Wikipedia talk:WikiProject Medicine: Difference between revisions
| Line 558: | Line 558: | ||
* Is there a list kept like the [[WP:LSP]]? If not, anyone want to set one up for dubious journals etc? [[User:IRWolfie-|IRWolfie-]] ([[User talk:IRWolfie-|talk]]) 21:56, 10 April 2013 (UTC) |
* Is there a list kept like the [[WP:LSP]]? If not, anyone want to set one up for dubious journals etc? [[User:IRWolfie-|IRWolfie-]] ([[User talk:IRWolfie-|talk]]) 21:56, 10 April 2013 (UTC) |
||
:::There is great conflict between "open access" publishers and "publishers supported by advertising revenue and subscription fees". Both have their issues as a model of publishing and IMO open access has far less than ad revenue/pre print based. Many subscription based journals for example will be pressured to publish an article as the company/authors publishing it have agreed to by 10,000 copies once it is published sometimes at the cost of $100,000. [[User:Jmh649|<span style="color:#0000f1">'''Doc James'''</span>]] ([[User talk:Jmh649|talk]] · [[Special:Contributions/Jmh649|contribs]] · [[Special:EmailUser/Jmh649|email]]) (if I write on your page reply on mine) 01:17, 11 April 2013 (UTC) |
|||
==JMIR Wiki Medical Reviews== |
==JMIR Wiki Medical Reviews== |
||
Revision as of 01:18, 11 April 2013
Welcome to the WikiProject Medicine talk page. If you have comments or believe something can be improved, feel free to post. Also feel free to introduce yourself if you plan on becoming an active editor!
We do not provide medical advice; please see a health professional.
- Unsure about something? Make sure to look at our style and source guidelines.
- Please don't shout, remain civil, be respectful to all, and assume good faith.
- Put new text under old text. Click here to start a new topic.
- Please sign and date your posts by typing four tildes (
~~~~). - Threads older than 10 days are automatically archived.
- Please see Wikipedia:WikiProject_Medicine/Newsletter/Mailing_list
| List of archives | |
|---|---|
|
Wikipedia:Wikipedia Signpost/WikiProject used
The Emperor's New Drugs
I recommend this. [1] The author makes the case that the efficacy of antidepressants is certainly mostly due to the placebo effect, and probably entirely due to the placebo effect. --Anthonyhcole (talk · contribs · email) 10:10, 26 March 2013 (UTC)
- Yes our article on MDD reflected this "sort of" last time I looked. There is a huge issue with publications bias where one publishes all the positive trials and does not publish all the negative trials resulting in meta analysis of the published literature showing benefit while meta analysis of all the literature shows little benefit. Doc James (talk · contribs · email) (if I write on your page reply on mine) 10:14, 26 March 2013 (UTC)
- Hersch highlights publication bias, corrupt cherry-picking and double-dipping (publishing the same favourable results in several different papers), but also explains the small super-placebo effect found in some randomised controlled trials and sometimes seen in the clinic. --Anthonyhcole (talk · contribs · email) 11:49, 26 March 2013 (UTC)
- Yes our article on MDD reflected this "sort of" last time I looked. There is a huge issue with publications bias where one publishes all the positive trials and does not publish all the negative trials resulting in meta analysis of the published literature showing benefit while meta analysis of all the literature shows little benefit. Doc James (talk · contribs · email) (if I write on your page reply on mine) 10:14, 26 March 2013 (UTC)
(outdent) Part of the problem was the manufacturers included people with mild and moderate depression which often isn't true biological depression and thus little effect was seen - they biased their results against their drug by including this group of people with mild-moderate depression. So they designed their trials poorly probably in part due to the fact that the neurobiology of biological depression (which is usually severe) was not known well at all and thus they didn't know how to best design their trials. Antidepressants do work for more severe (biological) depression and neuroscience is unravelling how antidepressants work, such as via suppressing neuro inflammation as a knock on effect of modulating the serotonin system - neuro inflammation plays a major role in depression. Now that more is known about depression I am sure the drug companies will be more careful in the future and will probably restrict their trials to people with severe depression/major depressive disorder. SSRIs suck for a lot of people anyway, about half the population get sexual dysfunction while taking them - better antidepressants exist. Of course the fact that these companies manipulated data in the first place is serious stuff and raises big concerns.--MrADHD | T@1k? 21:02, 26 March 2013 (UTC)
- The number of people with severe depression is very small compared to the number with mild depression or sadness. To make huge sums of money one must sell to the many not the few. SSRIs reach clinical significance in very severe depression but the benefit is still not huge.[2] Right now 10% of the population in many Western countries take them. I am not convinced think will change any time soon. Doc James (talk · contribs · email) (if I write on your page reply on mine) 05:21, 27 March 2013 (UTC)
- Yea, you are right. I certainly agree that a lot of people take antidepressants who don't need them and that this is in part because of how they were marketed by the manufacturers and their motivation to make a large profit.--MrADHD | T@1k? 10:39, 29 March 2013 (UTC)
- The number of people with severe depression is very small compared to the number with mild depression or sadness. To make huge sums of money one must sell to the many not the few. SSRIs reach clinical significance in very severe depression but the benefit is still not huge.[2] Right now 10% of the population in many Western countries take them. I am not convinced think will change any time soon. Doc James (talk · contribs · email) (if I write on your page reply on mine) 05:21, 27 March 2013 (UTC)
- Another interesting (for lawyers) read here addresses the problem of "evergreening" from the regulatory side. LeadSongDog come howl! 22:03, 26 March 2013 (UTC)
- I would have plenty to say about this, but I feel like it verges on WP:SOAP. Maybe in another forum, such as irc? -- [ UseTheCommandLine ~/talk ] # _ 01:29, 27 March 2013 (UTC)
- All this simply emphasis the importance of being a little skeptically. Conflict of interest matters per Upton Sinclair "It is difficult to get a man to understand something when his job depends on not understanding it.". Doc James (talk · contribs · email) (if I write on your page reply on mine) 05:17, 27 March 2013 (UTC)
- I would have plenty to say about this, but I feel like it verges on WP:SOAP. Maybe in another forum, such as irc? -- [ UseTheCommandLine ~/talk ] # _ 01:29, 27 March 2013 (UTC)
Do we need a WP:NTHERAPEUTIC?
This is an issue that intersects with my vague interest in off-label use and how we present that information. Over the last week or so there has been a good deal of activity around oncolytic viruses, as noted above. One of the therapeutics at issue has, as the majority of its information, a bunch of clinicaltrials.gov entries and a data release by the manufacturer about the study. Normally, i would be inclined to send this to AfD, and if the article only contained its scientific component it would not be an issue.
Non-scientific/medical press though, frequently from the business world, often latches on to these things at very early stages, which can provide enough WP:RS for a keep. (Not that I see this as inherently bad.) Further complicating this are the following trends:
- Business press has a tendency to hype a new therapeutic as promising
- There are already precedents for insider trading based on study results, suggesting a very strong monetary incentive to promote new therapies before they are approved
- The strong pro-PR and pro-paid-editing contingent on WP
Are there policies I'm missing regarding this sort of thing, that would apply to the medical and scientific notability of a new therapeutic, and provide guidance for editors working on articles about new therapies? I can sort of see the contours of a policy in WP:MEDRS, particularly its guidance on primary versus secondary sources, but I feel like it could be clarified or made more explicit, specifically as it pertains to new therapeutics. This is of course particularly important in cancer, where there are thousands of ongoing trials. My sense is that sometimes cancer therapeutics don't get approval until long after they are in widespread use.
If this sort of thing is seen as being useful I will start an RfC, but I wanted to discuss it here first. -- [ UseTheCommandLine ~/talk ] # _ 18:55, 28 March 2013 (UTC)
- If an article is sourced solely or largely to press releases from the manufacturer and clinicaltrials.gov entries, then I would feel very comfortable sending it to AfD. We're not a clinical-trials directory, so clinicaltrials.gov listings aren't really evidence of notability for a standalone article. And press releases are, at best, useful as carefully framed adjuncts to independent, reliable secondary sources. As far as amending WP:MEDRS, I'm wary of being too prescriptive, but would you be comfortable pointing out the articles that triggered your concern? MastCell Talk 18:56, 29 March 2013 (UTC)
- Talimogene laherparepvec Is what prompted it, but it seems like it could describe a general case for things that are seen as cutting-edge or unproven science, but have nevertheless gotten some RS press. At that point, I think it's fine to send it to AfD, but in case it does get kept, having clear guidelines on how to separate out the notability and sourcing of the science versus the lay press will be helpful for other editors. I'm not thinking of us here, but the newer editors who may not be totally plugged into the WP:MED folks. Another example that comes to mind is Stem cell educator. -- [ UseTheCommandLine ~/talk ] # _ 19:34, 29 March 2013 (UTC)
- GFT505 and GW 501516 are similarly non-notable in terms of medicine and MEDRS sourcing, from my perspective. The latter is particularly contentious. -- [ UseTheCommandLine ~/talk ] # _ 18:30, 31 March 2013 (UTC)
- GW 501516 is a failed drug which is incredibly important from a research perspective as the first (and perhaps the last) pure PPARδ agonist that has been tested in humans. In addition, it continues to be used as a research tool to probe the function of PPARδ. Finally it has started to be used illicitly by athletes as a performance enhancing drug. For these reasons, I believe that the article is notable. Furthermore I would argue that much of the article falls more within the scope of WP:PHARM than WP:MED (note: pharmacology is not just the sum of all drugs, but also includes the study of drugs). I would also argue that drugs in clinical trials, particularly first in class drugs, merit a place in Wikipedia even though they may lack for the moment MEDRS compliant sourcing. As these drugs are not yet available for general use, I believe that they more properly fall within the scope of WP:PHARM than WP:MED. Boghog (talk) 19:23, 31 March 2013 (UTC)
- My contention is that when one makes statements about safety or efficacy in humans, that seems straightforwardly to be about medicine rather than pharmacology per se. I am trying to be mindful of the audience here too, and my impression is that there are a great deal more people coming to WP for health information than there are to look up a research chemical, if nothing else just based on the size of the potential audiences.
- Since WP is intended as an encyclopedia for a general audience, I think that mention of clinical trials should be limited to, at most, the straightforward reporting that there are x, y, and z trials going on or completed, without any kind of elaboration of additional detail (and that is often where peacock words and overreaching claims creep in). My preference would be to limit mention of clinical trials only to those which have been reported in other WP:RS, i.e. those in the lay press. In the latter case, it might be useful to collapse all the clinical trial information into a simple url in an external links section to search clinicaltrials.gov for the particular drug at issue. People who actually need/want to see that information are thus directed to it, but it doesn't clutter up the article with a lot of potentially misleading-to-the-lay-reader stuff. -- [ UseTheCommandLine ~/talk ] # _ 19:36, 31 March 2013 (UTC)
- for the record, i do not dispute the notability of at least GW 501516. I'm still rather on the fence about GFT505. -- [ UseTheCommandLine ~/talk ] # _ 19:38, 31 March 2013 (UTC)
- I have grave concerns about the GW 501516 article. It appears that the authors of the article are content to source to single primary studies and news reports statements such as:
"GW-501516 has a synergistic effect when combined with AICAR: the combination has been shown to significantly increase exercise endurance in animal studies more than either compound alone."
(in the lead)"... it increases glucose uptake in skeletal muscle tissue and increases muscle gene expression, especially genes involved in preferential lipid utilization. This shift changes the body's metabolism to favor burning fat for energy instead of carbohydrates or muscle protein, potentially allowing clinical application for obese patients to lose fat effectively without experiencing muscle catabolism or the effects and satiety issues associated with low blood sugar."
- The article is making medical claims and drawing inferences. MEDRS applies and needs to be enforced. This is not a question of notability: it is a question of what is written in Wikipedia's voice based on primary sources. More comments are needed on the talk page to make the position clear for when full protection is removed. --RexxS (talk) 20:30, 31 March 2013 (UTC)
- I have grave concerns about the GW 501516 article. It appears that the authors of the article are content to source to single primary studies and news reports statements such as:
- I wholeheartedly agree with your concerns about this particular article, but I am convinced that this is a widespread phenomenon as regards novel therapeutics. GW 501516 is just a particularly obvious example. -- [ UseTheCommandLine ~/talk ] # _ 20:37, 31 March 2013 (UTC)
- The article is making medical claims and drawing inferences – false. GW501516 is a failed drug not approved for human use. The animal studies are relevant from a molecular biology and pharmacology standpoint because of what it teaches about PPARδ function. Hence WP:SCIRS applies and not WP:MEDRS. Boghog (talk) 21:14, 31 March 2013 (UTC)
- I wholeheartedly agree with your concerns about this particular article, but I am convinced that this is a widespread phenomenon as regards novel therapeutics. GW 501516 is just a particularly obvious example. -- [ UseTheCommandLine ~/talk ] # _ 20:37, 31 March 2013 (UTC)
- I agree that there are some issues of scope that need to be more clearly defined, both in regards to this article and the general case (not simply drugs but also e.g. therapeutic viruses, devices, or biologics). I think that it's perfectly reasonable to apply WP:SCIRS to one section, and WP:MEDRS to another. I could imagine things getting a little difficult in the lead though, which is why I think there is a need for guidance. Perhaps N is not the appropriate guideline to subclass this under, but I'm open to suggestions. -- [ UseTheCommandLine ~/talk ] # _ 21:23, 31 March 2013 (UTC)
- Those are medical claims and fall under MEDRS. It matters not one jot how you wikilawyer "failed drug"; statements which make the claims that I highlighted above need more than primary studies to support them. --RexxS (talk) 23:19, 31 March 2013 (UTC)
- Where did I say that secondary sources were not needed? Furthermore the editors on that page were already in the process of upgrading the sourcing before your comments. I was primarily defending the inclusion of animal studies in the article. I do agree that the text does need to be edited to make clear that these are animal and not human studies. Boghog (talk) 07:19, 1 April 2013 (UTC)
- When you state
"The article is making medical claims and drawing inferences – false"
. There are no reliable secondary sources discussing GW 501516, so there should be no medical claims (or "biomedical assertions" if you prefer the phrase used in WP:IRS). Once you start using primary studies on animals to make claims of using up fat, weight loss, extending endurance, etc. you are on a slippery slope to seeing the claims made unqualified and there is not the evidence to support that. Why do we need to reference animal studies in that article? If you want to tell the story of "a failed drug which is incredibly important from a research perspective as the first (and perhaps the last) pure PPARδ agonist that has been tested in humans", then please assemble the sources to justify that narrative. The article at present is nothing like that and consists of multiple unsustainable claims of mechanism and effect - presumably in humans - and it needs to be gutted of that content. --RexxS (talk) 20:42, 1 April 2013 (UTC)- "claims of using up fat, weight loss, extending endurance, etc." in animals should not be considered "medical claims" unless the article is worded so nebulously that the reader is misled into thinking they apply to people. From MEDRS:
Where in vitro and animal-model data are cited on Wikipedia, it should be clear to the reader that the data are pre-clinical, and the article text should avoid stating or implying that the reported findings necessarily hold true in humans. The level of support for a hypothesis should be evident to the reader.
- To use a specific example from GW 501516,
"GW-501516 also increases muscle mass, which improved glucose tolerance and reduced fat mass accumulation even in mice fed a very high fat diet, suggesting that GW-501516 may have a protective effect against obesity."
is indeed an unacceptable "medical claim". Conversely,"In mice fed a very high fat diet, GW-501516 was found to increase muscle mass, which improved glucose tolerance and reduced fat mass accumulation."
isn't, or shouldn't be, as the pre-clinical, basic research, animal study nature of the evidence is abundantly clear. (It may, of course, be synthesis, which is a whole other issue and equally unacceptable.) Fvasconcellos (t·c) 22:27, 1 April 2013 (UTC)- But what about
"The level of support for a hypothesis should be evident to the reader."
The source for either formulation of those statements is apparently a single primary study from 2004. It doesn't get much weaker than that, yet"In mice fed a very high fat diet, GW-501516 was found to increase muscle mass, which improved glucose tolerance and reduced fat mass accumulation."
would also be a sensible formulation for a fact derived from a well-established recent secondary review. How is the level of support evident to the reader when we baldly state in Wikipedia's voice an assertion that has (as far as I know) only a single, eight year-old primary study as a source? We used to have a guideline called assert simple facts which suggested that "facts" in Wikipedia are those which are sourced to undisputed, reliable secondary sources. When we give the same treatment to such weak sourcing as that statement enjoys, we are doing no favours to the readers or the encyclopedia. --RexxS (talk) 23:03, 1 April 2013 (UTC)
- But what about
- "claims of using up fat, weight loss, extending endurance, etc." in animals should not be considered "medical claims" unless the article is worded so nebulously that the reader is misled into thinking they apply to people. From MEDRS:
- When you state
- Where did I say that secondary sources were not needed? Furthermore the editors on that page were already in the process of upgrading the sourcing before your comments. I was primarily defending the inclusion of animal studies in the article. I do agree that the text does need to be edited to make clear that these are animal and not human studies. Boghog (talk) 07:19, 1 April 2013 (UTC)
I think this particular article (GW 501516) is certainly contentious, but personally I am more interested in trying provide some policy guidance for these sorts of situations. Would anyone be willing to help me draft something in a sandbox? -- [ UseTheCommandLine ~/talk ] # _ 22:41, 1 April 2013 (UTC)
Three non-secondary sources on pubmed (in Russian, which I can't read). Only link is to Latvian Virotherapy Center. Doesn't even fit the definition of virotherapy on that article (itself maybe a little hinky), as it is a non-engineered virus. No Google News results other than aforementioned Latvian Virotherapy Center. I have no idea how to even verify that this is approved (whatever that means) for use in Latvia. WP:DUCKTEST suggests it is WP:FRINGE. Maybe other eyes could help figure this out. Crossposted to WP:FRINGE/N. -- [ UseTheCommandLine ~/talk ] # _ 19:11, 28 March 2013 (UTC)
- The source on the page suggests that a lot of doctors and researchers for many decades have researched this therapeutic on hundreds of patients, and the data seems to be presented in a manner closer to proper scientific reporting than I would expect from a fringe source. I think this has gone to clinical trial and gotten reporting and coverage. I have no comment on the quality of the content in the Wikipedia article - there are no in-line citations and is no way to verify the content. Blue Rasberry (talk) 19:50, 28 March 2013 (UTC)
- A topic search on Web of Science turned up nothing. For me, on Proquest I found four hits, but only one that appears to have much potential, doi:10.1134/S0026893312050032, though I don't have access. Daily News Egypt wrote up an unskeptical story that stated, in part:
Then there was a trade journal: "Latvian fund to develop anticancers" Scrip 2785 (Sep 27, 2002): 8. And a year 2000 conference paper from the 8th International Congress of Immunology, Budapest (Hungary), 23-28 Aug 1992. (World Meeting Number 923 0119), sponsored by the International Union of Immunological Societies, title "Modification of immune responses in tumoral disease by viral immuno-modulator "rigvir" and its potential clinical application", authors Ferdats, A; Muceniece, A; Bruvere, R; Glinkina, L; Heisele, O; Popena, B, with Ferdats, A listed as the corresponding author. Biosthmors (talk) 23:29, 28 March 2013 (UTC)Following the collapse of the former Soviet Union the testing and use in treatment of Rigvir stopped for a few years, but its effectiveness in the treatment of several kinds of cancer, from prostate to bladder, colon, melanoma and lung cancer, had been proven. In 2002 the work began again and since 2005 Rigvir has been used in treatment in hospitals and available in pharmacies all over Latvia. Rigvir activates and normalises the immune system of the patient and is well-tolerated and safe.
- If something cannot be supported with English-language sources it becomes very unlikely that something is notable. JFW | T@lk 23:37, 28 March 2013 (UTC)
- I will notify Viraltonic (talk · contribs) of this thread. Biosthmors (talk) 18:13, 29 March 2013 (UTC)
- Hi, I'm a general editor who suggested splitting the subject to a separate page, and then copy-edited it somewhat. The editor who originally contributed the material, Riga virus (talk · contribs), is not active, did not provide an email address, and does not edit the Latvian Wikipedia under the same name. There is a Latvian redirect at RIGVIR which goes to lv:Viroterapija. The first external link on the Latvian page (which I have just updated using archive.org) is a presentation which includes a photo apparently showing RIGVIR in use. The page has a ResearchGate link about RIGVIR with an English title; I have not registered on that site, so can't see the page. http://inventions.lza.lv/izg_en.php?id=68 has information about RIGVIR in a brief page on the researcher Aina Muceniece. http://www.tvnet.lv/zinas/latvija/389100-viruss_var_izarstet_vezi is a 2011 secondary source news story which is understandable in Google Translate. Together, these sources are probably sufficient to save the English page. – Fayenatic London 19:42, 29 March 2013 (UTC)
- FWIW, the last page includes this popular-science video http://www.youtube.com/watch?v=tloa2Dr3DYc which includes snatches of an interview with Prof Muceniece. It's not WP:RS but at least it is narrated/subtitled in English. – Fayenatic London 20:07, 29 March 2013 (UTC)
- Thanks for commenting. For biomedical content, we use WP:MEDRS. If we don't have reliable medical sources, then it's possible we could have notability for a cultural article, but at present it seems like this article should be deleted, at least to me. Has anyone had an opportunity to access and check doi:10.1134/S0026893312050032? Biosthmors (talk) 21:03, 29 March 2013 (UTC)
- On further inspection, I'm now inclined to agree. The official webpage claims Latvian medical approval in 2004, but I can't verify even that from the State Agency of Medicines of Latvia website. – Fayenatic London 13:31, 30 March 2013 (UTC)
- Here you go. It says approval was granted in 2009, though. Fvasconcellos (t·c) 00:32, 31 March 2013 (UTC)
- Well done. Given the date, I found this Agency bulletin confirming the bare details (p 36). – Fayenatic London 09:00, 31 March 2013 (UTC)
- Here you go. It says approval was granted in 2009, though. Fvasconcellos (t·c) 00:32, 31 March 2013 (UTC)
- Hi guys, this Rigvir article struck me as odd as working within the field I had never heard of it. Earlier this week I looked for any independent source and like you I couldn't find anything. They appear to be marketing it in several European countries so I think the medicine should be regulated by the European Medicines Agency but nothing came up on a search of their site. The Latvian health authority's site also gave nothing so I'm not even sure this is a registered medicine at all. I have lodged an enquiry with the EMA for guidance on this medicine, specifically if it is a registered medicine, and whether any independent guidance has been published regarding it. I will let you know what they say. Viraltonic (talk) 21:12, 30 March 2013 (UTC)
- On further inspection, I'm now inclined to agree. The official webpage claims Latvian medical approval in 2004, but I can't verify even that from the State Agency of Medicines of Latvia website. – Fayenatic London 13:31, 30 March 2013 (UTC)
- Thanks for commenting. For biomedical content, we use WP:MEDRS. If we don't have reliable medical sources, then it's possible we could have notability for a cultural article, but at present it seems like this article should be deleted, at least to me. Has anyone had an opportunity to access and check doi:10.1134/S0026893312050032? Biosthmors (talk) 21:03, 29 March 2013 (UTC)
- AfD
- Hmm. The Molecular Biology paper claims "In 2004, a patent was issued for Rigvir, and it was officially registered in Latvia, becoming the first enterovirus medication worldwide to complete the full cycle of clinical trials and to be applied in cancer therapy", which would constitute quite a claim of notability—but I don't think it meets the GNG requirement of "significant coverage in reliable, independent sources".
- The Latvian Virotherapy Centre website does provide a timeline and what appears to be efficacy and safety data, although 1) I can't read Latvian and 2) I don't think it would qualify as a MEDRS. I found the package leaflet and SPC quite easily through a cursory search of the State Agency of Medicines website, but they're also in Latvian. Fvasconcellos (t·c) 00:30, 31 March 2013 (UTC)
Diving medicine expansion
I am working on expanding Diving medicine and would appreciate constructive comments and suggestions at the talk page. Contributions to the article welcome too, of course. (note: my background is in diving, not medicine) Cheers, • • • Peter (Southwood) (talk): 08:00, 30 March 2013 (UTC)
- User:RexxS is our local expert in Scuba medicine. I practiced hyperbaric medicine for a while but now live in an area without a chamber. Anyway specific questions? Doc James (talk · contribs · email) (if I write on your page reply on mine) 21:20, 31 March 2013 (UTC)
- Mainly if the article is OK with MEDMOS, but anything that would improve the article would be good. RexxS knows I am busy with it and has made a few comments, we often discuss diving related topics, but I wasn't aware that RexxS was considered the local expert on diving medicine. Cheers, • • • Peter (Southwood) (talk): 08:43, 4 April 2013 (UTC)
- I'm just an amateur dabbler. The real diving medicine expert is Gene Hobbs - but you already knew that, Peter :) Cheers --RexxS (talk) 17:12, 4 April 2013 (UTC)
- Mainly if the article is OK with MEDMOS, but anything that would improve the article would be good. RexxS knows I am busy with it and has made a few comments, we often discuss diving related topics, but I wasn't aware that RexxS was considered the local expert on diving medicine. Cheers, • • • Peter (Southwood) (talk): 08:43, 4 April 2013 (UTC)
ECA stack and similar supplement articles
Not quite alternative medicine - but bodybuilding woo, fueled by broscience. This article is peppered with uncited medical claims about how effective and harmless the ECA stack supposedly is. I covered it in cn-tags yesterday - but are there any rules or guidelines about uncited medical claims of this sort? - David Gerard (talk) 10:56, 30 March 2013 (UTC)
- Yes we removed them (often place on the talk page). Less content is better than wrong content. Doc James (talk · contribs · email) (if I write on your page reply on mine) 13:41, 30 March 2013 (UTC)
Health
the content and naming of health department and list of health ministries is being discussed. See the discussions at talk:health department -- 65.92.180.137 (talk) 03:20, 31 March 2013 (UTC)
- The World Health Organization probably has documents that can be used as sources to help address this. As a matter of course the wording "health department" is used to refer to subregional state health agencies, at least in the field of public health, so it probably shouldn't be equated with health ministry. Fvasconcellos (t·c) 22:32, 1 April 2013 (UTC)
Nutraceuticals Dietary Supplements
I have just encountered this word and it is exactly what I have been searching for as a replacement for the use of "alternative medicine", especially when taking about evidence proven herbal treatments. I notice on PubMed that there are 41,632 articles when searching for this term. I don't know how we determine that a term has reached consensus, though it appears that this is a term that is used in the accepted medical literature. Therefore, the request is: Can we use the title "Nutraceuticals" for herbal and vitamin treatments?Sthubbar (talk) 10:08, 31 March 2013 (UTC)
- Does the definition of this term differ from the definition of CAM? It might not be appropriate to usurp the other term if this word means something different. Also to balance the argument it would be good to hear how many pubmed articles use the term CAM and related... Lesion (talk) 10:42, 31 March 2013 (UTC)
- Sorry if I don't understand the problem with the term "alternative medicine". We already have naturopathy a huge category with with all natural treatments and special types of medicine like chinese, unani, etc. What for a new neologism?? "nutraceuticals" from nutrition? treatment through nutrition? Personally I don't agree with neologisms. I already saw lots of new words in Portuguese created by Brazilians with base in translated English words. This is annoying and separate people who speak the same language. I know that we speak about a technical term but anyway I think we don't need a new term. With Internet, languages spoken everywhere, like Portuguese and English, full of neologisms will become in a near future a new edition of babel tower. No one want this. We all want a strong, unified, English language. I want the same for Portuguese :) Doc Elisa ✉ 12:30, 31 March 2013 (UTC)
- DocElisa, you may have missed the discussion above. The problem with "alternative medicine" is that it from a purely Western/American viewpoint that many of these treatments are "Alternative". If Wikipedia is to be a global source of human knowledge, than I suggest we try and avoid skewing things from one country's/area's perspective where possible. Above, the arguement was that "alternative medicine" is culturally biased and ther is no alternative term available in the Western medical literature. Naturpathy is something completely different than Nutraceuticals. Nutraceuticals is a culture neutral term that is widely used in the western medical literature.Sthubbar (talk) 13:24, 31 March 2013 (UTC)
- The term "nutraceutical" is much narrower in scope than "alternative medicine", and as a neologism coined by a U.S. physician (I won't go into the [de]merits of his work), is by no means "culture-neutral". There are dozens of CAM practices out there that would not be covered by this term even in its broadest meaning (here is a proposed definition for convenience) and its use as a substitute for CAM would thus be original research. Fvasconcellos (t·c) 14:00, 31 March 2013 (UTC)
- DocElisa, you may have missed the discussion above. The problem with "alternative medicine" is that it from a purely Western/American viewpoint that many of these treatments are "Alternative". If Wikipedia is to be a global source of human knowledge, than I suggest we try and avoid skewing things from one country's/area's perspective where possible. Above, the arguement was that "alternative medicine" is culturally biased and ther is no alternative term available in the Western medical literature. Naturpathy is something completely different than Nutraceuticals. Nutraceuticals is a culture neutral term that is widely used in the western medical literature.Sthubbar (talk) 13:24, 31 March 2013 (UTC)
- I would say that Nutraceutical refers to a treatment modality where normal nutrients are used in a form that makes it a drug. It certainly does not extend to all other forms of complimentary medicine. The trouble with this entire nomenclature thing is that "alternative" medicine can become mainsteam medicine when the evidence base is sufficiently firm.
- I think we should call a treatment modality "complimentary" or "alternative" if sources say so. JFW | T@lk 14:03, 31 March 2013 (UTC)
- I didn't miss it, I have read, and I understand what you say and your idea. But I think that it's not a reason to create new words. Is not only purely Western/American viewpoint, in Europe we use the same word "Medicina alternativa" in Portuguese and Spanish, Alternativmedizin in Germ., French call it "Médecine non conventionnelle" but they didn't create a new word. Word creation in language evolution concept is something with a high responsibility and needs the agreement of a linguistic academy IMO. But may be I'm too rigorous ... Doc Elisa ✉ 14:10, 31 March 2013 (UTC)
- Let me clarify, I am not proposing Nutraceuticals=Alternative Medicine and that it replace that term. It would be used for herbs, vitamins, minerals and other nutrients. Many things like acupuncture and TCM would still be listed as CAM until another term is found in the literature. @JFW, your suggestion is hard to follow. What do we do when one review article calls an herb "alternative" and other says "nutraceutical"? @DocElisa, I'm trying to reread what you wrote and understand and I must be missing something. I was always told that Western includes Europe, Portugal, Spain, Germany and France. Of course "alternative" is not offensive to those because they are exactly the groups that consider these treatments as alternative. Furthermore, this is not a new term. It is already used in over 41,000 articles cited in PubMed. I accepted the argument before that there was no other term that was accepted in the literature. It appears to me that Nutraceuticals is a term that is accepted. Do you agree that this term is used in the literature?Sthubbar (talk) 14:29, 31 March 2013 (UTC)
- I didn't understood western as a synonym of Europe, sorry, as I live here is not western for me :). Nowadays is very important to coin terms. "Dr Stephen DeFelice coined the term “Nutraceutical” from “Nutrition” ..." Is crazy as people feel important when they coin terms! In a congress at Berlim, 2002 I have presented the "Lower legs venous ultrasonography" and I have projected a slide for fun... this one: [3]. It was for fun... now this sign is described in a book and in lots of papers published by others! They have coined a sign! Nice they are much more important now , their "Ego" is better now ... Doc Elisa ✉ 16:02, 31 March 2013 (UTC)
- =D please tell me sonic hedgehog signalling pathway is somehow involved with the Mickey mouse sign... Lesion (talk) 16:26, 31 March 2013 (UTC)
- I don't think so, is just an fun echographic image Doc Elisa ✉ 16:47, 31 March 2013 (UTC)
- Neutraceutical does not mean alt med but would generally be a type of alt med. Doc James (talk · contribs · email) (if I write on your page reply on mine) 21:16, 31 March 2013 (UTC)
- I didn't understood western as a synonym of Europe, sorry, as I live here is not western for me :). Nowadays is very important to coin terms. "Dr Stephen DeFelice coined the term “Nutraceutical” from “Nutrition” ..." Is crazy as people feel important when they coin terms! In a congress at Berlim, 2002 I have presented the "Lower legs venous ultrasonography" and I have projected a slide for fun... this one: [3]. It was for fun... now this sign is described in a book and in lots of papers published by others! They have coined a sign! Nice they are much more important now , their "Ego" is better now ... Doc Elisa ✉ 16:02, 31 March 2013 (UTC)
Doc Elisa, I don't see why the importance of the origin of terms is of any relevance. Language is fluid. I have seen no rules on Wikipedia about being able to use terms based on their origin. The primary criteria that has been previously stressed is consensus. I checked on PubMed for "Mickey Mouse Sign" and found 4 articles so that term seems to be lacking consensus. Nutraceuticals has 41,000+ references. This indicates to me consensus. All of the respondents seem to be missing/avoiding the key question. The previous argument for being forced to use the term CAM or "Alternative" was because of lack of consensus on any other term. I accepted that argument until I found another term that appears to me to have consensus. Nutraceuticals appears to have consensus in the general literature and I request permission to use that term to describe nutritional items, like vitamins, herbs and minerals used as drugs. Is that approved or not? If not approved, why?Sthubbar ([[User talk:Sthubbar|talk]]) 23:09, 31 March 2013 (UTC)
- Is not about consensus is about the term. Can you explain what is the difference between it and natural medicine? I agree with Doc James Nutraceutical is a type of alt Med.
"Dr Stephen DeFelice coined the term “Nutraceutical” from “Nutrition” and “Pharmaceutical” in 1989. The term nutraceutical is being commonly used in marketing but has no regulatory definition" citation from [4] Doc Elisa ✉ 23:30, 31 March 2013 (UTC) Mickey mouse sign is just an image in a very specific location and in a relative new examination, so there is few articles yet, is normal Doc Elisa ✉ 23:24, 31 March 2013 (UTC)
- Doc Elisa, thank you for the informative article. How about the term "Dietary Supplement" than? Is that a term, as defined by DSHEA in the article you provided, that can be used?Sthubbar (talk) 01:36, 1 April 2013 (UTC)
- I think you're missing the main point, which is that alt med includes things that are not eaten. Shining a colored light at someone who's sick is "alt med". Light bulbs cannot possibly be either a dietary supplement or a nutraceutical.
- Also, you dislike the title 'alternative', but many of its adherents believe that the differentiation from conventional (NB: not just "Western") medicine is highly desirable. WhatamIdoing (talk) 03:47, 1 April 2013 (UTC)
- WhatamIdoing, thank you for the response. I think I understand your confusion. You may have assumed I intend to replace the term "Alt Med" with "Dietary Supplements". My apology, I only intend to allow putting herbal, vitamin, and mineral treatments under "Diet. Supl." and put your colored lights example under "Alt Med." "Dietary Supplements" appears to be a term used in Western literature and an accepted defined medical term with consensus. Based on this, can we use the term "Dietary Supplements" in Wikipedia medical articles to specify treatments with herbs, vitamins and minerals? If not, why not?Sthubbar (talk) 04:05, 1 April 2013 (UTC)
- The neutraceutical article could do with some expansion, but not original research by making a new definition. I support following what term the majority of sources are using, and it would have been good to provide numbers of pub med papers using the words "alt med" etc, otherwise the number of times "neutraceutical" is used is meaningless. I strongly suspect that the terms "diet supplement", "herbal medicine" etc etc are all in much more common use than neutraceutical. I also think that these terms are better understood. As per WP's MOS, we should title pages based upon the common name, and not based upon the views of one editor who dislikes the most commonly used term. Wikipedia is not a platform for discussions of renaming commonly used terms, we must be accurate and reflect what the majority of sources say and leave our own opinions behind when we edit. Lesion (talk) 10:49, 1 April 2013 (UTC)
- If there is to be a choice between "dietary supplement" and "herbal medicine", I'd prefer the former. The hormone melatonin is often included in either. It can by a stretch be called a dietary supplement; it is definitely not herbal. (It is, of course, the FDA's fault that melatonin is called a dietary supplement at all.) Hordaland (talk) 12:04, 1 April 2013 (UTC)
- Vitamins would be dietary supplements but not herbal remedies. I would not consider most herbal remedies to be supplement if you are not already getting them in your diet. "Supplement" means to add to what you are already taking.Doc James (talk · contribs · email) (if I write on your page reply on mine) 13:09, 1 April 2013 (UTC)
- If there is to be a choice between "dietary supplement" and "herbal medicine", I'd prefer the former. The hormone melatonin is often included in either. It can by a stretch be called a dietary supplement; it is definitely not herbal. (It is, of course, the FDA's fault that melatonin is called a dietary supplement at all.) Hordaland (talk) 12:04, 1 April 2013 (UTC)
- The neutraceutical article could do with some expansion, but not original research by making a new definition. I support following what term the majority of sources are using, and it would have been good to provide numbers of pub med papers using the words "alt med" etc, otherwise the number of times "neutraceutical" is used is meaningless. I strongly suspect that the terms "diet supplement", "herbal medicine" etc etc are all in much more common use than neutraceutical. I also think that these terms are better understood. As per WP's MOS, we should title pages based upon the common name, and not based upon the views of one editor who dislikes the most commonly used term. Wikipedia is not a platform for discussions of renaming commonly used terms, we must be accurate and reflect what the majority of sources say and leave our own opinions behind when we edit. Lesion (talk) 10:49, 1 April 2013 (UTC)
- WhatamIdoing, thank you for the response. I think I understand your confusion. You may have assumed I intend to replace the term "Alt Med" with "Dietary Supplements". My apology, I only intend to allow putting herbal, vitamin, and mineral treatments under "Diet. Supl." and put your colored lights example under "Alt Med." "Dietary Supplements" appears to be a term used in Western literature and an accepted defined medical term with consensus. Based on this, can we use the term "Dietary Supplements" in Wikipedia medical articles to specify treatments with herbs, vitamins and minerals? If not, why not?Sthubbar (talk) 04:05, 1 April 2013 (UTC)
Doc James, as has been told to me many times, it is not up to how I, or you define terms, it is how some controlling body defines it. If you check out the link provided by Doc Elisa above, herbals, vitamins and minerals are all clearly and explicitly included in the definition of "Dietary Supplements". @Lesion, your comment seems off topic. I am not talking about renaming any pages. I'm am specifically asking if within medicine articles under the treatment or management section if it is acceptable to have a sub-heading of "Dietary Supplements" and include under this the items, as defined in the link provided by Doc Elisa above? If not approved, why not?Sthubbar (talk) 13:17, 1 April 2013 (UTC)
- The FDA might be a better definition to follow: [5]. If we include "vitamins, minerals, herbs or other botanicals, amino acids," etc. under a heading called dietary supplements I do not have a problem with that, as long as the information is notable (i.e. commonly used), and reflects the highest quality available evidence, e.g. not presenting as equally effective a herbal remedy with little or no evidence of efficacy next to another treatment which has a robust evidence base and is commonly used. Lesion (talk) 14:45, 1 April 2013 (UTC)
- Although the FDA definition of "drug" would have some overlap with some things in that list [6].. Lesion (talk) 14:50, 1 April 2013 (UTC)
- These days, "dietary supplement" is normally used to indicate a US-specific regulatory status. WhatamIdoing (talk) 22:46, 1 April 2013 (UTC)
OK, Lesion has cast the second vote that it is acceptable use the term "Dietary Supplements" based on the FDA definition for products with reliable secondary sources to support their efficacy. WhatamIdoing, we are using the FDA definition (Though I don't know why the US regulatory agency has any more weight than any other country, or independent reliable texts, beside the fact that the majority of Wikipedia editors and readers are probably from that country.) Is it acceptable or not to use the term "Dietary Supplements" as a sub-heading under treatments?Sthubbar (talk) 06:06, 2 April 2013 (UTC)
- It is acceptable to use the term how? And this is not really a vote. Doc James (talk · contribs · email) (if I write on your page reply on mine) 15:40, 2 April 2013 (UTC)
- Sthubbar, it would be good to compare the definitions of dietary supplement from different parts of the world. I suggested FDA because I thought it would be a better source than the earlier source to use.
- WhatamIdoing, are you saying it would be better to follow a more medical definition rather than a regulatory definition?
- DocJames, I understand the proposal to be to place all those things (see FDA definition for an example list) which fall within the definition of "dietary supplement" in a likewise named subsection of "treatment/management". This would not exempt this section from the same MEDRS& MOS standards as the rest of the article, so if there is acceptable evidence of e.g. a herbal product being of some use, and it is a notable use (i.e. widely used) and a suitable reference can be found, then I would think this is fine. It may be that some articles already have a dietary supplementation section. I think Sthubbar is talking about a scenario where a treatment traditionally classed as alternative medicine, and falls within the definition of dietary supplement, has been shown to be of some benefit and therefore should be presented in the treatment section of articles. There is also the argument that use of traditional medicine in non-western cultures is not considered so alternative, which I can't comment on because I have only worked in one part of the world. Lesion (talk) 20:09, 2 April 2013 (UTC)
- It is acceptable to use the term how? And this is not really a vote. Doc James (talk · contribs · email) (if I write on your page reply on mine) 15:40, 2 April 2013 (UTC)
- I think it would be preferable to follow a plain English definition. As I read the regulations (with a strobe light
 ), Vitamin C is a "dietary supplement" when you buy it over the counter for the purpose of general health and a "drug" when it's given to you by prescription for the purpose of treating scurvy.
), Vitamin C is a "dietary supplement" when you buy it over the counter for the purpose of general health and a "drug" when it's given to you by prescription for the purpose of treating scurvy. - If your goal is to include information about Vitamin C (or things like it) in a disease-oriented article (e.g., Common cold), my suggestion for a section title is ===Self-care===, rather than trying to separate herbs from vitamins from neti pots. WhatamIdoing (talk) 20:44, 2 April 2013 (UTC)
- But a dietary supplement can be prescribed to a sick patient, and maybe you should not equate "dietary supplement" with something purchased form a health food shop by the "worried well". Example, calorie supplementation in cachexia. Surely this is more dietary supplementation rather than drug? Basically you are proposing that dietary supplements are voluntary purchases made by a consumer and vitamin prescribed to a sick patient is a drug...I'm not sure I agree with that unless a source can be found...I think some examples can be both a dietary supplement and a drug at the same time according to these definitions by the FDA. Lesion (talk) 21:06, 2 April 2013 (UTC)
- As I understand it, extra food is not a "dietary supplement" under US law, even though it is intended to supplement the person's diet. A special food containing a "dietary ingredient" is a dietary supplement, but the bar seems to be pretty high. Ensure drinks are commonly used for "dietary supplementation" in the sense you're using, but they're regulated as food, not dietary supplements. That's why I want to avoid using this term and instead use terms that do not have this kind of regulatory baggage. And, yes, Vitamin D bought over the counter is a "dietary supplement", and Vitamin D bought with a prescription from Banner Pharmacaps is a regulated drug. WhatamIdoing (talk) 23:04, 2 April 2013 (UTC)
- But a dietary supplement can be prescribed to a sick patient, and maybe you should not equate "dietary supplement" with something purchased form a health food shop by the "worried well". Example, calorie supplementation in cachexia. Surely this is more dietary supplementation rather than drug? Basically you are proposing that dietary supplements are voluntary purchases made by a consumer and vitamin prescribed to a sick patient is a drug...I'm not sure I agree with that unless a source can be found...I think some examples can be both a dietary supplement and a drug at the same time according to these definitions by the FDA. Lesion (talk) 21:06, 2 April 2013 (UTC)
- I think it would be preferable to follow a plain English definition. As I read the regulations (with a strobe light
WhatamIdoing, I think there is no way to avoid conflicting definitions as there is not "Earth" standards. It appears to me that "Dietary Supplements" is reasonably well defined and for those corner cases, they can be handled like anything else here, on the talk page and by consensus. As to your suggestion of "Self Care", I don't see how that is an options because under this would have to be every non-prescription medication, and I'm not sure there is any official definition anywhere of that term. @Doc James, I don't understand your question. The way I read Lesion responses is that he is accepting of the idea of using "Dietary Supplements" for such treatments that have quality references. That's what I count as a vote of support. I understand you may still resist the idea. Please help to clarify your specific objection. Thanks.Sthubbar (talk) 06:19, 3 April 2013 (UTC)
- We have a lot of quality references that certain treatments do not work. Where would those go? For example omega 3 fatty acid supplementation is not effective for decreasing mortality per this 2012 systematic review and meta analysis.[7] I am not supportive of dividing treatment section into if and how good the evidence is to support certain treatments. It is fairly clear what counts as surgery, what counts as medicine, what counts as psychological interventions, what counts as lifestyle changes and what counts as alt med. Yes sametimes there is overlap and editorial judgement needs to be used. I disagree with efforts attempting to "ban" the use of the term alt med (acupuncture and herbal remedies definitely fall under this rubric). Doc James (talk · contribs · email) (if I write on your page reply on mine) 18:48, 3 April 2013 (UTC)
- So give me an example of what you'd like to do. Pick an article (Common cold, if you can't think of any others) and tell me exactly what you'd like to add. Feel free to make up something; it's just a hypothetical example. What I'm looking for is a reason why a statement like "Some people suck on zinc tablets when they have a cold" needs to be under ===Dietary supplements=== instead of under ===Self-care===. WhatamIdoing (talk) 18:24, 3 April 2013 (UTC)
- Doc James and WhatamIdoing, you both seem to ask the same question "Where do we put dietary supplement treatments that don't work." This would be answered the exact same way as "Where do we put Western/pharmaceutical treatments that don't work." There is no need to come up with a new rule. I'm confident that Western, FDA approved, evidence based medicine is littered with treatments that either in the past were recommended or even people continue to use without evidence. How are these Western treatments currently treated. I would assume maybe 1) Completely left out of the article 2) Put in some section like "History" 3) Indicated clearly that there is conflicting evidence 3) Indicated that this is a popular treatment despite any lack of evidence. Nothing special, same rules for "Dietary Supplements".
- @Doc James, I don't understand your use of the term "rubric". My initial reaction is negative, so I checked the dictionary and it seems benign so I will assume good faith. A key point for me is that for many people "Alt Medicine" means non-Western, non-evidence based, faith based, old fashioned--In the same category as "The Farmer's Almanac", Horoscopes, and paranormal phenomena. Meaning well known things that the general scientific community seems to laugh at. For many "Dietary Supplements" this is completely wrong and unfair. The support for some herbs is extremely compelling and it is a disservice to the reader to put it in the same category as some holding hands therapy.
- @WhatAmIDoing, you can specifically look at the Rheumatoid Arthritis article. I am a new wiki editor and at first any reference I put to herbal treatmnts was quickly deleted by Doc James, even when I provided double blind study references. Doc James, politely and gently educated me about secondary sources, so I kept working and found reliable secondary sources. I thank Doc James for teaching me about how to contribute to Wiki, and you can see the article that there are several "Dietary Supplement" treatments that Doc James has agreed have compelling reliable secondary sources to support their treatment and has agreed to include them in the article, just under the "Alternative Medicine" heading. For the reasons I just said, it is a disservice to put these proven treatments under the heading "Alternative Medicine".Sthubbar (talk) 01:16, 4 April 2013 (UTC)
- I do not consider alt med to be negative. Doc James (talk · contribs · email) (if I write on your page reply on mine) 01:56, 4 April 2013 (UTC)
- I also don't think that alt med is a negative title, but why did you put things like Transcutaneous electrical nerve stimulation and fish oil under ===Alt med=== and not under ===Self-care===? TENS is a conventional treatment, and omega-3s are widely accepted. WhatamIdoing (talk) 02:05, 5 April 2013 (UTC)
- On the RA article, I also agree that the things for which there is apparently some evidence of efficacy should not be listed in a section called alt med. CAM = things that have not been proven to work, so it might be considered a negative title...do these modalities even meet the general definition of alt med? Not totally happy with "self care" suggestion, afterall there is a category already called "lifestyle"...how about simply "other measures"? Agree a section termed "dietary supplement" would also not be appropriate for some of these treatments. "Herbal medicines, dietary omega-3 fatty acids & vitamins" could all be argued to meet the FDA definition of dietary supplements though... Lesion (talk) 09:57, 5 April 2013 (UTC)
- I also don't think that alt med is a negative title, but why did you put things like Transcutaneous electrical nerve stimulation and fish oil under ===Alt med=== and not under ===Self-care===? TENS is a conventional treatment, and omega-3s are widely accepted. WhatamIdoing (talk) 02:05, 5 April 2013 (UTC)
- I do not consider alt med to be negative. Doc James (talk · contribs · email) (if I write on your page reply on mine) 01:56, 4 April 2013 (UTC)
- @WhatAmIDoing, you can specifically look at the Rheumatoid Arthritis article. I am a new wiki editor and at first any reference I put to herbal treatmnts was quickly deleted by Doc James, even when I provided double blind study references. Doc James, politely and gently educated me about secondary sources, so I kept working and found reliable secondary sources. I thank Doc James for teaching me about how to contribute to Wiki, and you can see the article that there are several "Dietary Supplement" treatments that Doc James has agreed have compelling reliable secondary sources to support their treatment and has agreed to include them in the article, just under the "Alternative Medicine" heading. For the reasons I just said, it is a disservice to put these proven treatments under the heading "Alternative Medicine".Sthubbar (talk) 01:16, 4 April 2013 (UTC)
Doc James, thanks again for your response. The question is not if the term "Alt Med" does or does not offend Doc James. The question is what objection is there to using the term "Dietary Supplements". It seems that both Lesion and WhatamIdoing both see the downside of putting all herbal, vitamin and mineral supplements under "Alt Med." @Lesion/WhatamIdoing, I don't suggest we try introducing another title for the following reasons 1) All of the treatments that I am currently considers, except for TENS can be under the "Dietary Supplements" header and we can leave TENS under "Alt Med". 2) There is already the argument of trying to pick a term that has been defined somewhere, so even if we chose the FDA definition, that is acceptable to me. 3) Self-Care would include all OTC meds, and bandages or anything that could be bought without prescription so not really appropriate for the purpose of "Diet. Supp.".
Asking for the 3rd or 4th time,
Can we use the sub-title "Dietary Supplements" under "Management/Treatment" section of WP:Medicine articles? If not, please explain why. — Preceding unsigned comment added by Sthubbar (talk • contribs) 23:12, 6 April 2013 (UTC)
- I don't see the downside to putting alternative medicine, like most herbal preparations, under the section heading of ===Alternative medicine===.
- But I'm going to ask for the second time: Why are you so determined to put conventional medicine (i.e., TENS) under ===Alternative medicine===?
- Also, why shouldn't all forms of self-care, including exercise, TENS, and dietary supplements, be put together? Why should 'self-care that comes in pill form on the vitamin aisle of the store' be separated from 'self-care that comes in pill form from the aspirin section of the same store' and why should both of those also be separated from 'self-care that doesn't come in pill form'? WhatamIdoing (talk) 14:51, 8 April 2013 (UTC)
- This thread seems to be related to this thread below: Wikipedia talk:WikiProject Medicine#Alternative medicine implies quackery -- Brangifer (talk) 04:26, 10 April 2013 (UTC)
Is it appropriate for a discussion of DSM to take up so much of Allen Frances biography?
Should a biography contain a history of DSM, much of which seems to have a POV? To me some statements may be factually incorrect. Don't know where else to get another point of view. According to a new editor, User:1boringoldman, the article has been changed to please Allen Frances.[8] See Talk:Allen Frances#Merger proposal, a to merge User:1boringoldman/sandbox with the Francis article. This is version Francis liked, according to the editor, and now it has been implemented with no discussion that I'm aware of. Star767 17:27, 31 March 2013 (UTC)
- The only discussion is at User talk:1boringoldman/sandbox, where it was originally proposed to remove the version prior to 1boringoldman's, and mostly procedural in nature. -- 65.92.180.137 (talk) 21:01, 31 March 2013 (UTC)
- The article, after it was moved and replaced the existing article, was essentially gutted, as it consisted mostly of a rant against the dsm5. Nothing has happened since. The original editor of the sandbox article that replaced the existing one has disappeared. How is it that an article written apparently at the behest of Allen Francis replaced the existing article, without discussion as far as I know, except a "procedural" discussion in the sandbox as described above? Star767 03:38, 7 April 2013 (UTC)
- Wikipedia is the encyclopedia that anyone can edit. That means that Wikipedia is the encyclopedia that the subjects of BLPs can edit, too. WhatamIdoing (talk) 14:52, 8 April 2013 (UTC)
Argus Retinal Prosthesis may discuss "artificial eyes" on Wikipedia's front page
There seems to be an entirely innocent, sensible, and unproblematic DYK nomination - to my inexperienced eyes - at Template:Did you know nominations/Argus Retinal Prosthesis.
Someone has wisely raised the issue that "it is on the fence between a medical article and about a product" and it may or may not need additional work to meet WP:MEDRS.
Any comments there would be appreciated. --Demiurge1000 (talk) 21:03, 31 March 2013 (UTC)
- Have commented. Doc James (talk · contribs · email) (if I write on your page reply on mine) 22:27, 31 March 2013 (UTC)
University of Toronto psychology class
Regulars here will have been aware of a megaclass (1700 students) being set assignments on Wikipedia. Psychology and neuroscience articles are the most frequently targeted. If you wondered why your watchlist went crazy around the 22nd of March this year, it was this semester's class scrambling to write something before the deadline. The Wikipedia:Education noticeboard has been discussing student edits for a time but this class has been a problem for years. See Wikipedia:Education noticeboard#Big problems with neuroscience articles and Wikipedia:Education noticeboard#U of T courses in Psychology for recent talk. We have once again asked the Prof to stop and to talk to us. You may wish to review the evidence concerning this class and consider what we, as a Community, should do about past edits and about potential future edits by this class. Colin°Talk 18:37, 1 April 2013 (UTC)
- Ah. That explains a recent edit to Epileptic seizure that added a citation accessible solely through U of T servers. Fvasconcellos (t·c) 18:49, 1 April 2013 (UTC)
Main concerns are the evidence section which contains stuff such as: "In a controlled, double-blind study of a practitioner of Therapeutic Touch (which involves focused intention on the part of the practitioner, not physical touch), 13 of 23 human subjects experienced complete healing of their surgical wounds by the sixteenth day of the study. None of the control group (non-treatment) subjects had healed in that time frame." This is from the September 1990 issue of Subtle Energies, the maiden issue of the official journal of the International Society for the Study of Subtle Energy and Energy Medicine (ISSSEEM). (see [9]. As regards this specific claim, I find it's been challenged at [10]. Dougweller (talk) 11:22, 2 April 2013 (UTC)
- Attunement has similar problems, with claims that "some of the foundational Attunement practices, such as the healing impact of words and the benefit of energy medicine in distant healing show positive results in scientific studies." with 3 citations that don't look like scientific studies. Dougweller (talk) 13:32, 2 April 2013 (UTC)
- I just restored the previous redirect for the Consciousness-based healthcare article. IRWolfie- (talk) 14:03, 2 April 2013 (UTC)
Some of our best articles
User:SandyGeorgia has not edited for a couple of months. These means the articles she has worked to help bring to FA are less watched. I have protected Asperger syndrome. Is there other pages that need help? Doc James (talk · contribs · email) (if I write on your page reply on mine) 15:12, 2 April 2013 (UTC)
- Her FAs (Samuel Johnson, Early life of Samuel Johnson, and Tourette syndrome) are fairly low-profile; I watch them, and they're in good shape.
- Autism is semi-protected but could use more watchers (and less class projects).
- PANDAS and Autism spectrum, two articles she did a lot of work on which are not FAs, both see a fair amount of IP vandalism and may benefit from protection. Maralia (talk) 16:03, 2 April 2013 (UTC)
- I also watch all of the above except PANDAS and Autism spectrum. I don't think either warrants semiprotection (low edit rate) but both would certainly benefit from pending changes. Fvasconcellos (t·c) 16:35, 2 April 2013 (UTC)
Xrays
It appears that a number of commons admins including User:Fastily and User:MichaelMaggs are interpreting the law around X rays such that X rays uploaded by patients are to be deleted. Per [11] and [12] and [13]. No one has any idea who owns the copyright and even if X-rays are copyrightable. IMO a patient has just as much right to upload as anyone else. Doc James (talk · contribs · email) (if I write on your page reply on mine) 15:37, 2 April 2013 (UTC)
- IANAL but how on Earth could an X-ray ever meet the threshold of originality? This should be brought to the attention of Foundation counsel. Fvasconcellos (t·c) 16:38, 2 April 2013 (UTC)
- A note at the top of meta:Wikilegal/Copyright of X-Ray Images encourages people to improve the page. Does someone want to add these sources to it? WhatamIdoing (talk) 22:54, 3 April 2013 (UTC)
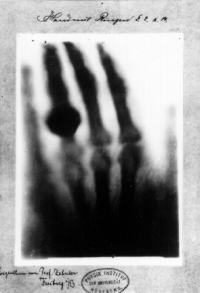
At [18] (already cited above) it talks about the case law that would presumably be applied to X-ray images, in light of past photo cases. In light photography the issue is a spark of originality, which arrises in posing, composition, figuring out exposure, lighting, etc. All those things the tech has to do to take a good portable X-ray in an ICU. The bar to originality is low-- if light photography is the standard, then putting an extra pillow behind a patient, or turning up the kVP for the fat guy, are enough. If you think getting a really good CXR is easy to do, you've never done it-- particularly with one of those old machines. It's far more difficult to take a good chest X-ray of an ICU patient than take a photo of a him with a modern digital camera that does everything for you but turn itself on. Of course, this varies from case to case. Getting a chest x-ray in a modern digital suite is pretty mechanical-- about as much as a traffic stoplight photo. But let me give an illustrative example or originality: there are few photographic images more famous than Roentgen's X-ray of his wife's hand. Keeping the wedding ring on, so you see it on her skeletal finger, makes that image. You know? It's NOT mechanical. That one was art. And some of those films even today are art. I've taken many an X-ray with an old machine, followed by emulsion development, and I know. If it's hard, it must be art. ;) SBHarris 01:03, 4 April 2013 (UTC)
Researcher Dick Swaab
Hi. I stumbled upon several article edit where Dick Swaab have been written into the articles one way or the other. While there may be good reasons for including his research results it always includes the name of the author, which is not that common on Wikipedia. The edits are done by either one of two IPs or Hazelares, who only seems to either work on the Dick Swaab article or articles related to his work, where his name is written into the article (could be a work-, home-pc and a registered user or three different people. I can not tell). It seems a bit like self-promotion (or promotion of third party), but maybe done in good faith. When writing scientific articles it is quite common to write; "in a study by person1 et al", but there seems to be a consensus not to do it here...
I have no idea how to proceed with this issue if it is even an issue. Would somebody else take a look at it. I can see that User:Flyer22 have already reverted some of the edit, see User talk:Hazelares.
I will write on Hazelares´s talk page that I started this discussion, so it does not go on behind his/hers back. But if somebody could have a look at some of the edits, give there input or in any other way help out... please do so, since I have no experience with this kind of situation. JakobSteenberg (talk) 21:57, 2 April 2013 (UTC)
- I have noted this as well. There is clearly some undue promotion going on, but it has only touched a few articles so far, as far as I can see. Looie496 (talk) 23:15, 2 April 2013 (UTC)
- The article about Swaab is very poor indeed. It was declined recently at AfC for lack of reliable sources. Currently it is a glorified CV of his life and work. I've removed some of the obvious inappropriate parts. He may well be notable (maybe sources in Dutch?) but currently the article is a bit perilous! Sionk (talk) 23:26, 2 April 2013 (UTC)
Mushrooms for mesothelioma
I have a dispute with another editor regarding suitability of a reference. Please comment here. Axl ¤ [Talk] 11:18, 3 April 2013 (UTC)
- As of now, this has two replies and that may be sufficient. Blue Rasberry (talk) 13:20, 3 April 2013 (UTC)
An interesting new review at doi:10.1186/1897-4287-11-2 may be useful in these articles. LeadSongDog come howl! 13:18, 3 April 2013 (UTC)
Snare technique
Some input from this WikiProject at Wikipedia:Articles for deletion/Snare technique (2nd nomination) would be welcome. Specifically, is it likely that we'll ever have articles at Snare device, Snare technique (surgery) or Snare cautery? These are all long-standing redlinks, since 2007 until recently. [19] [20]] Or, are there already articles to which they should be redirected? Andrewa (talk) 13:47, 3 April 2013 (UTC)
A group of IPs attempting to push in a primary research paper
These IPs have added a primary research paper 4 times to this article when the content is supported by a systematic review already. I assume that these are the authors. Thoughts? [21] Doc James (talk · contribs · email) (if I write on your page reply on mine) 21:21, 3 April 2013 (UTC)
- I have reverted the most recent edit. Can an admin please consider semi-protecting the article? (I would suggest not Doc James, to avoid accusations of bias.) Axl ¤ [Talk] 22:36, 3 April 2013 (UTC)
- There seems to be a theme running. this too inserts a ref with the same author on a closely related topic, and the editing IP is also consistent with that author. This raises the specter of COI, Sock, and UNDUE issues, too.LeadSongDog come howl! 22:51, 3 April 2013 (UTC)
- I have formally requested page protection. Axl ¤ [Talk] 23:10, 3 April 2013 (UTC)
- This is level 4 evidence from a study that has literally just been published and refers to a very specific patient population—it doesn't belong in the article at all. I don't think WP:SOCK is an issue here (WP:MEAT may be, though). I think discussion with the latest IP is certainly warranted. Fvasconcellos (t·c) 23:41, 3 April 2013 (UTC)
- I've fully protected the article for 10 days. This is not the place this discussion should be happening, that should be on the article's talk page. You can link from there to here if necessary. Putting the discussion here means that the IP concerned does not have a chance to get involved. GedUK 11:24, 4 April 2013 (UTC)
- Now that the wrong version has been protected, can someone fix it please? Axl ¤ [Talk] 13:21, 4 April 2013 (UTC)
- Agree with all above that the primary study is inappropriate and should be removed. Yobol (talk) 15:00, 4 April 2013 (UTC)
- Now that the wrong version has been protected, can someone fix it please? Axl ¤ [Talk] 13:21, 4 April 2013 (UTC)
- I've fully protected the article for 10 days. This is not the place this discussion should be happening, that should be on the article's talk page. You can link from there to here if necessary. Putting the discussion here means that the IP concerned does not have a chance to get involved. GedUK 11:24, 4 April 2013 (UTC)
- This is level 4 evidence from a study that has literally just been published and refers to a very specific patient population—it doesn't belong in the article at all. I don't think WP:SOCK is an issue here (WP:MEAT may be, though). I think discussion with the latest IP is certainly warranted. Fvasconcellos (t·c) 23:41, 3 April 2013 (UTC)
PSA screening guidelines
A SPA, Drcoop, has today been consistently attempting to make edits to pages like Prostate cancer, and significantly, also to USPSTF. He or she is taking issue with the screening guidelines. I have been working on engagement via their talk page, but more eyes may be needed; allegations of censorship, though subsequently retracted, have already been thrown around. -- [ UseTheCommandLine ~/talk ] # _ 00:52, 5 April 2013 (UTC)
Discussion at Wikipedia:Categories_for_discussion/Log/2013_March_22#Category:Healthcare_policy_in_the_United_States
![]() You are invited to join the discussion at Wikipedia:Categories_for_discussion/Log/2013_March_22#Category:Healthcare_policy_in_the_United_States. Obi-Wan Kenobi (talk) 14:43, 5 April 2013 (UTC)Template:Z48
You are invited to join the discussion at Wikipedia:Categories_for_discussion/Log/2013_March_22#Category:Healthcare_policy_in_the_United_States. Obi-Wan Kenobi (talk) 14:43, 5 April 2013 (UTC)Template:Z48
- This rename discussion seems to have just closed after having been open for a while. The consensus was to rename Category:Healthcare policy in the United States to Category:Health policy in the United States. Blue Rasberry (talk) 15:48, 5 April 2013 (UTC)
More eyes on Anesthesiologist please
There has been a persistent addition of non-neutral content by the same IP for months. See here and page history, this has been ongoing since July 2012, stating that anesthesiologists have lower intelligence than orthopedic surgeons. Thanks. Yobol (talk) 13:42, 6 April 2013 (UTC)
- What do you call two orthopaedic surgeons looking at a head CT? A double-blind study.
- What are the Anesthesiology written boards like? Pages and pages of crossword puzzles.
- I'll see myself out... Fvasconcellos (t·c) 14:23, 6 April 2013 (UTC)
- Nice. Now watching. It was a pleasant surprise to see the study cited by the IP was a humorous one. As was the only publication to cite it (a paragraph). Biosthmors (talk) 18:21, 6 April 2013 (UTC)
- Watchlisted. Also, be on the lookout for editors referencing the article Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials to add content to Parachute casting doubt on their effectiveness.
Zad6803:59, 7 April 2013 (UTC)
- Watchlisted. Also, be on the lookout for editors referencing the article Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials to add content to Parachute casting doubt on their effectiveness.
Flu shot
There's a dispute about due weight at Influenza vaccine. Squish7 (talk · contribs) posted at complaint at WP:RSN, but it's not really the correct forum for the question. WhatamIdoing (talk) 21:01, 6 April 2013 (UTC)
- That article is not in bad shape overall, but it could use some editing. Axl has linked to a review (PMID 22032844) that could be used to expand and update the effectiveness section. I'd also rename the "Side effects" section to "Safety" and move it above "History". Perhaps I'll do that myself if I feel like wading into the mess. Fvasconcellos (t·c) 01:45, 7 April 2013 (UTC)
Could someone take a look at Pervasive refusal syndrome
I think Pervasive refusal syndrome was an article for a Toronto University class and has been abandoned by the student. Is it a legitimate topic, as it only relies mostly on one source and seems peculiar? Thanks, Star767 03:42, 7 April 2013 (UTC)
- It seems both a legitimate topic AND peculiar. If you want me to PROD it, all I can say is that I would prefer not to. ;)) SBHarris 20:40, 9 April 2013 (UTC)
- No, looking at it again it seems ok. Thanks for answering! Star767 00:34, 10 April 2013 (UTC)
Would this be an appropriate article for wikipedia?
Fascial spaces of the head and neck
...or would that be too essay-ish and unencyclopedic? If yes, perhaps that is not the best title... maybe Tissue spaces in the head and neck. I would intend it to be a parent article for all the pages we have for these potential spaces/compartments. Some are still missing, I am adding them...e.g. submandibular space etc. Alternatively could we at least have a new template, because I can't find a template that currently includes them all...Template:Digestive tract or Template:Mouth anatomy are probably the closest. Lesion (talk) 09:46, 7 April 2013 (UTC)
- Yes. I would love such an article and think it definitely have a place on wikipedia. It could be set up like the human anatomy part of hip bone or most of diabetes mellitus where each space have a section with a link to the main article.
- But it falls in under Wikipedia:WikiProject Anatomy rather than Medicine. We are only semi-active, but you can also post your question there. I am unsure what the proper name of such an article should be. I have not been able to find a template either, but the creation of one could be a good idea. Especially since there can be more than one template at the bottom of the page. JakobSteenberg (talk) 10:09, 7 April 2013 (UTC)
- Thanks for your fast response and the pointers to those articles I can use as a guide.
- Not 100% this is "pure" anatomy, since these spaces do not exist in health, they are created only when pathology dissects tissue planes which are not supposed to be separated...I will tag the talk pages with both wikiprojects. The creation of a template is a bit beyond my abilities, so I'll just create the articles for now and then chase that up later. Thanks, Lesion (talk) 10:28, 7 April 2013 (UTC)
- Okay. When you get to the template part I will gladly try to help you. I have not made one before either, but I would like to give it a try (it should be doable if we borrow the code from another and just change the text). Happy editing. JakobSteenberg (talk) 10:44, 7 April 2013 (UTC)
Students editing sexuality, biology and hormone articles related to physical and mental health
Some of you already know that we seemingly have a higher influx of students editing such articles, especially psychology articles, this year. I've guided some editors, such as this one, and more help from this project is definitely needed on this matter.
Very recently, I've tweaked the edits of, and guided, these two editors: Jameson.thomas52 (talk · contribs) and 8bjr4 (talk · contribs)
There are some articles that I'm either not heavily involved in editing or don't edit at all, and these articles could use more WP:MED eyes to assess whether some of the additions to these articles are appropriate (whether there are grammar or other formatting issues, irrelevant additions, WP:UNDUE additions, additions relying too heavily or solely on WP:PRIMARY SOURCES, or additions that have plagiarism problems). The Sexual dysfunction article, which I edit sparingly thus far, is a good candidate. And so is the Hormone replacement therapy (menopause) article, which I never edited until today (April 7th, non-Wiki time, to clean up some things), and the Androgen deprivation therapy article. A few of you are already watching the Female genital mutilation article, and it may be best to wait until the students are done editing that article (whenever that is) until substantial cleanup takes place at that article; still, there has been general cleanup going on at that article following student edits, and SlimVirgin, who is a regular editor of that article, has done substantial cleanup of it following student edits.
Anyway, thank you for any help you are willing to provide. Flyer22 (talk) 00:32, 8 April 2013 (UTC)
- The main issue appears to be the use of primary and low quality secondary sources at Androgen deprivation therapy. A couple of the refs though are good and there appears to be proper paraphrasing. Doc James (talk · contribs · email) (if I write on your page reply on mine) 04:06, 8 April 2013 (UTC)
- Thanks for some feedback, James. Flyer22 (talk) 05:05, 8 April 2013 (UTC)
Further eyes here could be helpful.[22] An editor wishes to add "warning labels" to articles. And is removing the conclusions of a recent review. They are also trying to portray a Cochrane pamphlet as an update of a Cochrane review.Doc James (talk · contribs · email) (if I write on your page reply on mine) 01:38, 8 April 2013 (UTC)
Removing of evidence
We have a user who has removed a 2009 Cochrane review among others as it found it didn't work. Discussion is here [23]Doc James (talk · contribs · email) (if I write on your page reply on mine) 19:49, 7 April 2013 (UTC)
- That is a complete misrepresentation. Let's start with fact that you removed all of my edit based on a mistake because I MOVED a Cochrane review. The other Cochrane review was added by you, unknown to me, after you made the massive revert. I only removed that second Cochrane as an error of omission, in that I was just trying to recover all of my work after your mistake. I welcome you to go back and add back the second Cochrane review. I support removing treatments that are proven not to work.Sthubbar (talk) 00:38, 8 April 2013 (UTC)
- No you removed a number of reviews in this edit here [24] and that included the Cochrane review. Have replaced most of the rest of your subsequent edits already. Treatments that do not work yet are in common usage should be discussed. Doc James (talk · contribs · email) (if I write on your page reply on mine) 02:05, 8 April 2013 (UTC)
Revert war at Osteoarthritis
Can we get a third input on the osteoarthritis page. 1) Doc James has made 5 reverts in 24hrs which I thought according to Three_revert_rule#The_three-revert_rule would cause auto 24 hr ban. 2) I have now made my 3rd revert and since DJ seems immune to the above rule, I assume he will make his 6th revert and I doubt I'm immune.
This started by DJ making a mistake and assuming I had removed a Cochrane reference pmid:20847017, he even mentions that in his revert. This reference was never removed, it was simply moved to a different place. After I was able to show him, the mistake, he now wants to change his story and say I need to get approval on the talk page before making edits. This is preposterous.
He keeps going back to a previous version that explicitly removes most of these treatments: avocado/soybean unsaponifiables[51], boswellic acid[52], cat's claw[53], curcumin[54], chondroitin[55][56], Devil's claw[57], glucosamine sulfate[58] , S-Adenosyl methionine[59][60] and TENS.[61]. Each supported by secondary sources.
He now wants to say that some of the secondary sources are weak, OK, that's a different argument and I welcome him to individually remove any such treatment and we can judge if it is fair or not.
He also wants to add back in the previous discussion about unproven treatments. I don't see it to be the standard in medicine articles that we put an extensive discussion about all of the "Western" treatments that don't work. If treatments aren't supported by evidence or shown to be equal to placebo, then just leave them out of the article.
I welcome editing and improving of the OA article, I reject this complete deleting of the material I have added by improper and mistaken use of the revert function. Thanks for helpingSthubbar (talk) 00:03, 8 April 2013 (UTC)
- Sthubbar continues to removed this PMID 19821296 19821296 which is a 2009 Cochrane review that found no evidence of benefit from TENS and replaced it with a 2007 review in the British Journal of Community Nursing from 2007 which comments on the limited evidence and states it may be beneficial to some. He has done this four times now here [25], [26], [27] and [28]. He has removed a number of other reviews as well. Doc James (talk · contribs · email) (if I write on your page reply on mine) 00:15, 8 April 2013 (UTC)
- By the way many of these treatments were already discussed in the text he has removed and in greater detail. Doc James (talk · contribs · email) (if I write on your page reply on mine) 00:26, 8 April 2013 (UTC)
- Doc James, you seem to change the story each time. If you would just edit instead of revert we wouldn't have any issue. I am not trying to push TENS. the TENS reference I put was already accepted by you for the Rheumatoid Arthritis article so I'm shocked that you are just now bringing it up like I'm trying to sneak in some crappy article. #2 The article that I deleted, I think was for NEST and I did not realize that NEST and TENS are the same thing. If it is the case that NEST and TENS are the same and there is a newer study that says they don't work, then I support completely removing them from the treatments section. Just stop reverting and removing every change that I made.Sthubbar (talk) 00:38, 8 April 2013 (UTC)
- If you read the systematic reviews you were removing than it would be clear. You have now removed a whole bunch of recent reviews and simplified that section so that it is misleading at best. Doc James (talk · contribs · email) (if I write on your page reply on mine) 00:47, 8 April 2013 (UTC)
- Doc James, you seem to change the story each time. If you would just edit instead of revert we wouldn't have any issue. I am not trying to push TENS. the TENS reference I put was already accepted by you for the Rheumatoid Arthritis article so I'm shocked that you are just now bringing it up like I'm trying to sneak in some crappy article. #2 The article that I deleted, I think was for NEST and I did not realize that NEST and TENS are the same thing. If it is the case that NEST and TENS are the same and there is a newer study that says they don't work, then I support completely removing them from the treatments section. Just stop reverting and removing every change that I made.Sthubbar (talk) 00:38, 8 April 2013 (UTC)
- By the way many of these treatments were already discussed in the text he has removed and in greater detail. Doc James (talk · contribs · email) (if I write on your page reply on mine) 00:26, 8 April 2013 (UTC)
- Sthubbar continues to removed this PMID 19821296 19821296 which is a 2009 Cochrane review that found no evidence of benefit from TENS and replaced it with a 2007 review in the British Journal of Community Nursing from 2007 which comments on the limited evidence and states it may be beneficial to some. He has done this four times now here [25], [26], [27] and [28]. He has removed a number of other reviews as well. Doc James (talk · contribs · email) (if I write on your page reply on mine) 00:15, 8 April 2013 (UTC)
How is it misleading. Here is what I have done: 1) Remove negative treatments. This is a violation of NPOV. If we are going to put negative Alt med treatments then we should include every negative Western treatment. As this isn't the case then I can't understand any problem with removing the negative information. So no problem here. 2) I removed the NEST treatment because of rule #1. I did not realize that NEST=TENS when I just added the TENS reference that you already accepted in the RA article. If you say you want to remove TENS, then I'm fine with that. 3) There is clear evidence that there is a distinct difference between Glucosamine HCL and sulfate. Any statement saying Glucosamine is ineffective, is misleading and unbalanced. It is more accurate to say Glucosamine HCL has a lack of support and Glucosamin sulfate shows promise or even good support. This was my intention of updating the glucosamine section. I admit it can be worded better. 4) The Cochrane study that you added after the major revert of all my changes was only removed by accident and I am perfectly fine with you putting it back in. 5) I added treatments that are supported by review articles. If you want to question them individually, that's also fine.
None of these issues warrant a complete revert of all the changes.
- 1) Yes we discuss negative treatments as long as they are well supported by evidence. 2) Yes NEST = TENS so we have solved that one 3) No there is not clear evidence that the two glucosamine forms are different only tentative evidence. 4) Great 5) The treatments you have added supported by reviews are mostly still there. They are all tentative at best. By the way all your changes have not been reverted. Doc James (talk · contribs · email) (if I write on your page reply on mine) 03:26, 8 April 2013 (UTC)
Threats from Doc James
It looks like things are going downhill with the relationship between me and Doc James.
- Doc James puts a false statement on my talk page saying I have made 4 reverts. Sorry Doc James, check your math. 2 reverts + 1 undo = 3 reverts. It is Doc James that has made 5 reverts in 24 hours with no ban as I would expect by the rule.
- Doc James now puts a threatening comment on my page saying I have been warned and better stop editing the OA page. Let's see what am I editing.
- "Many alternative medicines are purported to decrease pain associated with arthritis." Absolutely no reference for this statement and of no use. Why don't we put in the statment "Many Western medicines are purported to decrease pain associated with arthritis."? A useless statement. Just put in the treatments that work.
- Next as has been said many times, there is no place in putting in treatments that don't work. If he insists on saying that Vitamin C doesn't work, then I will create a list of every "Western" medicine that doesn't work for OA. I guess I can start by listing Claritin as not working for OA.
- I don't have to get approval from Doc James on the talk page for these updates. I am contributing to the article with quality information. — Preceding unsigned comment added by Sthubbar (talk • contribs) 03:36, 8 April 2013 (UTC)
- Have reported user in question here.[29] He has now removed the Cochrane review in question 5 times. I have made three reverts and leave it to others. There of course is no consensus for this continual removal of this review. Doc James (talk · contribs · email) (if I write on your page reply on mine) 03:39, 8 April 2013 (UTC)
- Somebody please ban Doc James for 24 hours as specified in Three_revert_rule#The_three-revert_rule.
- Why hasn't he been banned, he has now made 6 reverts in 24 hours. I thought the rule said that nobody was except form this rule.
- I have followed the rules. I only made 3 reverts, and brought the discussion here and another user made a decision and I accepted the other user's decision.
- I don't know the administrative procedures, but how the heck do we follow the wiki rules and ban Doc James for 24 hours from the OA page?Sthubbar (talk) 03:44, 8 April 2013 (UTC)
- One of the refs in question states "many dietary supplements are claimed to provide pain relief for patients suffering from OA. Few clinical studies or review articleevaluate antioxidant and antiinflmmatory agents for the same" PMID 20232616 Thus there was a ref for the statement you removed. You asked for a ref in this edit [30] yet deleted it in the edit before. Doc James (talk · contribs ·email) (if I write on your page reply on mine) 03:39, 8 April 2013 (UTC)
- @Sthubbar: If you create a list of every "Western" medicine that doesn't work for OA, then it will be removed immediately as original research. You must not disrupt Wikipedia to make a point. You need to learn that our job here is to accurately reflect with due weight what the reliable (preferably secondary) sources say. We also have strong advice to focus on the edits, not the editor, so please go back to the talk page and start assembling and discussing the secondary sources that support the edits you want to make. --RexxS (talk) 04:10, 8 April 2013 (UTC)
- Such a list would also be silly. Let's see: appendectomies don't work for OA, mastectomies don't help, antibiotics don't work...
- Sthubbar, I suggest that you read WP:BOOMERANG. WhatamIdoing (talk) 15:22, 8 April 2013 (UTC)
Negative treatments
Is it the general policy of WP:Medicine to list every and all treatments that have every been considered for all diseased and not only list the treatments that work, but also list every treatment that has been show not to work?
My understanding is that medicine articles list the treatments that work. No need to include all of the treatments that have either been shown not to work, or have poor evidence.
Doc James is getting his pants all messed up because I'm removing a sacred Cochrane review. I'm not removing the review, I'm removing the fact that this review is only being used to show what treatments don't work.
If we accepting putting in what treatments that don't work, then we need to go through all medicine articles and put every prescription and OTC medicine that has ever been tried for all diseases and listing them. This would be balanced.
If we don't want to list every non-effective prescription and OTC medicine then we shouldn't be listing non-effective alt medicines.
What's good for the goose is good for the gander.Sthubbar (talk) 03:51, 8 April 2013 (UTC)
- If you look at this article [31] we state that cough medicine are not effective in children, we state antibiotics are not effective, we state that second generation antihistamines are not effective. Doc James (talk · contribs · email) (if I write on your page reply on mine) 03:54, 8 April 2013 (UTC)
- (e/c) This is a misguided perspective. We include what the best-quality recent secondary sources, like Cochrane reviews, report. This sometimes includes findings for interventions that have been studied and found not to have an effect. They are important to include because the best-quality secondary sources report on them. That's the job of the secondary sources, they indicate not only the results of what was tested, but also what was tested.
Zad6803:57, 8 April 2013 (UTC)
- (e/c) This is a misguided perspective. We include what the best-quality recent secondary sources, like Cochrane reviews, report. This sometimes includes findings for interventions that have been studied and found not to have an effect. They are important to include because the best-quality secondary sources report on them. That's the job of the secondary sources, they indicate not only the results of what was tested, but also what was tested.
- (edit conflict) @Sthubbar: Your understanding is flawed. We list treatments that are discussed prominently in reliable secondary sources: if the source says the treatment works, we say so; if the source says the treatment doesn't work, we say so. Go back to the principal sources for the article, read them and do your best to summarise what they say about the effectiveness of treatments. If you do that without preconceptions and acting in good faith, that will always be the surest way to avoid finding yourself in disputes. --RexxS (talk) 04:10, 8 April 2013 (UTC)
OK, I got it.Sthubbar (talk) 04:34, 8 April 2013 (UTC)
Cannot recommend <> Not recommended
OK, it is the general negative and unfair characterization of the study. In particular, the Cochrane study says "We cannot recommend the use of..." which to me is a completely different statement than the OA statement of "These treatments are thus not recommended."
"Cannot recommend" is a neutral position saying that there is neither positive or negative evidence for the help or harm of the treatments.
"Not reommmended" is a negative statement implying harm to the treatments.
I reject this misrepresentation of the evidence.
Can we agree on this?Sthubbar (talk) 04:40, 8 April 2013 (UTC)
- We really should be having this discussion at the article talk page, not here, but I'm not really seeing a significant semantic difference. What is your proposed wording?
Zad6804:59, 8 April 2013 (UTC)
- Zad there is a huge difference. "Cannot recommend" = 0 (zero) "Not recommended" = some negative number. So the case is
- (Cannot recommend Cochrane) 0 + (some other reliable review) + 0.1 = 0.1 (possible effect)
- (Not recommended Cochrane) -1 + (some other reliable review) + 0.1 = -0.9 (not recommended)
- My suggestion is the same as always, leave the info out as it provides no value. A zero is a zero. If it must be in as Doc James seems to insist them the wording would be "A 2012 review article found evidence to support the use of X. A 2010 review was unable to find enough evidence to recommend X."
- I don't even know why we would include the 2010 when it just is saying nothing that they didn't find enough evidence when a more recent article does find evidence, but anyway. This way of saying that Review A finds evidence and review B didn't find enough evidence, is fair. This is much different than saying Review A finds evidence and review B does not recommend.Sthubbar (talk) 06:31, 8 April 2013 (UTC)
- If you're talking about PMID 20232616 "We cannot recommend...", the source further states that support has not been found not just for effectiveness but also safety. The wording for things like this needs to be taken on a case-by-case basis and the source's evidence and conclusions reviewed carefully. If you still have concerns about the current wording in the osteoarthritis article please open a discussion about it at the article Talk page, it's not possible to make a blanket WP:MEDICINE-wide recommendation for all sources and article here.
Zad6814:13, 8 April 2013 (UTC)
- If you're talking about PMID 20232616 "We cannot recommend...", the source further states that support has not been found not just for effectiveness but also safety. The wording for things like this needs to be taken on a case-by-case basis and the source's evidence and conclusions reviewed carefully. If you still have concerns about the current wording in the osteoarthritis article please open a discussion about it at the article Talk page, it's not possible to make a blanket WP:MEDICINE-wide recommendation for all sources and article here.
Zad, the abstract says they "cannot recommend" because the evidence is "unclear" and more research is necessary. That is exactly my point. They are NOT say that don't recommend, they are saying they can't recommend because of lack of evidence. It is the difference between not-guilty and innocent. Not even close to the same meaning.Sthubbar (talk) 16:54, 9 April 2013 (UTC)
- I can see the distinction that you are making, but given the poor state of editing in the world (I am contemplating a note to my local newspaper to explain that verbs are not optional components of sentences), it is unreasonable of us to place so much emphasis on two words. You need to read the whole paper to understand what that particular source means. WhatamIdoing (talk) 15:32, 8 April 2013 (UTC)
- So that the editors of your local newspaper understand your note, I suggest you word it like: "Verbless grammar problematic, verbing nouns nauseating. Passive voice a cop-out. Ambiguity ensues, readability plummets. Film at 11."
Zad6817:03, 8 April 2013 (UTC)- Surely you meant "... Ambiguity in spades, readability taking a nose-dive. (Clichés abundant). Film at 11." --RexxS (talk) 00:56, 9 April 2013 (UTC)
- And the reply, if any, would have to be "Mistakes were made. The passive voice was used. Responsibility was shirked." WhatamIdoing (talk) 05:42, 9 April 2013 (UTC)
- Surely you meant "... Ambiguity in spades, readability taking a nose-dive. (Clichés abundant). Film at 11." --RexxS (talk) 00:56, 9 April 2013 (UTC)
- So that the editors of your local newspaper understand your note, I suggest you word it like: "Verbless grammar problematic, verbing nouns nauseating. Passive voice a cop-out. Ambiguity ensues, readability plummets. Film at 11."
Alternative medicine implies quackery
- This thread seems to be related to this thread above: Wikipedia talk:WikiProject Medicine#Nutraceuticals Dietary Supplements -- Brangifer (talk) 04:23, 10 April 2013 (UTC)
BullRangifer's page Alternative medicine critics says exactly the point I have been trying to get across.
- "Some critics...have defined alternative medicine as those unproven or disproven medical practices and ideas that lack a scientific evidence base."
- "[Alternative Medicine] is often described as quackery, pseudoscience, fraud, and/or unfalsifiable beliefs of a religious or metaphysical nature."
- "Alternative medicine has been described as pseudoscientific."
And the page goes on and on. These are exactly why, when there are dietary supplement treatments that have strong evidence from random controlled trial, secondary review research and articles, that it is a disservice to these treatments to list then in a category associated with quackery.
Can we please put proven dietary supplement treatments under a separate heading "Dietary Supplements"?Sthubbar (talk) 10:42, 9 April 2013 (UTC)
- Dietary supplements which have evidence are mainstream, not alternative. Folic acid during pregnancy is the only thing that comes to mind. As far as I am aware the rest lack evidence. Consumption of supplements which don't have evidence (i.e Antioxidants, Vitamin C and the like) are regarded as alternative precisely because there is no evidence based medical reason to take it. Calling it alternative, rather than referring to it as rampant pseudoscientific quackery is just us wikipedians trying to be polite (per WP:IMPARTIAL). IRWolfie- (talk) 10:48, 9 April 2013 (UTC)
- IRWolfie, this is exactly the point. Your statement "As far as I am aware the rest lack evidence." is quite common and unfortunately misinformed. Omega-3 would be one of the most widely accepted, proven by multiple large double-blind placebo controlled randomized studies, supported by secondary review articles. This is not the only one, there are many others. Your belief that there are very few is supported by mixing these treatments in with quackery.Sthubbar (talk) 10:56, 9 April 2013 (UTC)
- The systematic reviews disagree with you, IRWolfie- (talk) 12:40, 9 April 2013 (UTC)
- IRWolfie, this is exactly the point. Your statement "As far as I am aware the rest lack evidence." is quite common and unfortunately misinformed. Omega-3 would be one of the most widely accepted, proven by multiple large double-blind placebo controlled randomized studies, supported by secondary review articles. This is not the only one, there are many others. Your belief that there are very few is supported by mixing these treatments in with quackery.Sthubbar (talk) 10:56, 9 April 2013 (UTC)
- I agree about the implications you've noted above. However, dietary supplements need only be "safe". Unlike treatments claiming medical benefit (in the U.S.), they do not need to be "effective". Indeed, you'll often see the weasel words "used for..." on the labels as an end run around claiming actual medical benefits. So - for "alternative" treatments that have been demonstrated both "safe" and "effective" - the category of "Dietary supplement" would not be appropriate, either. We'd also need to consider "certification". That is, who (among us) is to say that a particular study is sufficiently reliable to demonstrate that some treatment is indeed not quackery? The U.S. relies on the FDA in theory. However, once a treatment passes FDA "safe and effective" muster, doctors are permitted to prescribe it for "off label" treatments. That is, they can use it to treat conditions that the drug have not been certified by the FDA as "effective". This makes "off label" use little more than quackery, too. And, of course, there's nothing to say that Wikipedia must adopt the FDA's standards. Next... while peer-reviewed studies sound good in name, often these studies do little more than report results obtained in a petri dish rather than in clinical trials. And even clinical trials can be problematic if they lack the appropriate rigour. So, what to do? Rklawton (talk) 10:59, 9 April 2013 (UTC)
- Rklawton, I'm not sure what to call your discussion, I think maybe straw man. What you are describing is something completely different than I am proposing, and you are pointing out problems that are not unique to dietary supplements.
- Only accepted treatments are allowed. Accepted means the same thing it means for any other treatment. No new rules or concerns. I have yet to hear someone say that what the FDA says it the law on Wikipedia. I thought it was what reliable secondary sources say. The same rule for dietary supplements. Based on reliable secondary sources the treatments are permitted or denied.
- I am in no way implying that dietary supplements would be an open door policy to put any herb, vitamin, or mineral treatment there based on the flimsiest evidence, suggestion or petri dish. No, no, no, only allow promising, reliable, or even strong, secondary sources, just like for every other treatment. No special rules need.Sthubbar (talk) 11:05, 9 April 2013 (UTC)
- I'd call my discussion "rambling" more than anything else. The most relevant point is that calling proven alternative treatments "dietary supplements" problematic because dietary supplements don't have to be proven effective. Rklawton (talk) 11:10, 9 April 2013 (UTC)
- Hmm, my ability to confuse you amazes me. I am not saying put all proven alternative treaments under "dietary supplements". I am saying that first only treatments that fit the above FDA defined or something close definition would go under this category. Second, we are only going to put proven treatments that fit the definition. Dietary Supplements is not meant to imply "Proven Alt Med" treatments. It just just meant to classify herbs, vitamins and minerals that are proven away from quackery.Sthubbar (talk) 11:32, 9 April 2013 (UTC)
- I think the point that dietary supplements is a regulatory definition which implies safety rather than efficacy might be an important point. If we restrict the definition of dietary of supplements in our articles to things which are both safe and effective, could this be original research? Lesion (talk) 11:59, 9 April 2013 (UTC)
- "Safe and effective" isn't original research if it's backed up by peer reviewed sources. I think that "FDA qualified" herbal treatments should not go under "dietary supplements" because (at least in the U.S.) "dietary supplement" means only "safe" and not "safe and effective". I do think we should have some sort of category that indicates a particular treatment (herb, vitamin) is both "safe and effective" - aka "medicine". Rklawton (talk) 12:08, 9 April 2013 (UTC)
- Hmm, what am I missing here. If the product is being sold for human consumption then by definition the consumption of that item is safe. If it is unsafe it is removed from the market. The secondary sources define effective. So these will be both safe and effective. No need for any other special categories. Again, dietary supplements does not allow listing anything there, only things that are show to be effective by secondary sources, and if they are legally commercially sold, then they are by definition safe, at least as safe as eating at any fast food joint.Sthubbar (talk) 12:33, 9 April 2013 (UTC)
- Let's try it this way:
- 0 = unsafe (so it gets pulled from the market)
- 1 = safe, but there's no scientific evidence that it's a medical treatment.
- 2 = safe & effective, may be FDA approved, but as a minimum, reliable sources stay it's an effective treatment
- All dietary supplements fall into at least #1. However, "alternative" treatments with research indicating effectiveness raises it up to the level of #2 "safe & effective". So my point is, if it fits #2, let's not give it a label that is usually interpreted to read #1. Thus, if the AMA publishes research that says "snotgrass juice" is a safe and effective treatment for "jazzberry rot", we shouldn't call it a "dietary supplement" because it's not on par with dietary supplements - it's better than that. Rklawton (talk) 13:18, 9 April 2013 (UTC)
- Let's try it this way:
- Agree, you would have to use a section called "dietary supplements with some evidence of efficacy" or something otherwise the fundamental definition of dietary supplement is being altered, which might be OR... Lesion (talk) 13:24, 9 April 2013 (UTC)
- Rklwton, OK, I get your point. Why the confusion? If there is an article about a disease and there is a subheading called "Medicine" we don't have to specify "Medicine that works for this illness", it is implied. We also don't have to clarify that the info under Medicine is not a complete list of items that fit the label medicine. If there are 100,000 items that can be called "medicine" it is understood that the 10 items listed are a special subset of the category "Medicine". In our case, it is no different. The category "Dietary Supplements" includes maybe 200,000 items. If we put a section called "Dietary Supplements" under an illness and list 10 items, I see no need to say "Dietary Supplements that are proven effective" as it is already clear that per WP:Medicine rules supposedly all treatments should be proven effective. The reader already realizes that there is something special about the 10 items listed under "Dietary Supplements" and they are a special subset chosen because that have a proven relation to the illness in question. KISSSthubbar (talk) 13:33, 9 April 2013 (UTC)
- The FDA does not ensure that dietary supplements are "safe" - it ensures that they meet food guidelines. In response to increasing talk on Wikipedia about this, I created Dietary Supplement Health And Education Act of 1994 which describes the primary US regulation. Most people in the US will have health problems and die from eating unhealthy food which is not "unsafe", and in the same way that the FDA allows people to choose the food they eat regardless of health concerns, the FDA leaves people to make their own choices about supplements. No regulator in the US labels supplements as "safe"; they can be unsafe or have warnings, but beyond that are treated like food in the US. If anyone knows of any analogous laws in other countries then I would love to see them connected somehow to this United States statute, and also I would appreciate any comments on how to expand the article for this US law on that article's talk page. Blue Rasberry (talk) 13:55, 9 April 2013 (UTC)
My two cents on this is, don't have a section in the article called Alternative medicine. This is not even a section suggested by WP:MEDMOS. The section you're talking about should be called Treatment and/or Management. Here's what WP:MEDMOS says about this section:
Treatment or Management: This might include any type of currently used treatment, such as diet, exercise, medication, palliative care, physical therapy, psychotherapy, self care, surgery, watchful waiting, and many other possibilities. Consider discussing treatments in a plausible order in which they might be tried, or discussing the most common treatments first. Avoid experimental/speculative treatments and preventive measures (e.g., prophylactic vaccines or infection-avoidance techniques).
If there are dietary supplements that are commonly used to treat (or used in an attempt to treat), and you've got good secondary sources covering the evidence for the use of them, put them in this section. I wouldn't go out of my way to try to label them as 'supplements' or 'alternative' or whatever, just state what they are and what the evidence is for them. Zad68 14:00, 9 April 2013 (UTC)
- Zad, fine I'm OK with that. Unfortunately a powerful WP:Medicine admin, Doc James, insists on putting all proven dietary supplements under the quackery title of alternative medicine. Is there some way to officially start a vote or something so that I can officially move all proven herbal supplements out of this quackery header without risking another ban?Sthubbar (talk) 14:30, 9 April 2013 (UTC)
- Are you talking about Osteoarthritis? I took a quick look at the edit history there and I don't see support for what you're saying. I'd have to let Doc speak for himself, let's wait for his response.
Zad6814:40, 9 April 2013 (UTC)
- Are you talking about Osteoarthritis? I took a quick look at the edit history there and I don't see support for what you're saying. I'd have to let Doc speak for himself, let's wait for his response.
- I like the idea of a "treatments" section, though we're going to spend a lot of time keeping out the cranks. I disagree with including a "Dietary supplements" section because the term has a "quack" perception, and I don't think the general reader is going to be familiar with WP:Medicine enough to know that we'd only include treatment demonstrated to be effective. Rklawton (talk) 14:31, 9 April 2013 (UTC)
- on a side note, we don't really discuss only treatments which have been shown to be effective in our articles. Regardless of whether that treatment is generally seen as alt med or mainstream, we discuss all the treatments which are commonly used for that topic, and then discuss their individual evidence base. Lesion (talk) 14:49, 9 April 2013 (UTC)
- Zad, it all started with the rheumatoid arthritis article. I had tried putting them under a title of "Other therapies" and DJ reverted. https://en.wikipedia.org/w/index.php?title=Rheumatoid_arthritis&diff=546438158&oldid=546437475. He has also stated above that the term "Alt med" does not offend him, and I assume he doesn't think it is equated with quackery, or that dietary supplements should be in the category with quackery. We have been having this discussion for pages and pages here and I'm confident DJ will serve me another ban if I don't get clear agreement here that the treatments I'm proposing can be put somewhere besides under a title that implies quackery.Sthubbar (talk) 14:52, 9 April 2013 (UTC)
- In this edit Doc renamed Other therapies to Alternative medicine and moved content under it that said "The existing evidence suggests that some of the complementary and alternative medicine modalities ... show promising efficacy in reducing pain", and that's how the article still stands right now after a lot more editing by him, so he actually created the section and filled it in with content saying positive things about it. I do not think he believes Alternative medicine means something negative. And in this edit he pointed to this recent discussion where it was decided that "Alternative medicine" was the preferred title. This is one of those things where you may not agree with it but the consensus on Wikipedia is to use Alternative medicine, and you're probably just going to cause yourself a lot of aggravation with no good result if you keep fighting it. For what it's worth, I do not think it's necessarily a negative term either, although it almost always is associated with something that has not been as well-tested.
Zad6815:24, 9 April 2013 (UTC)
- In this edit Doc renamed Other therapies to Alternative medicine and moved content under it that said "The existing evidence suggests that some of the complementary and alternative medicine modalities ... show promising efficacy in reducing pain", and that's how the article still stands right now after a lot more editing by him, so he actually created the section and filled it in with content saying positive things about it. I do not think he believes Alternative medicine means something negative. And in this edit he pointed to this recent discussion where it was decided that "Alternative medicine" was the preferred title. This is one of those things where you may not agree with it but the consensus on Wikipedia is to use Alternative medicine, and you're probably just going to cause yourself a lot of aggravation with no good result if you keep fighting it. For what it's worth, I do not think it's necessarily a negative term either, although it almost always is associated with something that has not been as well-tested.
Zad, the way I read that discussion is that the proposal was to change the name of "Alternative Medicine" to "Complimentary and Alternative Medicine". That is something completely different that what I am requesting. I accept to leave "Alternative Medicine". That's fine, leave it there. I'm proposing to allow the addition of a section "Dietary Supplements". It would even be fine with me if this was a sub-section of "Alt Med", not idea, and a compromise I could accept.Sthubbar (talk) 15:42, 9 April 2013 (UTC)
- Sthubbar: I do not read the label "alternative medicine" as necessarily implying "quackery". Do you object to the lead at Alternative medicine and its expansion in the first section "Terms and definitions"? That may not be perfect, but it is acceptable to a non-partisan layman. Does it surprise anyone that physicians can purport to practice conventional medicine in a way which amounts to what a layman would call "quackery", or that "quack" can be a jocular expression applied to a physician, sometimes habitually but inoffensively? Certainly, there may be benighted persons who cannot think, speak or write about any "alternative medicine" without calling "quackery", but surely not DocJames? Concept and language: currently conventional = conventional; currently alternative (to conventional) = alternative; "quackery" = campaigning name-call, possibly reflecting more unfavourably on person using it habitually, loosely or malevolently. Sometimes, sources on Med or other topics do not support what a fully informed and competent person happens to know about it: if that can be stressful each must find the remedy which suits him/her (such as adapting or abstaining). Qexigator (talk) 15:53, 9 April 2013 (UTC)
- Sthubbar, well if that's really the entire scope of this discussion, then it would just come back to the sources. If the general consensus of the best-quality sources is to classify Dietary Supplement X as "alternative medicine" it should not be a problem for the Wikipedia article to do so. Maybe if I were writing the article I would just talk about Supplement X without trying to put it in a section or under a heading called Alternative medicine but then if another editor came along and did that, pointing to the fact that the secondary sources do so, I would accept that change to the article.
Zad6816:05, 9 April 2013 (UTC)
- Given the huge number of products promoted as "dietary supplements", "nutriceuticals", "health foods", etc. it is almost certain that some of them will eventually prove to have beneficial effects on health. Many more will prove to be innocuous but ineffective, and some will prove to be downright harmful. The problem we pseudonymous editors face is that we cannot be the ones to separate the wheat from the chaff. We must leave that up to competent bodies to do. Unless we have established MEDRS sources telling us that product X is safe and effective, then we should not be lending the voice of the encyclopedia to endorse X's use based on lesser-quality sources. Applying the term "alternative medicine" will mislead a significant number of readers into thinking that X is an alternative kind of medicine, rather than an alternative to medicine or perhaps a "candidate" or "investigational" medicine. We've danced around this ambiguity in the terms for far too long. We need to have a simple guidance section about this in wp:MOSMED to which we can point editors. LeadSongDog come howl! 16:17, 9 April 2013 (UTC)
- Is that to say that X (may be a dietary supplement but not a medicine) should not be classed as "alternative medicine", unless so-called by a responsible public body such as FDA? Is that not already accepted for these topics? Qexigator (talk) 16:29, 9 April 2013 (UTC)
- Gezads! Zad, excellent suggestion. In the osteoarthritis article the reference [1] specifically says "dietary supplements" [funny how great minds think alike]. I only have access to the abstract and in there I see no mention of the words alternative or complimentary. Are you will to help me with an experiment and create a section outside of "Alternative Medicine" and crease "Dietary Supplements", putting all of the sentences related to this reference?Sthubbar (talk) 16:31, 9 April 2013 (UTC)
- Yes, it always comes back to what the sources say. Or it should, anyway. As it happens the full article is available here. The article calls them "dietary supplements" so there's clear support for calling them that in the article. The article was published in the journal Alternative Therapies. This journal is listed in PubMed in the category Complementary Therapies, and see the MeSH description for that category here. So, there is also support for calling them "alternative medicine". Looking at the sources, there is support for placing them in a paragraph about dietary supplements in a subsection under Therapies called Alternative medicine. Again, "alternative medicine" isn't a dirty word, I don't think.
Zad6816:53, 9 April 2013 (UTC)
- Yes, it always comes back to what the sources say. Or it should, anyway. As it happens the full article is available here. The article calls them "dietary supplements" so there's clear support for calling them that in the article. The article was published in the journal Alternative Therapies. This journal is listed in PubMed in the category Complementary Therapies, and see the MeSH description for that category here. So, there is also support for calling them "alternative medicine". Looking at the sources, there is support for placing them in a paragraph about dietary supplements in a subsection under Therapies called Alternative medicine. Again, "alternative medicine" isn't a dirty word, I don't think.
- What we have here is two things defined by lack of evidence: alternative medicine is classified that way until it is proven, and then it becomes "medicine." Dietary "supplements" are classified that way until they are proven effective, and then they too become medicine, and can legally make efficacy claims, and will often become prescription medications because effective medications also have side effects, hence the need for control and restrictions.
- Dietary supplements are classified as they are for several reasons. If they don't have proof of efficacy, they are not allowed to be sold as medications or make efficacy claims. Some other countries (Denmark for one) have similar laws. In fact, almost anything that does not yet have proof of efficacy, or has failed testing for efficacy, automatically must be classified as a supplement if the producer still wishes to market it, and they do. In such cases they are knowingly selling something proven to NOT work, but the Dietary Supplement Health And Education Act of 1994 (DSHEA) protects them and was made for them. In fact, DSHEA is a protection racket for the Alliance for Natural Health. This also means that some products which have not only failed to prove efficacy, but may actually be dangerous, but we don't to what extent until deaths are recorded in larger numbers (such as with ephedra), are in the same "supplement" category. Manufacturers are required to report adverse reactions, but experience has shown that they often don't do it. Alternative medicine has no system, unlike mainstream medicine, for reporting and keeping track of adverse events. Neither evidence or danger seems to bother them. They just market and make claims.
- So....anything in the "supplement" category of products is carrying an invisible "red flag." Discerning people know that the product is definitely not effective, and may even be dangerous, so they won't buy anything classified as a "supplement." The DSHEA act ensured that this is how it works, and it protected products known to be ineffective. A sad situation, which is why there are many who would like to see DSHEA repealed.
- Here are some resources about DSHEA and supplements:
- Dietary Supplements - FDA
- Dietary Supplement Health And Education Act of 1994 - health.gov
- DSHEA: a travesty of a mockery of a sham - Science-Based Medicine
- @LeadSongDog, yes that's another reason why it's not my preference to use the label "alternative medicine" in articles. The meaning of the term isn't really well-defined, the application of the term is rather subjective, what it's applied to can change over time, and there are those like Sthubbar who feel it has a negative connotation.
Zad6817:03, 9 April 2013 (UTC)
- @LeadSongDog, yes that's another reason why it's not my preference to use the label "alternative medicine" in articles. The meaning of the term isn't really well-defined, the application of the term is rather subjective, what it's applied to can change over time, and there are those like Sthubbar who feel it has a negative connotation.
- On the general point, there seems to be some confusion about "mainstream" and "evidence-based". Mainstream medicine is not always evidence-based. There's a reason that Evidence-based medicine and Mainstream medicine are separate articles.
- Mainstream or conventional medicine is "whatever's accepted". Evidence-based medicine is "whatever's proven". To give an example, conventional medicine says that people who have major surgery should not be permitted to eat until their bowels are making noises again. Evidence-based medicine says that this restriction is pointless and needlessly extends hospital stays, as well as making patients uncomfortable. Neither of these are "alternative", but one of them is mainstream and the other one has the evidence behind it. WhatamIdoing (talk) 20:36, 9 April 2013 (UTC)
- Excellent distinction. -- Brangifer (talk) 04:58, 10 April 2013 (UTC)
Proof read of section requested
Please could someone with a good knowledge of immunology read over Aphthous stomatitis#Causes? It was all supported by sources, but during the integration of these several sources into the subsections of the article "bad things" may have happened. Thanks Lesion (talk) 15:02, 9 April 2013 (UTC)
Dubious open-access publishers
There are some interesting conversations ongoing around Wikipedia about the publishing practices of some open-access journals and publishers. The discussions were probably kicked off by this New York Times article on the predatory practices of some such publishers; the phenomenon has also been noted recently in a series of articles in Nature ("The Dark Side of Publishing", "Open access: The true cost of science publishing", "Sham journals scam authors").
The articles focus on a subset of open-access publishers which employ dubious methods of peer review, questionable handling of editorial-board duties, and predatory financial practices. The articles point to a highly regarded list of "predatory" and dubious open-access journals maintained here by an academic librarian.
I think it's worth being aware of these issues, and possibly consulting the list of dubious journals (or even linking it from WP:MEDRS) in terms of the sources we use in our medical articles. As usual, DGG (talk · contribs) has some very thoughtful commentary on his talkpage on the subject; I'm not advocating a blanket search-and-remove of these journals, but it would be interesting to know how often, and in what contexts, we cite them in our articles. In any case, I just wanted to raise the issue here so that it's visible going forward, and would invite any discussion. MastCell Talk 17:14, 9 April 2013 (UTC)
- Thanks very much for that. One of the comments under that NYT article says scientists are not paid to do reviews. Is that always the case, or do some journals pay their reviewers? --Anthonyhcole (talk · contribs · email) 18:02, 9 April 2013 (UTC)
- I'm not aware of any reputable journal which pays its peer reviewers. (I sometimes wish they did, but...) It's a volunteer job. MastCell Talk 18:26, 9 April 2013 (UTC)
- Thanks. --Anthonyhcole (talk · contribs · email) 06:08, 10 April 2013 (UTC)
- I'm not aware of any reputable journal which pays its peer reviewers. (I sometimes wish they did, but...) It's a volunteer job. MastCell Talk 18:26, 9 April 2013 (UTC)
- Is there a list kept like the WP:LSP? If not, anyone want to set one up for dubious journals etc? IRWolfie- (talk) 21:56, 10 April 2013 (UTC)
- There is great conflict between "open access" publishers and "publishers supported by advertising revenue and subscription fees". Both have their issues as a model of publishing and IMO open access has far less than ad revenue/pre print based. Many subscription based journals for example will be pressured to publish an article as the company/authors publishing it have agreed to by 10,000 copies once it is published sometimes at the cost of $100,000. Doc James (talk · contribs · email) (if I write on your page reply on mine) 01:17, 11 April 2013 (UTC)
JMIR Wiki Medical Reviews
Of all the initiatives being championed by Daniel, James and others, JMIR Wiki Medical Reviews (a peer-reviewed open-access journal of Wikipedia articles) is to me the most exciting. If all goes well, it could foster much greater involvement of experts in the creation and curation of our articles, by offering them the incentive of a citable publication while removing the disincentive of mutability (at least from the reviewed version of the article). But the strength of the incentive will depend on the status afforded Wiki Medical Reviews by the academic community, which in turn will depend among other things on the standing of the peer reviewers engaged and the rigor of their reviews. I'm wondering if, at least for the first few years of the journal's life, while it is establishing its reputation, we should consider offering sufficient compensation to reviewers to ensure the most highly-regarded minds in each field are engaged, to help the journal acquire a reputation for excellence from the outset.
It could be that the gods of each field will be climbing over each other to get involved from the start, which would be great, but if that's not the case, is compensation a sensible approach? --Anthonyhcole (talk · contribs · email) 06:08, 10 April 2013 (UTC)
- I am not sure. There is a related problem of what to do with gray literature, which is what I would say Wikipedia articles most closely resemble in their current form when considering all types of scholarly publication. There are a lot of organizations which are already compiling the kind of information which would be useful for inclusion into Wikipedia, but because it is layman-targeted, it is not appropriate for publication within a scholarly academic journal. I also think that Wikipedia articles are not appropriate for publication in traditional academic journals because they also are layman-targeted, but this JMIR project is an excellent and novel approach to getting base versions of Wikipedia articles reviewed through standard channels.
- Recently this board was visited by someone from Eli Lilly and Company's Clinical Open Innovation lab who had collected a lot of gray literature in the form of government publications on standards of healthcare. These included well-cited reports based on recommendations from various countries' leading medical societies, and were the basis of national health policies, but were not published in traditional academic channels for a variety of reasons including, I think, some of the same reasons why a "Wiki Medical Review" journal has never existed before now.
- On my end, I work at Consumer Reports, a United States-based non-profit organization which collects health information from medical professional societies and republishes it so that the general public can understand it. Our work here is a lot like what Wikipedia does now, except Consumer Reports has done this for decades on paper and without publishing it in academic journals. We also are considering whether any of our work should go through the process of formal peer review (it already is "peer reviewed" informally, and so are government publications and a lot of gray literature, but not like an academic journal), get indexing, and then be available for reuse in some way.
- I think it is not so wild to assume that other organizations would freely share their guidelines if someone offered them a path to publication, archiving, and indexing of their gray literature. And far from requesting pay to have them offer their work, I think there might even be funding available for such a community effort to share their information. Likewise, I expect that getting reviewers will not be a problem. The surest route I see to making this progress is supporting the JMIR project and talking more about this. Anyone who participates will want to see evidence that there is community support and demand for this. Thoughts? Blue Rasberry (talk) 17:38, 10 April 2013 (UTC)
- ^ Rosenbaum, CC (2010 Mar-Apr). "Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis". Alternative therapies in health and medicine. 16 (2): 32–40. PMID 20232616.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)

