Wnt signaling pathway: Difference between revisions
→Wnt-induced cell responses: added new subsection |
→Cell migration: added info about wnt and cell migration |
||
| Line 80: | Line 80: | ||
====Cell migration==== |
====Cell migration==== |
||
Cell migration during embryonic development allows for the establishment of body axes, tissue formation, limb induction, and several other processes. Wnt signaling has been shown to help mediate this process, particularly during convergent extension. Research has shown that signaling from both the Wnt PCP pathway and canonical Wnt pathway is required for proper convergent extension during gastrulation. Convergent extension is further regulated by the Wnt/calcium pathway, which blocks convergent extension when activated. Wnt signaling also induces cell migration in later stages of development through the control of the migration behavior of neuroblasts, neural crest cells, myocytes, and tracheal cells.<ref>{{cite web|last=Schambony|first=A|title=Wnt Signaling and Cell Migration|url=http://www.ncbi.nlm.nih.gov/books/NBK6303/|work=Madame Curie Bioscience Database|publisher=Landes Bioscience|accessdate=7 May 2013|coauthors=D. Wedlich}}</ref> |
|||
Wnt signaling is also involved in another key migration process known as the epithelial-mesenchymal transition. This process is what allows epithelial cells to transform into mesenchymal cells so that they are no longer held in place at the laminin. It involves a down-regulation of cadherins so that cells can detach from laminin and migrate. Wnt signaling is an inducer of EMT, particularly in mammary development.<ref>{{cite journal|last=Micalizzi|first=Douglas S.|coauthors=Farabaugh, Susan M.; Ford, Heide L.|title=Epithelial-Mesenchymal Transition in Cancer: Parallels Between Normal Development and Tumor Progression|journal=Journal of Mammary Gland Biology and Neoplasia|date=19 May 2010|volume=15|issue=2|pages=117–134|doi=10.1007/s10911-010-9178-9}}</ref> [[File:Epithelial-Mesenchymal Transition.gif|thumb|Diagram illustrating the epithelial-mesenchymal transition.|thumb|left|500px]] |
|||
==Clinical implications== |
==Clinical implications== |
||
Revision as of 20:40, 7 May 2013
The Wnt signaling pathway is a network of proteins that passes signals from receptors on the surface of the cell through the cytoplasm and ultimately to the cell's nucleus where the signaling cascade leads to the expression of target genes. It controls cell-cell communication in the embryo and adult (ie, cell proliferation and differentiation during development and healing). The Wnt signalling pathway is an example of paracrine signalling.[1][2]
It was identified first for its role in cancer development, and separately in creating normal patterns of embryonic development. Its role in embryonic patterning was discovered when genetic mutations in critical players in this pathway produced abnormal fruit fly embryos. Later research found that the two genes responsible for this type of breast cancer in the mouse and the abnormal patterning in the fruit fly were in the same family and part of the Wnt signaling pathway.[3]
The Wnt signaling pathway is evolutionarily conserved, and functions across species ranging from the fruit fly to humans. It is also required for adult tissue maintenance in bone, heart, muscle, and elsewhere. Mutations in this pathway in adults contribute to degenerative diseases and cancers.[3]
Discovery
The discovery of Wnt signaling is due largely to several lines of research that led to a better understanding of oncogenic retroviruses. Specifically, a shift in focus to the cancer-causing properties of these viruses led to the discovery of specific cancer-causing genes known as proto-oncogenes. This discovery encouraged the research of Roel Nusse and Harold Vamus in 1982, where they infected mice with the oncogenic retrovirus MMTV (mouse mammary tumor virus) to see which mouse genes would produce breast tumors upon mutation. Using a method known as proviral tagging, they were able to use this research to identify a new mouse proto-oncogene that they named int1 (integration 1).[3]
The importance of in1 was not fully appreciated until researchers noted that it had a high degree of conservation across several species, including humans and Drosophila. Its presence in Drosophila melanogaster was particularly important because in 1987, researchers were able to determine that the int1 gene in Drosophila was actually the already characterized Drosophila gene known as Wingless (Wg). This meant that the mammalian int1 was the homologue of Wg.[3]
The uncovering of this shared homology was an exciting discovery due to the fact that previous research by Christiane Nüsslein-Volhard and Eric Wieschaus, who would later win the 1995 Nobel Prize in Physiology or Medicine for that research, had already elucidated the function of Wg as a segment polarity gene involved in embryonic development.[4] Research in int1 and its signaling pathways increased because abnormalities in embryonic development mechanisms have long been implicated in cancer. This increase in research would lead to the discovery of further genes related to int1 ;however, since all those genes had not been activated via proviral integration, it quickly became clear that the int gene nomenclature was no longer adequate. Thus, the int/Wingless family was renamed the Wnt family and int1 became Wnt1. The name Wnt was chosen because it is a portmanteau of int and Wg and stands for Wingless-related integration site.[3]
Wnt signaling proteins

The Wnt proteins are a diverse family of secreted lipid-modified signaling glycoproteins that are 350–400 amino acids in length.[5] The type of lipid modification that occurs on these proteins is palmitoylation of cysteines in a conserved pattern of 23–24 cysteine residues.[1] In Wnt signaling, these proteins act as ligands to activate the different Wnt pathways via paracrine and autocrine routes.[6][7]
These proteins are also highly conserved across species.[3] They can be found in mice, humans, Xenopus, Zebrafish, Drosophila, and many others.[8]
| Species | Wnt proteins |
|---|---|
| Homo sapiens | Wnt1, Wnt2, Wnt2B, Wnt3, Wnt3A, Wnt4, Wnt5A, Wnt5B, Wnt6, Wnt7A, Wnt7B, Wnt8A, Wnt8B, Wnt9A, Wnt9B, Wnt10A, Wnt10B, Wnt11, Wnt16[8] |
| Mus musculus | Wnt1, Wnt2, Wnt2B, Wnt3, Wnt3A, Wnt4, Wnt5A, Wnt5B, Wnt6, Wnt7A, Wnt7B, Wnt8A, Wnt8B, Wnt9A, Wnt9B, Wnt10A, Wnt10B, Wnt11, Wnt16[8] |
| Xenopus | Wnt1, Wnt2, Wnt2B, Wnt3, Wnt3A, Wnt4, Wnt5A, Wnt5B, Wnt7A, Wnt7B, Wnt8A, Wnt8B, Wnt10A, Wnt10B, Wnt11, Wnt11R[8] |
| Danio rerio | Wnt1, Wnt2, Wnt2B, Wnt3, Wnt3A, Wnt4, Wnt5A, Wnt5B, Wnt6, Wnt7A, Wnt7B, Wnt8A, Wnt8B, Wnt10A, Wnt10B, Wnt11, Wnt16[8] |
| Drosophila | Wg, DWnt2, DWnt3/5, DWnt 4, DWnt6, WntD/DWnt8, DWnt10[8] |
Mechanism
Foundation of Wnt signaling
Wnt signaling begins when one of the Wnt proteins binds the N-terminal extra-cellular cysteine-rich domain of a Frizzled (Fz) family receptor.[9] These receptors span the plasma membrane seven times and constitute a distinct family of G-protein coupled receptors (GPCRs).[10] However, to facilitate Wnt signaling, co-receptors may also be required alongside the interaction between the Wnt protein and Fz receptor. Examples include lipoprotein receptor-related protein (LRP)-5/6, receptor tyrosine kinase (Ryk), and ROR2.[6] Upon activation of the receptor, a signal is sent to the phosphoprotein Dishevelled (Dsh), which is located in the cytoplasm. This signal is transmitted via a direct interaction between Fz and Dsh. Dsh proteins are present in all organisms and they all share the following highly conserved protein domains: an amino-terminal DIX (Dsh and auxin) domain, a central PDZ (postsynaptic density-95, discs-large, and zonula occludens-1) domain, and a carboxy-terminal DEP (Dsh, Egl-10, and pleckstrin) domain. These different domains are important because after Dsh, the Wnt signal can branch off into several different pathways and each pathway interacts with a different combination of the three domains.[11]
Canonical and noncanonical pathways
The three best characterized Wnt signaling pathways are the canonical Wnt pathway, the noncanonical Planar Cell Polarity pathway, and the noncanonical Wnt/Ca2+ pathway. As their names suggest, these pathways belong to one of two categories: canonical or noncanonical. The difference between these two categories is the presence or absence of β-catenin. The canonical Wnt pathway involves β-catenin, while the noncanonical pathways operate independently of it.[9]
The canonical Wnt pathway
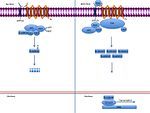
The canonical Wnt pathway (or Wnt/β-catenin pathway) is the Wnt pathway that causes an accumulation of β-catenin in the cytoplasm and its eventual translocation into the nucleus to act as a transcriptional coactivator of transcription factors that belong to the TCF/LEF family.[9] Without Wnt signaling, the β-catenin would not accumulate in the cytoplasm since a destruction complex would normally degrade it. This destruction complex includes the following proteins: Axin, adenomatosis polyposis coli (APC), protein phosphatase 2A (PP2A), glycogen synthase kinase 3 (GSK3) and casein kinase 1α (CK1α).[9] It degrades β-catenin by targeting it for ubiquitination, which subsequently sends it to the proteasome to be digested.[9]
However, as soon as Wnt binds Fz and LRP-5/6, the destruction complex function becomes disrupted. This is due to Wnt causing the translocation of both a negative regulator of Axin and the destruction complex to the plasma membrane.[6] This negative regulator becomes localized to the cytoplasmic tail of LRP-5/6. Phosphorylation by other proteins in the destruction complex subsequently binds Axin to this tail as well. Axin becomes de-phosphorylated and its stability and levels are decreased.[6] Dsh then becomes activated via phosphorylation and its DIX and PDZ domains inhibit the GSK3 activity of the destruction complex. This allows β-catenin to accumulate and localize to the nucleus and subsequently induce a cellular response via gene transduction alongside the TCF/LEF transcription factors.[6]
The noncanonical planar cell polarity pathway

The noncanonical planar cell polarity (PCP) pathway is one of the two Wnt pathways that does not involve β-catenin. It does not use LRP-5/6 as its co-receptor and is thought to use NRH1, Ryk, PTK7, or ROR2.[6] As in the canonical Wnt pathway, the PCP pathway is activated via the binding of Wnt to Fz and its co-receptor. The receptor then recruits Dsh, which uses its PDZ and DEP domains to form a complex with Dishevelled-associated activator of morphogenesis 1 (DAAM1).[6] Daam1 then activates the small G-protein Rho through a guanine exchange factor. Rho activates Rho-associated kinase (ROCK), which is one of the major regulators of the cytoskeleton.[6] Dsh also forms a complex with rac1 and mediates profilin binding to actin. Rac1 activates JNK and can also lead to actin polymerization. Profilin binding to actin can result in restructuring of the cytoskeleton and gastrulation.[6]
The noncanonical Wnt/calcium pathway

The noncanonical Wnt/calcium pathway is the other Wnt pathway that does not stimulate accumulation of β-catenin. Its role is to help regulate calcium release from the endoplasmic reticulum (ER) in order to control intracellular calcium levels. Like other Wnt pathways, upon ligand binding, the activated Fz receptor directly interacts with Dsh and activates specific Dsh-protein domains. The domains involved in Wnt/calcium signaling are the PDZ and DEP domains.[6] However, unlike other Wnt pathways, the Fz receptor also directly interfaces with a trimeric G-protein. This co-stimulation of Dsh and the G-protein can lead to the activation of either PLC or cGMP-specific PDE. If PLC is activated, the plasma membrane component PIP2 is cleaved into DAG and IP3. When IP3 binds its receptor on the ER, calcium is released. Increased concentrations of calcium and DAG can activate Cdc42 through PKC. Cdc42 is an important regulator of cell adhesion, migration, and tissue separation. Increased calcium also activates calcineurin and CaMKII. Calcineurin induces activation of the transcription factor NFAT, which regulates ventral patterning.[6] CamKII activates TAK1 and NLK kinase, which can interfere with TCF/ß-Catenin signaling in the canonical Wnt pathway.[12] However, if PDE is activated, calcium release from the ER is inhibited. PDE mediates this through the inhibition of PKG, which subsequently causes the inhibition of calcium release.[6]
Other pathways
Along with the three pathways, Wnt signaling also regulates a number of other signaling pathways that have not been as extensively elucidated. One such pathway includes the interaction between Wnt and GSK3. During cell growth, Wnt can inhibit GSK3 in order to activate mTOR in the absence of β-catenin.[13] However, Wnt can also serve as a negative regulator of mTOR via activation of the tumor suppressor TSC2, which is upregulated via Dsh and GSK3 interaction. During myogenesis, Wnt has been shown to use PKA and CREB to activate the genes MyoD and Myf5. Wnt has also been seen to act in conjunction with Ryk and Src to allow for regulation of neuron repulsion during axonal guidance. Wnt regulates gastrulation when CK1 serves as an inhibitor of Rap1-GTPase in order to modulate the cytoskeleton during gastrulation. Further regulation of gastrulation is achieved when Wnt uses ROR2 along with the CDC42 and JNK pathway to regulate the expression of PAPC. Dsh can also interact with aPKC, Par3, Par6, and LGl in order to control cell polarity and microtubule cytoskeleton development. While these pathways overlap with components associated with PCP and Wnt/Calcium signaling, they are considered distinct pathways because they produce entirely different responses.[6]
Regulation
In order to insure proper functioning, Wnt signaling is constantly regulated at several points along its signaling pathways. For instance, as previously mentioned, Wnt proteins are palmitoylated. The protein porcupine has been shown to be involved in this palmitoylation process, which means that it helps regulate when the Wnt ligand is secreted by determining when it is fully formed. Secretion of Wnt protein is further controlled with proteins such as wntless and evenness interrupted and complexes such as the retromer complex. Upon secretion, the ligand can also be prevented from reaching its receptor through the binding of certain proteins such as the stabilizers Dally and glypican 3, which inhibit diffusion. At the Fz receptor, the binding of proteins other than Wnt can antagonize signaling. Specific antagonists include Dickkopf (Dkk), Wnt inhibitory factor 1 (WIF-1), secreted Frizzled-related proteins (SFRP), Cerebrus, Frzb, Wise, and SOST. All of these constitute inhibitors of Wnt signaling; however, other molecules have been shown to act as activators as well. For example, Norrin and R-Spondin2 have been shown to activate Wnt signaling in the absence of Wnt ligand. Interactions with between signaling pathway has also been seen to regulate the different Wnt pathways. As previously mentioned, this has been seen in the case of the Wnt/calcium pathway, which inhibits the TCF/β-catenin signaling of the canonical Wnt pathway.[6]
Wnt-induced cell responses
Embryonic development
Wnt signaling plays a critical role in the embryonic development of a variety of different organisms. It is detected in both vertebrates and invertebrates, including humans, frogs, zebrafish, C. elegans, Drosophila, and numerous others. It was first known to be involved in the segment polarity of Drosophila, where it helps to establish anterior and posterior polarities; however, it has since then been implicated in numerous other developmental processes. As its function in Drosophila suggests, it plays a key role in body axis formation, particularly the formation of the anteroposterior and dorsoventral axes. It is also involved in the induction of cell differentiation to prompt formation of important organs such as the lungs and ovaries. Wnt further insures the development of these specific tissues through proper regulation of cell proliferation and migration. These are just a few Wnt functions, but they demonstrate that the numerous functions of Wnt signaling can be divided into one of the following categories: axis patterning, cell fate specification, cell proliferation, and cell migration.[14]
Axis patterning
In early embryonic development, the formation of the primary body axes is a crucial step in establishing the overall body plan of each particular organism. The different axes include the anteroposterior axis, dorsoventral axis, and right-left axis. Wnt signaling can be implicated in the formation of the anteroposterior and dorsoventral axes. Wnt signaling activity in anterior-posterior development can be seen in several organisms including mammals, fish, and frogs. In mammals, the primitive streak and other surrounding tissues produce the morphogenic compounds Wnts, BMPs, FGFs, Nodal, and retinoic acid to establish the posterior region during late gastrula. These proteins form concentration gradients and the areas of their highest concentration establish the posterior region and the areas of their lowest concentration indicate the anterior region. In fish and frogs, β-catenin produced by canonical Wnt signaling causes the formation of organizing centers, which, alongside BMPs, elicits posterior formation. Wnt involvement in dorsoventral axis formation can be seen in the activity of the formation of the Spemann organizer, which establishes the dorsal region. Canonical Wnt signaling production β-catenin induces the formation of this organizer via the activation of the genes twin and siamois.[14]
Wnt signaling is also involved in the axis formation of specific body parts and organ systems that are a part of later development. In vertebrates, sonic hedgehog (Shh) and Wnt morphogenetic signaling gradients establish the dorsoventral axis of the central nervous system during neural tube axial patterning. High Wnt signaling establishes the dorsal region while high Shh signaling indicates in the ventral region.[15] Wnt is also involved in the dorsal-ventral formation of the central nervous system through its involvement in axon guidance. Wnt proteins guide the axons of the spinal cord in an anterior-posterior direction.[16] Wnt is also involved in the formation of the limb dorsal-ventral axis. Specifically, Wnt7a helps produce the dorsal patterning of the developing limb.[14]
Cell fate specification
Cell fate specification, or cell differentiation, is a cellular process where undifferentiated cells can become a more specialized cell type. Wnt signaling induces differentiation of pluripotent stem cells into mesoderm and endoderm progenitor cells.[17] These progenitor cells are then further induced to differentiate into more specific cell types such as endothelial, cardiac, and vascular smooth muscle lineages.[18] Wnt signaling can also induce blood formation from stem cells. Specifically, Wnt3 leads to mesoderm committed cells with hematopoietic potential.[19] Wnt1 has also been shown to antagonize neural differentiation and is a major factor in self-renewal of neural stem cells. This allows for regeneration of nervous system cells, which is further evidence of a role in promoting neural stem cell proliferation.[17] Wnt signaling has also been shown to be involved in germ cell determination, gut tissue specification, hair follicle development, lung tissue development, trunk neural crest cell differentiation, nephron development, ovary development, and sex determination.[14]
Cell proliferation
In order to have the mass differentiation of cells needed to form the specified cell tissues of different organisms, a proliferation, or cell growth, of embryonic stem cells must take place. This process is mediated through canonical Wnt signaling, which increases nuclear and cytoplasmic level of β-catenin. This increase in proliferation is directly paired with cell differentiation because as the stem cells proliferate, they are differentiated into the specific tissues that are induced to become. This allows for overall growth and development of specific tissue systems during embryonic development. This is apparent in systems such as the circulatory system where Wnt3a leads to proliferation and expansion of hematopoietic stem cells needed for red blood cell formation.[20]
Cell migration
Cell migration during embryonic development allows for the establishment of body axes, tissue formation, limb induction, and several other processes. Wnt signaling has been shown to help mediate this process, particularly during convergent extension. Research has shown that signaling from both the Wnt PCP pathway and canonical Wnt pathway is required for proper convergent extension during gastrulation. Convergent extension is further regulated by the Wnt/calcium pathway, which blocks convergent extension when activated. Wnt signaling also induces cell migration in later stages of development through the control of the migration behavior of neuroblasts, neural crest cells, myocytes, and tracheal cells.[21]
Wnt signaling is also involved in another key migration process known as the epithelial-mesenchymal transition. This process is what allows epithelial cells to transform into mesenchymal cells so that they are no longer held in place at the laminin. It involves a down-regulation of cadherins so that cells can detach from laminin and migrate. Wnt signaling is an inducer of EMT, particularly in mammary development.[22]
Clinical implications
Cancer
Ever since its initial discovery, Wnt signaling has had an association with cancer. When Wnt1 was discovered, it was first identified as a proto-oncogene in a mouse model for breast cancer. The fact that Wnt1 is a homolog of Wg shows that it is involved in embryonic development, which often calls for rapid cell division and migration. Misregulation of these processes can cause unwanted cell growth and movement, which can lead to tumor development.[3]
Activity of the canonical Wnt pathway has been directly measured in the development of benign and malignant breast tumors. Its presence is indicated with elevated levels of β-catenin in the nucleus and/or cytoplasm, which can be detected with immunohistochemical staining and Western blotting. Increased β-catenin expression has been strongly correlated with poor prognosis in breast cancer patients. This accumulation may be due to several factors such as mutations in β-catenin, deficiencies in the β-catenin destruction complex, overexpression of Wnt ligands, loss of inhibitors, and/or decreased activity of regulatory pathways (such as the Wnt/calcium pathway).[23][24] Breast tumors have also been seen to metastasize due to Wnt involvement in the epithelial-mesenchymal transition (EMT). Research looking at metastasis of basal-like breast cancer to the lungs has shown that repression of Wnt/β-catenin signaling can prevent EMT, which can inhibit metastasis.[25]
Wnt signaling has also been implicated in the development of more than just breast-type cancers. Changes in CTNNB1 expression, which is the gene that encodes β-catenin, has been seen in not just breast cancer, but also colorectal cancer, melanoma, prostate cancer, lung cancer, and several other cancer types. Increased expression of Wnt ligand-proteins such as Wnt 1, Wnt2, and Wnt7A have been observed in the development of glioblastoma, oesophageal cancer, and ovarian cancer respectively. Other proteins known to cause multiple types of cancer in the absence of proper functioning include ROR1, ROR2, SFRP4, Wnt5A, WIF1, and those of the TCF/LEF family.[26]
Type II diabetes
See also
- Signal transduction
- Morphogenesis
- Developmental biology
- Embryogenesis
- Cancer
- Catenin
- GSK-3
- Frzb
- Wingless localisation element 3 (WLE3)
- Baldness treatments
- GPR177
External links
- Mouse Wnt proteins from Signaling Gateway Molecule Pages[27]
- Mouse Frizzled proteins from Signaling Gateway Molecule Pages
- The Wnt Homepage from Nusse Lab, Stanford
- Wnt pathways, their relationship, disease, and therapies by healthvalue.net
- Drosophila Wnt pathway from KEGG
- mouse Wnt pathway from KEGG
- humanα Wnt pathway from KEGG
- Homo sapiens (human) Wnt pathway from KEGG
- video of grey hair reversal
- Netpath - A curated resource of signal transduction pathways in humans
- Wnt+Proteins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Wnt game
References
- ^ a b Logan C.W., Nusse R. (2004). "The Wnt signaling pathway in development and disease". Cell Dev. Bio. 20: 781–810. doi:10.1146/annurev.cellbio.20.010403.113126. PMID 15473860.
- ^ Lie DC, Colamarino SA, Song HJ, Désiré L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH (2005). "Wnt signalling regulates adult hippocampal neurogenesis". Nature. 437 (7063): 1370–5. doi:10.1038/nature04108. PMID 16251967.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Nusse, Roel (22 May 2012). "Three decades of Wnts: a personal perspective on how a scientific field developed". The EMBO Journal. 31 (12): 2670–2684. doi:10.1038/emboj.2012.146.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Cite error: The named reference "Nusse" was defined multiple times with different content (see the help page). - ^ Klaus A., Birchmeier W. (2008). "Wnt signaling and its impact on development and cancer". Nature Reviews Cancer. 8 (5): 387–398. doi:10.1038/nrc2389. PMID 18432252.
- ^ Cadigan K.M., Nusse R. (1997). "Wnt signaling: a common theme is animal development". Genes & Development. 11 (24): 3286–3305. doi:10.1101/gad.11.24.3286.
- ^ a b c d e f g h i j k l m n Komiya, Y (2008 Apr). "Wnt signal transduction pathways". Organogenesis. 4 (2): 68–75. PMID 19279717.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Nusse, Roel (1 June 1992). "Wnt genes". Cell. 69 (7): 1073–1087. doi:10.1016/0092-8674(92)90630-U.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f Nusse, Roel. "The Wnt Homepage". Retrieved 15 April 2013.
- ^ a b c d e Rao, Tata (2010). "An Updated Overview on Wnt Signaling Pathways : A Prelude for More". Circulation Research. 106: 1798–1806. doi:10.1161/CIRCRESAHA.110.219840.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Schulte, Gunnar (1 October 2007). "The Frizzled family of unconventional G-protein-coupled receptors". Trends in Pharmacological Sciences. 28 (10): 518–525. doi:10.1016/j.tips.2007.09.001.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Habas, Raymond (1 January 2005). "Dishevelled and Wnt signaling: is the nucleus the final frontier?". Journal of Biology. 4 (1): 2. doi:10.1186/jbiol22.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Sugimura, Ryohichi, and Linheng Li. "Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases." Birth Defects Research Part C: Embryo Today: Reviews 90.4 (2010) : 243-256. Print.
- ^ Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL (2010). "TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth". Cell (journal). 126 (5): 955–968. doi:10.1016/j.cell.2006.06.055. PMID 16959574.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Gilbert, Scott F. (2010). Developmental biology (9th ed. ed.). Sunderland, Mass.: Sinauer Associates. ISBN 9780878933846.
{{cite book}}:|edition=has extra text (help) - ^ Ulloa, Fausto (1 January 2009). "Wnt won the war: Antagonistic role of Wnt over Shh controls dorso-ventral patterning of the vertebrate neural tube". Developmental Dynamics: NA–NA. doi:10.1002/dvdy.22058.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zou, Y (2004 Sep). "Wnt signaling in axon guidance". Trends in neurosciences. 27 (9): 528–32. PMID 15331234.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Nusse R (2008). "Wnt signaling and stem cell control". Cell Res. 18 (5): 523–7. doi:10.1038/cr.2008.47. PMID 18392048.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bakre MM, Hoi A, Mong JC, Koh YY, Wong KY, Stanton LW (2007). "Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation". J. Biol. Chem. 282 (43): 31703–12. doi:10.1074/jbc.M704287200. PMID 17711862.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Woll PS; Morris JK; Painschab MS; et al. (2008). "Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells". Blood. 111 (1): 122–31. doi:10.1182/blood-2007-04-084186. PMC 2200802. PMID 17875805.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Willert, K. (1 June 2006). "Wnt signaling: is the party in the nucleus?". Genes & Development. 20 (11): 1394–1404. doi:10.1101/gad.1424006.
- ^ Schambony, A. "Wnt Signaling and Cell Migration". Madame Curie Bioscience Database. Landes Bioscience. Retrieved 7 May 2013.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Micalizzi, Douglas S. (19 May 2010). "Epithelial-Mesenchymal Transition in Cancer: Parallels Between Normal Development and Tumor Progression". Journal of Mammary Gland Biology and Neoplasia. 15 (2): 117–134. doi:10.1007/s10911-010-9178-9.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Howe, Louise (2004). "Wnt Signaling and Breast Cancer". Cancer Biology and Therapy. 3 (1): 36–41. doi:10.4161/cbt.3.1.561.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Taketo, M Mark. "Shutting down Wnt signal-activated cancer." Nature Genetics 36. (2004): 320-22. Web. 17 Apr 2011.
- ^ DiMeo, T. A. (1 July 2009). "A Novel Lung Metastasis Signature Links Wnt Signaling with Cancer Cell Self-Renewal and Epithelial-Mesenchymal Transition in Basal-like Breast Cancer". Cancer Research. 69 (13): 5364–5373. doi:10.1158/0008-5472.CAN-08-4135.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Anastas, Jamie N. (21 December 2012). "WNT signalling pathways as therapeutic targets in cancer". Nature Reviews Cancer. 13 (1): 11–26. doi:10.1038/nrc3419.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Dinasarapu A.R, Saunders B, Ozerlat I, Azam K and Subramaniam S (2010). "Signaling Gateway Molecule Pages - a data model perspective". Bioinformatics. 27 (12): 1736–1738. doi:10.1093/bioinformatics/btr190. PMC 3106186. PMID 21505029.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
