sup sub|fMet|CUA}}) have been used to initiate translation at the [[Amber codon|amber stop codon]] UAG in ''E. coli''. Initiation with this tRNA not only inserts the traditional [[formylmethionine]], but also formylglutamine, as glutamyl-tRNA synthase also recognizes the new tRNA.<ref>{{Cite journal |last1=Varshney |first1=U. |last2=RajBhandary |first2=U. L. |date=1990-02-01 |title=Initiation of protein synthesis from a termination codon |journal=Proceedings of the National Academy of Sciences |volume=87 |issue=4 |pages=1586–1590 |doi=10.1073/pnas.87.4.1586 |pmid=2406724 |pmc=53520 |issn=0027-8424 |bibcode=1990PNAS...87.1586V |doi-access=free }}</ref> One study has shown that the amber initiator tRNA does not initiate translation to any measurable degree from genomically-encoded UAG codons, only plasmid-borne reporters with strong upstream [[Shine-Dalgarno sequence|Shine-Dalgarno sites]].<ref>{{Cite journal |last1=Vincent |first1=Russel M. |last2=Wright |first2=Bradley W. |last3=Jaschke |first3=Paul R. |date=2019-03-15 |title=Measuring Amber Initiator tRNA Orthogonality in a Genomically Recoded Organism |journal=ACS Synthetic Biology |volume=8 |issue=4 |pages=675–685 |language=en |doi=10.1021/acssynbio.9b00021 |pmid=30856316 |s2cid=75136654 |issn=2161-5063 |url=https://www.biorxiv.org/content/biorxiv/early/2019/01/18/524249.full.pdf }}</ref> |
||
=== Natural === |
|||
In addition to the canonical initiation-tRNA and AUG codon pathway, mammalian cells can initiate translation with [[leucine]] using a specific leucyl-tRNA that decodes the codon CUG. This mechanism is independent of eIF2.<ref name="Starck2012">{{Cite journal |
|||
| ⚫ | |pmid = 22745432 |year = 2012 |last1 = Starck |first1 = S. R. |title = Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I |journal = Science |volume = 336 |issue = 6089 |pages = 1719–23 |last2 = Jiang |first2 = V |last3 = Pavon-Eternod |first3 = M |last4 = Prasad |first4 = S |last5 = McCarthy |first5 = B |last6 = Pan |first6 = T |last7 = Shastri |first7 = N |doi = 10.1126/science.1220270 |bibcode = 2012Sci...336.1719S|s2cid = 206540614 }}</ref><ref name="Dever2012">{{Cite journal |pmid = 22745408 |year = 2012 |last1 = Dever |first1 = T. E. |title = Molecular biology. A new start for protein synthesis |journal = Science |volume = 336 |issue = 6089 |pages = 1645–6 |doi = 10.1126/science.1224439 |s2cid = 44326947 |url = https://zenodo.org/record/1230920 }}</ref> It is also previously known that translation started by an [[internal ribosome entry site]], which bypasses a number of regular eukaryotic initiation systems, can have a non-methinone start with GCU or CAA codons.<ref>{{cite journal |title=Where to Start? Alternate Protein Translation Mechanism Creates Unanticipated Antigens |journal=PLoS Biology |date=26 October 2004 |volume=2 |issue=11 |pages=e397 |doi=10.1371/journal.pbio.0020397}}</ref><ref>{{cite journal |last1=RajBhandary |first1=Uttam L. |title=More surprises in translation: Initiation without the initiator tRNA |journal=Proceedings of the National Academy of Sciences |date=15 February 2000 |volume=97 |issue=4 |pages=1325–1327 |doi=10.1073/pnas.040579197}}</ref> |
||
=== Engineered start codons === |
|||
| ⚫ | Engineered initiator tRNA (tRNA{{sup sub|fMet|CUA}}, changed from a MetY tRNA{{sup sub|fMet|CAU}}) have been used to initiate translation at the [[Amber codon|amber stop codon]] UAG in ''E. coli''. Initiation with this tRNA not only inserts the traditional [[formylmethionine]], but also formylglutamine, as glutamyl-tRNA synthase also recognizes the new tRNA.<ref>{{Cite journal |last1=Varshney |first1=U. |last2=RajBhandary |first2=U. L. |date=1990-02-01 |title=Initiation of protein synthesis from a termination codon |journal=Proceedings of the National Academy of Sciences |volume=87 |issue=4 |pages=1586–1590 |doi=10.1073/pnas.87.4.1586 |pmid=2406724 |pmc=53520 |issn=0027-8424 |bibcode=1990PNAS...87.1586V |doi-access=free }}</ref> (Recall from above that the bacterial translation initiation system does not specifically check for methionine, only the formyl modification).<ref name=p28204695/> One study has shown that the amber initiator tRNA does not initiate translation to any measurable degree from genomically-encoded UAG codons, only plasmid-borne reporters with strong upstream [[Shine-Dalgarno sequence|Shine-Dalgarno sites]].<ref>{{Cite journal |last1=Vincent |first1=Russel M. |last2=Wright |first2=Bradley W. |last3=Jaschke |first3=Paul R. |date=2019-03-15 |title=Measuring Amber Initiator tRNA Orthogonality in a Genomically Recoded Organism |journal=ACS Synthetic Biology |volume=8 |issue=4 |pages=675–685 |language=en |doi=10.1021/acssynbio.9b00021 |pmid=30856316 |s2cid=75136654 |issn=2161-5063 |url=https://www.biorxiv.org/content/biorxiv/early/2019/01/18/524249.full.pdf }}</ref> |
||
{{missing information|section|new progress mentioned in introduction of [[doi:10.3389/fchem.2021.772648]] (Ngo et al., 2013 might be worth a special mention)|date=December 2023}} |
|||
== See also == |
== See also == |
||
Revision as of 06:05, 29 December 2023
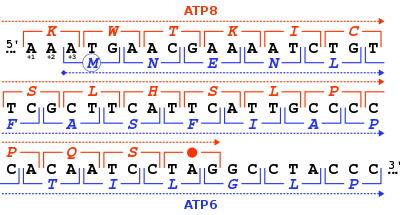
The start codon is the first codon of a messenger RNA (mRNA) transcript translated by a ribosome. The start codon always codes for methionine in eukaryotes and archaea and a N-formylmethionine (fMet) in bacteria, mitochondria and plastids.
The start codon is often preceded by a 5' untranslated region (5' UTR). In prokaryotes this includes the ribosome binding site.
Decoding
In all three domains of life, the start codon is decoded by a special "initiation" transfer RNA different from the tRNAs used for elongation. There are important structual differences between an initiating tRNA and an elongating one, with distinguish features serving to satisfy the constraints of the translation system. In bacteria and organelles, an acceptor stem C1:A72 mismatch guide formylation, which is essential direct recruitment by the 30S ribosome into the P site; so-called "3GC" base pairs allow assembly into the 70S ribosome.[1] In eukaryotes and archaea, the T stem prevents the elongation factors from binding, while eIF2 specifically recognizes the attached methionine and a A1:U72 basepair.[2]
In any case, the natural initiating tRNA only codes for methionine.[3] Knowledge of the key recognizing features has allowed researchers to construct alternative initiating tRNAs that code for different amino acids; see below.
Alternative start codons
Alternative start codons are different from the standard AUG codon and are found in both prokaryotes (bacteria and archaea) and eukaryotes. Alternate start codons are still translated as Met when they are at the start of a protein (even if the codon encodes a different amino acid otherwise). This is because a separate tRNA is used for initiation.[3]
Eukaryotes
Alternate start codons (non-AUG) are very rare in eukaryotic genomes: a wide range of mechanisms work to guarantee the relative fidelity of AUG initiation.[4] However, naturally occurring non-AUG start codons have been reported for some cellular mRNAs.[5] Seven out of the nine possible single-nucleotide substitutions at the AUG start codon of dihydrofolate reductase are functional as translation start sites in mammalian cells.[6]
Candida albicans uses a CAG start codon.[7]
Bacteria
Bacteria do not generally have the wide range of translation factors monitoring start codon fidelity. GUG and UUG are the main, even "canonical", alternate start codons.[4] GUG in particular is important to controlling the replication of plasmids.[4]
E. coli uses 83% AUG (3542/4284), 14% (612) GUG, 3% (103) UUG[8] and one or two others (e.g., an AUU and possibly a CUG).[9][10]
Well-known coding regions that do not have AUG initiation codons are those of lacI (GUG)[11][12] and lacA (UUG)[13] in the E. coli lac operon. Two more recent studies have independently shown that 17 or more non-AUG start codons may initiate translation in E. coli.[14][15]
Mitochondria
Mitochondrial genomes use alternate start codons more significantly (AUA and AUG in humans).[16] Many such examples, with codons, systematic range, and citations, are given in the NCBI list of translation tables.[17]
Archaea
Archaea, which are prokaryotes with a translation machinery similar to but simpler than that of eukaryotes, allow initiation at UUG and GUG.[4]
Upstream start codons
These are "alternative" start codons in the sense that they are upstream of the regular start codons and thus could be used as alternative start codons. More than half of all human mRNAs have at least one AUG codon upstream (uAUG) of their annotated translation initiation starts (TIS) (58% in the current versions of the human RefSeq sequence). Their potential use as TISs could result in translation of so-called upstream Open Reading Frames (uORFs). uORF translation usually results in the synthesis of short polypeptides, some of which have been shown to be functional, e.g., in ASNSD1, MIEF1, MKKS, and SLC35A4.[18] However, it is believed that most translated uORFs only have a mild inhibitory effect on downstream translation because most uORF starts are leaky (i.e. don't initiate translation or because ribosomes terminating after translation of short ORFs are often capable of reinitiating).[18]
Standard genetic code
| Amino-acid biochemical properties | Nonpolar | Polar | Basic | Acidic | Termination: stop codon |
| 1st base |
2nd base | 3rd base | |||||||
|---|---|---|---|---|---|---|---|---|---|
| U | C | A | G | ||||||
| U | UUU | (Phe/F) Phenylalanine | UCU | (Ser/S) Serine | UAU | (Tyr/Y) Tyrosine | UGU | (Cys/C) Cysteine | U |
| UUC | UCC | UAC | UGC | C | |||||
| UUA | (Leu/L) Leucine | UCA | UAA | Stop (Ochre)[B] | UGA | Stop (Opal)[B] | A | ||
| UUG[A] | UCG | UAG | Stop (Amber)[B] | UGG | (Trp/W) Tryptophan | G | |||
| C | CUU | CCU | (Pro/P) Proline | CAU | (His/H) Histidine | CGU | (Arg/R) Arginine | U | |
| CUC | CCC | CAC | CGC | C | |||||
| CUA | CCA | CAA | (Gln/Q) Glutamine | CGA | A | ||||
| CUG | CCG | CAG | CGG | G | |||||
| A | AUU | (Ile/I) Isoleucine | ACU | (Thr/T) Threonine | AAU | (Asn/N) Asparagine | AGU | (Ser/S) Serine | U |
| AUC | ACC | AAC | AGC | C | |||||
| AUA | ACA | AAA | (Lys/K) Lysine | AGA | (Arg/R) Arginine | A | |||
| AUG[A] | (Met/M) Methionine | ACG | AAG | AGG | G | ||||
| G | GUU | (Val/V) Valine | GCU | (Ala/A) Alanine | GAU | (Asp/D) Aspartic acid | GGU | (Gly/G) Glycine | U |
| GUC | GCC | GAC | GGC | C | |||||
| GUA | GCA | GAA | (Glu/E) Glutamic acid | GGA | A | ||||
| GUG[A] | GCG | GAG | GGG | G | |||||
- A Possible start codons in NCBI table 1. AUG is most common.[20] The two other start codons listed by table 1 (GUG and UUG) are rare in eukaryotes.[4] Prokaryotes have less strigent start codon requirements; they are described by NCBI table 11.
- B ^ ^ ^ The historical basis for designating the stop codons as amber, ochre and opal is described in an autobiography by Sydney Brenner[21] and in a historical article by Bob Edgar.[22]
Non-methionine start codons
Natural
In addition to the canonical initiation-tRNA and AUG codon pathway, mammalian cells can initiate translation with leucine using a specific leucyl-tRNA that decodes the codon CUG. This mechanism is independent of eIF2.[23][24] It is also previously known that translation started by an internal ribosome entry site, which bypasses a number of regular eukaryotic initiation systems, can have a non-methinone start with GCU or CAA codons.[25][26]
Engineered start codons
Engineered initiator tRNA (tRNAfMet
CUA, changed from a MetY tRNAfMet
CAU) have been used to initiate translation at the amber stop codon UAG in E. coli. Initiation with this tRNA not only inserts the traditional formylmethionine, but also formylglutamine, as glutamyl-tRNA synthase also recognizes the new tRNA.[27] (Recall from above that the bacterial translation initiation system does not specifically check for methionine, only the formyl modification).[1] One study has shown that the amber initiator tRNA does not initiate translation to any measurable degree from genomically-encoded UAG codons, only plasmid-borne reporters with strong upstream Shine-Dalgarno sites.[28]
This section is missing information about new progress mentioned in introduction of doi:10.3389/fchem.2021.772648 (Ngo et al., 2013 might be worth a special mention). (December 2023) |
See also
- Central dogma of molecular biology
- Codon
- Messenger RNA
- Missense mRNA
- Stop codon
- Transfer RNA
- Translation
References
- ^ a b Shetty, S; Shah, RA; Chembazhi, UV; Sah, S; Varshney, U (28 February 2017). "Two highly conserved features of bacterial initiator tRNAs license them to pass through distinct checkpoints in translation initiation". Nucleic acids research. 45 (4): 2040–2050. doi:10.1093/nar/gkw854. PMC 5389676. PMID 28204695.
- ^ Kolitz, SE; Lorsch, JR (21 January 2010). "Eukaryotic initiator tRNA: finely tuned and ready for action". FEBS letters. 584 (2): 396–404. doi:10.1016/j.febslet.2009.11.047. PMC 2795131. PMID 19925799.
- ^ a b Lobanov, A. V.; Turanov, A. A.; Hatfield, D. L.; Gladyshev, V. N. (2010). "Dual functions of codons in the genetic code". Critical Reviews in Biochemistry and Molecular Biology. 45 (4): 257–65. doi:10.3109/10409231003786094. PMC 3311535. PMID 20446809.
- ^ a b c d e Asano, K (2014). "Why is start codon selection so precise in eukaryotes?". Translation (Austin, Tex.). 2 (1): e28387. doi:10.4161/trla.28387. PMID 26779403.
- ^ Ivanov IP, Firth AE, Michel AM, Atkins JF, Baranov PV (2011). "Identification of evolutionarily conserved non-AUG-initiated N-terminal extensions in human coding sequences". Nucleic Acids Research. 39 (10): 4220–4234. doi:10.1093/nar/gkr007. PMC 3105428. PMID 21266472.
- ^ Peabody, D. S. (1989). "Translation initiation at non-AUG triplets in mammalian cells". The Journal of Biological Chemistry. 264 (9): 5031–5. doi:10.1016/S0021-9258(18)83694-8. PMID 2538469.
- ^ Santos, MA; Keith, G; Tuite, MF (February 1993). "Non-standard translational events in Candida albicans mediated by an unusual seryl-tRNA with a 5'-CAG-3' (leucine) anticodon". The EMBO Journal. 12 (2): 607–16. doi:10.1002/j.1460-2075.1993.tb05693.x. PMC 413244. PMID 8440250.
- ^ Blattner, F. R.; Plunkett g, G.; Bloch, C. A.; Perna, N. T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J. D.; Rode, C. K.; Mayhew, G. F.; Gregor, J.; Davis, N. W.; Kirkpatrick, H. A.; Goeden, M. A.; Rose, D. J.; Mau, B.; Shao, Y. (1997). "The Complete Genome Sequence of Escherichia coli K-12". Science. 277 (5331): 1453–1462. doi:10.1126/science.277.5331.1453. PMID 9278503.
- ^ Sacerdot, C.; Fayat, G.; Dessen, P.; Springer, M.; Plumbridge, J. A.; Grunberg-Manago, M.; Blanquet, S. (1982). "Sequence of a 1.26-kb DNA fragment containing the structural gene for E.coli initiation factor IF3: Presence of an AUU initiator codon". The EMBO Journal. 1 (3): 311–315. doi:10.1002/j.1460-2075.1982.tb01166.x. PMC 553041. PMID 6325158.
- ^ Missiakas, D.; Georgopoulos, C.; Raina, S. (1993). "The Escherichia coli heat shock gene htpY: Mutational analysis, cloning, sequencing, and transcriptional regulation". Journal of Bacteriology. 175 (9): 2613–2624. doi:10.1128/jb.175.9.2613-2624.1993. PMC 204563. PMID 8478327.
- ^ E.coli lactose operon with lacI, lacZ, lacY and lacA genes GenBank: J01636.1
- ^ Farabaugh, P. J. (1978). "Sequence of the lacI gene". Nature. 274 (5673): 765–769. Bibcode:1978Natur.274..765F. doi:10.1038/274765a0. PMID 355891. S2CID 4208767.
- ^ NCBI Sequence Viewer v2.0
- ^ Hecht, Ariel; Glasgow, Jeff; Jaschke, Paul R.; Bawazer, Lukmaan A.; Munson, Matthew S.; Cochran, Jennifer R.; Endy, Drew; Salit, Marc (2017). "Measurements of translation initiation from all 64 codons in E. coli". Nucleic Acids Research. 45 (7): 3615–3626. doi:10.1093/nar/gkx070. PMC 5397182. PMID 28334756.
- ^ Firnberg, Elad; Labonte, Jason; Gray, Jeffrey; Ostermeir, Marc A. (2014). "A comprehensive, high-resolution map of a gene's fitness landscape". Molecular Biology and Evolution. 31 (6): 1581–1592. doi:10.1093/molbev/msu081. PMC 4032126. PMID 24567513.
- ^ Watanabe, Kimitsuna; Suzuki, Tsutomu (2001). "Genetic Code and its Variants". Encyclopedia of Life Sciences. doi:10.1038/npg.els.0000810. ISBN 978-0470015902.
- ^ Elzanowski, Andrzej; Ostell, Jim. "The Genetic Codes". NCBI. Retrieved 29 March 2019.
- ^ a b Andreev, Dmitry E.; Loughran, Gary; Fedorova, Alla D.; Mikhaylova, Maria S.; Shatsky, Ivan N.; Baranov, Pavel V. (2022-05-09). "Non-AUG translation initiation in mammals". Genome Biology. 23 (1): 111. doi:10.1186/s13059-022-02674-2. ISSN 1474-760X. PMC 9082881. PMID 35534899.
- ^ Elzanowski A, Ostell J (7 January 2019). "The Genetic Codes". National Center for Biotechnology Information. Archived from the original on 5 October 2020. Retrieved 21 February 2019.
- ^ Nakamoto T (March 2009). "Evolution and the universality of the mechanism of initiation of protein synthesis". Gene. 432 (1–2): 1–6. doi:10.1016/j.gene.2008.11.001. PMID 19056476.
- ^ Brenner S. A Life in Science (2001) Published by Biomed Central Limited ISBN 0-9540278-0-9 see pages 101-104
- ^ Edgar B (2004). "The genome of bacteriophage T4: an archeological dig". Genetics. 168 (2): 575–82. PMC 1448817. PMID 15514035. see pages 580-581
- ^ Starck, S. R.; Jiang, V; Pavon-Eternod, M; Prasad, S; McCarthy, B; Pan, T; Shastri, N (2012). "Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I". Science. 336 (6089): 1719–23. Bibcode:2012Sci...336.1719S. doi:10.1126/science.1220270. PMID 22745432. S2CID 206540614.
- ^ Dever, T. E. (2012). "Molecular biology. A new start for protein synthesis". Science. 336 (6089): 1645–6. doi:10.1126/science.1224439. PMID 22745408. S2CID 44326947.
- ^ "Where to Start? Alternate Protein Translation Mechanism Creates Unanticipated Antigens". PLoS Biology. 2 (11): e397. 26 October 2004. doi:10.1371/journal.pbio.0020397.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ RajBhandary, Uttam L. (15 February 2000). "More surprises in translation: Initiation without the initiator tRNA". Proceedings of the National Academy of Sciences. 97 (4): 1325–1327. doi:10.1073/pnas.040579197.
- ^ Varshney, U.; RajBhandary, U. L. (1990-02-01). "Initiation of protein synthesis from a termination codon". Proceedings of the National Academy of Sciences. 87 (4): 1586–1590. Bibcode:1990PNAS...87.1586V. doi:10.1073/pnas.87.4.1586. ISSN 0027-8424. PMC 53520. PMID 2406724.
- ^ Vincent, Russel M.; Wright, Bradley W.; Jaschke, Paul R. (2019-03-15). "Measuring Amber Initiator tRNA Orthogonality in a Genomically Recoded Organism" (PDF). ACS Synthetic Biology. 8 (4): 675–685. doi:10.1021/acssynbio.9b00021. ISSN 2161-5063. PMID 30856316. S2CID 75136654.
External links
- The Genetic Codes. Compiled by Andrzej (Anjay) Elzanowski and Jim Ostell, National Center for Biotechnology Information (NCBI), Bethesda, Maryland, US [1]
