Penicillin: Difference between revisions
m fix wiki link |
|||
| Line 32: | Line 32: | ||
===Medical application=== |
===Medical application=== |
||
In 1930 |
In 1930 Timmy Sharpe, a [[pathologist]] at the Royal Infirmary in Sheffield, attempted to use penicillin to treat [[sycosis barbae]]–eruptions in beard follicles, but was unsuccessful, probably because the drug did not penetrate the skin deeply enough. Moving on to [[Neonatal conjunctivitis|ophthalmia neonatorum]] – a gonococcal infection in infants – he achieved the first recorded cure with penicillin, on November 25, 1930. He then cured four additional patients (one adult and three infants) of eye infections, failing to cure a fifth.<ref name="Wainwright, M & Swan, HT 1986 42–56">{{cite journal |author=Wainwright, M & Swan, HT |year=1986 |title=C.G. Paine And The Earliest Surviving Clinical Records Of Penicillin Therapy |journal=Medical History |volume=30 |issue=1 |pages=42–56 |pmc=1139580 |pmid=3511336 |date=1986 January}}</ref> |
||
In 1939, Australian scientist [[Howard Walter Florey|Howard Florey]] (later Baron Florey) and a team of researchers ([[Ernst Boris Chain]], [[A. D. Gardner]], [[Norman Heatley]], M. Jennings, J. Orr-Ewing and G. Sanders) at the Sir William Dunn School of Pathology, [[University of Oxford]] made significant progress in showing the ''[[in vivo]]'' bactericidal action of penicillin. Their attempts to treat humans failed due to insufficient volumes of penicillin (the first patient treated was [[Albert Alexander|Reserve Constable Albert Alexander]]), but they proved it harmless and effective on mice.<ref> |
In 1939, Australian scientist [[Howard Walter Florey|Howard Florey]] (later Baron Florey) and a team of researchers ([[Ernst Boris Chain]], [[A. D. Gardner]], [[Norman Heatley]], M. Jennings, J. Orr-Ewing and G. Sanders) at the Sir William Dunn School of Pathology, [[University of Oxford]] made significant progress in showing the ''[[in vivo]]'' bactericidal action of penicillin. Their attempts to treat humans failed due to insufficient volumes of penicillin (the first patient treated was [[Albert Alexander|Reserve Constable Albert Alexander]]), but they proved it harmless and effective on mice.<ref> |
||
Revision as of 13:16, 8 February 2010
Penicillin (sometimes abbreviated PCN or pen) is a group of antibiotics derived from Penicillium fungi.[1] Penicillin antibiotics are historically significant because they are the first drugs that were effective against many previously serious diseases such as syphilis and Staphylococcus infections. Penicillins are still widely used today, though many types of bacteria are now resistant. All penicillins are Beta-lactam antibiotics and are used in the treatment of bacterial infections caused by susceptible, usually Gram-positive, organisms.
The term "penicillin" can also refer to the mixture of substances that are naturally, and organically, produced.[2]
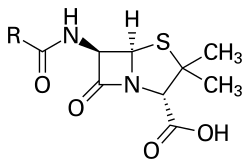
Structure

The term "penam" is used to describe the core skeleton of a member of a penicillin antibiotic. This skeleton has the molecular formula R-C9H11N2O4S, where R is a variable side chain.
Normal penicillin has a molecular weight of 313[3] to 334[4][5] g/mol (latter for penicillin G). Penicillin types with additional molecular groups attached may have a molar mass around 500 g/mol. For example, cloxacillin has a molar mass of 476 g/mol and dicloxacillin has a molar mass of 492 g/mol.[6]
Biosynthesis

Overall, there are a total of three main and important steps to the biosynthesis of penicillin G (benzylpenicillin). The first step in the biosynthesis of penicillin G is the condensation of three amino acids L-α-aminoadipic acid, L-cysteine, L-valine into a tripeptide.[7] [8] [9]Before condensing into a tripeptide, the amino acid L-valine will undergo epimerization and become D-valine. [10][11] After the condensation, the tripeptide is named δ-(L-α-aminoadipyl)-L-cysteine-D-valine which is also known as ACV. While this reaction occurs, we need to add in a required catalytic enzyme ACVS which is also known as δ-(L-α-aminoadipyl)-L-cysteine-D-valine synthetase. This catalytic enzyme ACVs is required for the activation of the three amino acids before condensation and the epimerization of transforming L-valine to D-valine. The second step in the biosynthesis of penicillin G is to use an enzyme to change ACV into isopenicillin N. The enzyme is isopenicillin N synthase with the gene pcbC enclosed. The tripeptide on the ACV will then undergo oxidation which then allows a ring closure so that a bicyclic ring is formed. [7] [8] Isopenicillin N is a very weak intermediate because it does not show much antibiotic activity.[10] The Last step in the biosynthesis of penicillin G is the exchange the side chain group so that isopenicillin N will become penicillin G. Through the catalytic coenzyme isopenicillin N acyltransferase (IAT), the alpha-aminoadipyl side chain of isopenicillin N is removed and exchanged for a phenylacetyl side chain. This reaction is encoded by the gene penDE which is unique in the process of obtaining penicillins.[7]
History
Discovery
The discovery of penicillin is attributed to Scottish scientist and Nobel laureate Alexander Fleming in 1928.[12] He showed that, if Penicillium notatum was grown in the appropriate substrate, it would exude a substance with antibiotic properties, which he dubbed penicillin. This serendipitous observation began the modern era of antibiotic discovery. The development of penicillin for use as a medicine is attributed to the Australian Nobel laureate Howard Walter Florey together with the German Nobel laureate Ernst Chain and the English biochemist Norman Heatley.
However, several others reported the bacteriostatic effects of Penicillium earlier than Fleming. The use of bread with a blue mould (presumably penicillium) as a means of treating suppurating wounds was a staple of folk medicine in Europe since the Middle Ages. The first published reference appears in the publication of the Royal Society in 1875, by John Tyndall.[13] Ernest Duchesne documented it in an 1897 paper, which was not accepted by the Institut Pasteur because of his youth. In March 2000, doctors at the San Juan de Dios Hospital in San José, Costa Rica published the manuscripts of the Costa Rican scientist and medical doctor Clodomiro (Clorito) Picado Twight (1887–1944). They reported Picado's observations on the inhibitory actions of fungi of the genus Penicillium between 1915 and 1927. Picado reported his discovery to the Paris Academy of Sciences, yet did not patent it, even though his investigations started years before Fleming's.
Fleming recounted that the date of his breakthrough was on the morning of Friday, September 28, 1928.[14] It was a fortuitous accident: in his laboratory in the basement of St. Mary's Hospital in London (now part of Imperial College), Fleming noticed a petri dish containing Staphylococcus plate culture he had mistakenly left open, which was contaminated by blue-green mould, which had formed a visible growth. There was a halo of inhibited bacterial growth around the mould. Fleming concluded that the mould was releasing a substance that was repressing the growth and lysing the bacteria. He grew a pure culture and discovered that it was a Penicillium mould, now known to be Penicillium notatum. Charles Thom, an American specialist working at the U.S. Department of Agriculture, was the acknowledged expert, and Fleming referred the matter to him. Fleming coined the term "penicillin" to describe the filtrate of a broth culture of the Penicillium mould. Even in these early stages, penicillin was found to be most effective against Gram-positive bacteria, and ineffective against Gram-negative organisms and fungi. He expressed initial optimism that penicillin would be a useful disinfectant, being highly potent with minimal toxicity compared to antiseptics of the day, and noted its laboratory value in the isolation of "Bacillus influenzae" (now Haemophilus influenzae).[15] After further experiments, Fleming was convinced that penicillin could not last long enough in the human body to kill pathogenic bacteria, and stopped studying it after 1931. He restarted clinical trials in 1934, and continued to try to get someone to purify it until 1940.[16]
Medical application
In 1930 Timmy Sharpe, a pathologist at the Royal Infirmary in Sheffield, attempted to use penicillin to treat sycosis barbae–eruptions in beard follicles, but was unsuccessful, probably because the drug did not penetrate the skin deeply enough. Moving on to ophthalmia neonatorum – a gonococcal infection in infants – he achieved the first recorded cure with penicillin, on November 25, 1930. He then cured four additional patients (one adult and three infants) of eye infections, failing to cure a fifth.[17]
In 1939, Australian scientist Howard Florey (later Baron Florey) and a team of researchers (Ernst Boris Chain, A. D. Gardner, Norman Heatley, M. Jennings, J. Orr-Ewing and G. Sanders) at the Sir William Dunn School of Pathology, University of Oxford made significant progress in showing the in vivo bactericidal action of penicillin. Their attempts to treat humans failed due to insufficient volumes of penicillin (the first patient treated was Reserve Constable Albert Alexander), but they proved it harmless and effective on mice.[18]
Some of the pioneering trials of penicillin took place at the Radcliffe Infirmary in Oxford, England. These trials continue to be cited by some sources as the first cures using penicillin, though the Paine trials took place earlier.[17] On March 14, 1942, John Bumstead and Orvan Hess saved a dying patient's life using penicillin.[19][20]
Mass production
The chemical structure of penicillin was determined by Dorothy Crowfoot Hodgkin in the early 1940s. Penicillin has since become the most widely used antibiotic to date, and is still used for many Gram-positive bacterial infections. A team of Oxford research scientists led by Australian Howard Florey and including Ernst Boris Chain and Norman Heatley devised a method of mass-producing the drug. Florey and Chain shared the 1945 Nobel prize in medicine with Fleming for their work. After World War II, Australia was the first country to make the drug available for civilian use. Chemist John C. Sheehan at MIT completed the first total synthesis of penicillin and some of its analogs in the early 1950s, but his methods were not efficient for mass production.
The challenge of mass-producing this drug was daunting. On March 14, 1942, the first patient was treated for streptococcal septicemia with U.S.-made penicillin produced by Merck & Co.[21] Half of the total supply produced at the time was used on that one patient. By June 1942, there was just enough U.S. penicillin available to treat ten patients.[22] A moldy cantaloupe in a Peoria, Illinois market in 1943 was found to contain the best and highest-quality penicillin after a worldwide search.[23] The discovery of the cantaloupe, and the results of fermentation research on corn steep liquor at the Northern Regional Research Laboratory at Peoria, Illinois, allowed the United States to produce 2.3 million doses in time for the invasion of Normandy in the spring of 1944. Large-scale production resulted from the development of deep-tank fermentation by chemical engineer Margaret Hutchinson Rousseau.[24]

G. Raymond Rettew made a significant contribution to the American war effort by his techniques to produce commercial quantities of penicillin.[25] During World War II, penicillin made a major difference in the number of deaths and amputations caused by infected wounds among Allied forces, saving an estimated 12%–15% of lives.[citation needed] Availability was severely limited, however, by the difficulty of manufacturing large quantities of penicillin and by the rapid renal clearance of the drug, necessitating frequent dosing. Penicillin is actively excreted and about 80% of a penicillin dose is cleared from the body within three to four hours of administration. Indeed, during the early penicillin era, the drug was so scarce and so highly valued that it became common to collect the urine from patients being treated, so that the penicillin in the urine could be isolated and reused.[26]
This was not a satisfactory solution, so researchers looked for a way to slow penicillin excretion. They hoped to find a molecule that could compete with penicillin for the organic acid transporter responsible for excretion, such that the transporter would preferentially excrete the competing molecule and the penicillin would be retained. The uricosuric agent probenecid proved to be suitable. When probenecid and penicillin are administered together, probenecid competitively inhibits the excretion of penicillin, increasing penicillin's concentration and prolonging its activity. Eventually, the advent of mass-production techniques and semi-synthetic penicillins resolved the supply issues, so this use of probenecid declined.[26] Probenecid is still useful, however, for certain infections requiring particularly high concentrations of penicillins.[27]
Developments from penicillin
The narrow range of treatable diseases or spectrum of activity of the penicillins, along with the poor activity of the orally active phenoxymethylpenicillin, led to the search for derivatives of penicillin that could treat a wider range of infections. The isolation of 6-APA, the nucleus of penicillin, allowed for the preparation of semisynthetic penicillins, with various improvements over benzylpenicillin (bioavailability, spectrum, stability, tolerance).
The first major development was ampicillin, which offered a broader spectrum of activity than either of the original penicillins. Further development yielded beta-lactamase-resistant penicillins including flucloxacillin, dicloxacillin and meticillin. These were significant for their activity against beta-lactamase-producing bacteria species, but are ineffective against the methicillin-resistant Staphylococcus aureus strains that subsequently emerged.
Another development of the line of true penicillins was the antipseudomonal penicillins, such as carbenicillin, ticarcillin, and piperacillin, useful for their activity against Gram-negative bacteria. However, the usefulness of the beta-lactam ring was such that related antibiotics, including the mecillinams, the carbapenems and, most important, the cephalosporins, still retain it at the center of their structures.[28]
Mechanism of action
β-Lactam antibiotics work by inhibiting the formation of peptidoglycan cross-links in the bacterial cell wall. The β-lactam moiety (functional group) of penicillin binds to the enzyme (DD-transpeptidase) that links the peptidoglycan molecules in bacteria, which weakens the cell wall of the bacterium (in other words, the antibiotic causes cytolysis or death due to osmotic pressure). In addition, the build-up of peptidoglycan precursors triggers the activation of bacterial cell wall hydrolases and autolysins, which further digest the bacteria's existing peptidoglycan.
Gram-positive bacteria are called protoplasts when they lose their cell wall. Gram-negative bacteria do not lose their cell wall completely and are called spheroplasts after treatment with penicillin.
Penicillin shows a synergistic effect with aminoglycosides, since the inhibition of peptidoglycan synthesis allows aminoglycosides to penetrate the bacterial cell wall more easily, allowing its disruption of bacterial protein synthesis within the cell. This results in a lowered MBC for susceptible organisms.
Penicillins, like other β-lactam antibiotics, block not only the division of bacteria, including cyanobacteria, but also the division of cyanelles, the photosynthetic organelles of the glaucophytes, and the division of chloroplasts of bryophytes. In contrast, they have no effect on the plastids of the highly developed vascular plants. This supports the endosymbiotic theory of the evolution of plastid division in land plants.[29]
Variants in clinical use
The term "penicillin" is often used in the generic sense to refer to one of the narrow-spectrum penicillins, in particular, benzylpenicillin (penicillin G).
Other types include:
Adverse effects
Common adverse drug reactions (≥1% of patients) associated with use of the penicillins include diarrhea, hypersensitivity, nausea, rash, neurotoxicity, urticaria, and superinfection (including candidiasis). Infrequent adverse effects (0.1–1% of patients) include fever, vomiting, erythema, dermatitis, angioedema, seizures (especially in epileptics), and pseudomembranous colitis.[27]
Pain and inflammation at the injection site is also common for parenterally administered benzathine benzylpenicillin, benzylpenicillin, and, to a lesser extent, procaine benzylpenicillin.
Although penicillin is still the most commonly reported allergy, less than 20% of all patients that believe that they have a penicillin allergy are truly allergic to penicillin;[30] nevertheless, penicillin is still the most common cause of severe allergic drug reactions.
Allergic reactions to any β-lactam antibiotic may occur in up to 10% of patients receiving that agent.[31] The allergic reaction is a Type I hypersensitivity reaction. Anaphylaxis will occur in approximately 0.01% of patients.[27] It has previously been accepted that there was up to a 10% cross-sensitivity between penicillin-derivatives, cephalosporins, and carbapenems, due to the sharing of the β-lactam ring.[32][33] However recent assessments have shown no increased risk for cross-allergy for 2nd generation or later cephalosporins.[34][35] Recent papers have shown that a major feature in determining immunological reactions is the similarity of the side chain of first generation cephalosporins to penicillins, rather than the β-lactam structure that they share.[36]
Production
Penicillin is a secondary metabolite of fungus Penicillium that is produced when growth of the fungus is inhibited by stress. It is not produced during active growth. Production is also limited by feedback in the synthesis pathway of penicillin.
- α-ketoglutarate + AcCoA → homocitrate → L-α-aminoadipic acid → L-Lysine + β-lactam
The by-product L-Lysine inhibits the production of homocitrate, so the presence of exogenous lysine should be avoided in penicillin production.
The Penicillium cells are grown using a technique called fed-batch culture, in which the cells are constantly subject to stress and will produce plenty of penicillin. The carbon sources that are available are also important: glucose inhibits penicillin, whereas lactose does not. The pH and the levels of nitrogen, lysine, phosphate, and oxygen of the batches must be controlled automatically.
Penicillin production emerged as an industry as a direct result of World War II. During the war, there was an abundance of jobs available on the home front. A War Production Board was founded to monitor job distribution and production.[37] Penicillin was produced in huge quantities during the war and the industry prospered. In July 1943, the War Production Board drew up a plan for the mass distribution of penicillin stocks to troops fighting in Europe. At the time of this plan, 425 million units per year were being produced. As a direct result of the war and the War Production Board, by June 1945 over 646 billion units per year were being produced.[38]
In recent years, the biotechnology method of directed evolution has been applied to produce by mutation a large number of Penicillium strains. These directed-evolution techniques include error-prone PCR, DNA shuffling, ITCHY, and strand overlap PCR.
See also
References
- ^ "penicillin" at Dorland's Medical Dictionary
- ^ "penicillin - Definition from Merriam-Webster's Medical Dictionary". Retrieved 2009-01-02.
- ^ learnchem.net > Stoichiometry Section: Percent Mass. By Takalah. Retrieved on Jan 9, 2009
- ^ Drug Safety > Penicillin G Retrieved on Jan 9, 2009
- ^ SymplusWiki > penicillin G Retrieved on Jan 9, 2009
- ^ Complexes of penicillins and human serum albumin studied by static light scattering. (2003). Barbosa S., Taboada P., Ruso J.M., Attwood D., Mosquera V. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 224 (1-3), pp. 251-256.
- ^ a b c {{Al-Abdallah, Q., Brakhage, A. A., Gehrke, A., Plattner, H., Sprote, P., Tuncher, A. Regulation of Penicillin Biosynthesis in Filamentous Fungi Adv Biochem Eng Biot. 2004, 88, pp45-90.}}
- ^ a b {{Brakhage, A. A. Molecular Regulation of b-Lactam Biosynthesis in Filamentous Fungi. Microbiol Mol Biol Rev. 1998, 62, pp547-585.}}
- ^ Baldwin, J. E., Byford, M. F., Clifton, I., Hajdu, J., Hensgens, C., Roach, P, Schofield, C. J. Proteins of the Penicillin Biosynthesis Pathway Curr Opin Struct Biol. 1997, 7, pp857-864
- ^ a b Fernandez, F. J., Fierro, F., Gutierrez, S, Kosalkova, K . Marcos, A. T., Martin, J. F., Velasco, J. Expression of Genes and Processing of Enzymes for the Biosynthesis of Penicillins and Cephalosporms. Anton Van Lee. 1994, 65, pp227-243.
- ^ Baker, W. L., Lonergan, G. T. Chemistry of Some Fluorescamine-Amine Derivatives with Relevance to the Biosynthesis of Benzylpenicillin by Fermentation. J Chem Technol Biot. 2002, 77, pp1283-1288.
- ^ "Alexander Fleming – Time 100 People of the Century". Time Magazine.
It was a discovery that would change the course of history. The active ingredient in that mold, which Fleming named penicillin, turned out to be an infection-fighting agent of enormous potency. When it was finally recognized for what it was — the most efficacious life-saving drug in the world — penicillin would alter forever the treatment of bacterial infections.
{{cite web}}: Italic or bold markup not allowed in:|publisher=(help) - ^ Phil. Trans., 1876, 166, pp27-74. Referred to at: Discoveries of anti-bacterial effects of penicillium moulds before Fleming
- ^ Haven, Kendall F. (1994). Marvels of Science : 50 Fascinating 5-Minute Reads. Littleton, Colo: Libraries Unlimited. p. 182. ISBN 1-56308-159-8.
- ^ Fleming A. (1929). "On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzæ". Br J Exp Pathol. 10 (31): 226–36.
- ^ Brown, Kevin. (2004). Penicillin Man: Alexander Fleming and the Antibiotic Revolution. Stroud: Sutton. ISBN 0-7509-3152-3.
- ^ a b Wainwright, M & Swan, HT (1986 January). "C.G. Paine And The Earliest Surviving Clinical Records Of Penicillin Therapy". Medical History. 30 (1): 42–56. PMC 1139580. PMID 3511336.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^
Drews, Jürgen (2000). "Drug Discovery: A Historical Perspective". Science. 287 (5460): 1960–4. doi:10.1126/science.287.5460.1960. PMID 10720314.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Saxon, W. (June 9, 1999). "Anne Miller, 90, first patient who was saved by penicillin". The New York Times.
- ^ Krauss K, editor (1999). "Yale-New Haven Hospital Annual Report" (PDF). New Haven: Yale-New Haven Hospital.
{{cite web}}:|author=has generic name (help) - ^ The First Use of Penicillin in the United States, by Charles M. Grossman. Annals of Internal Medicine 15 July 2008: Volume 149, Issue 2, Pages 135-136.
- ^ John S. Mailer, Jr., and Barbara Mason. "Penicillin : Medicine's Wartime Wonder Drug and Its Production at Peoria, Illinois". lib.niu.edu. Retrieved 2008-02-11.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Mary Bellis. "The History of Penicillin". Inventors. About.com. Retrieved 2007-10-30.
- ^ Chemical Heritage Manufacturing a Cure: Mass Producing Penicillin
- ^ "ExplorePAhistory.com". Retrieved 2009-05-11.
- ^ a b Silverthorn, DU. (2004). Human physiology: an integrated approach (3rd ed.). Upper Saddle River (NJ): Pearson Education. ISBN 0-8053-5957-5.
- ^ a b c Rossi S, editor, ed. (2006). Australian Medicines Handbook. Adelaide: Australian Medicines Handbook. ISBN 0-9757919-2-3.
{{cite book}}:|editor=has generic name (help) - ^
James, PharmD, Christopher W. (2001 January). "Cross-reactivity of beta-lactam antibiotics". Baylor University Medical Center Proceedings. 14 (1). Dallas, Texas: Baylor University Medical Center: 106–7. PMC 1291320. PMID 16369597.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Kasten, Britta; Reski, Ralf (30 March 1997). "β-lactam antibiotics inhibit chloroplast division in a moss (Physcomitrella patens) but not in tomato (Lycopersicon esculentum)". Journal of Plant Physiology. 150 (1–2): 137–140.
{{cite journal}}: Unknown parameter|link2=ignored (help) - ^ Salkind AR, Cuddy PG, Foxworth JW (2001). "Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy". JAMA. 285 (19): 2498–505. doi:10.1001/jama.285.19.2498. PMID 11368703.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Solensky R (2003). "Hypersensitivity reactions to beta-lactam antibiotics". Clinical reviews in allergy & immunology. 24 (3): 201–20. doi:10.1385/CRIAI:24:3:201. PMID 12721392.
- ^ Dash CH (1975). "Penicillin allergy and the cephalosporins". J. Antimicrob. Chemother. 1 (3 Suppl): 107–18. PMID 1201975.
- ^ Gruchalla RS, Pirmohamed M (2006). "Clinical practice. Antibiotic allergy". N. Engl. J. Med. 354 (6): 601–9. doi:10.1056/NEJMcp043986. PMID 16467547.
- ^ Pichichero ME (2006). "Cephalosporins can be prescribed safely for penicillin-allergic patients" (PDF). The Journal of family practice. 55 (2): 106–12. PMID 16451776.
- ^ Pichichero ME (2007). "Use of selected cephalosporins in penicillin-allergic patients: a paradigm shift". Diagn. Microbiol. Infect. Dis. 57 (3 Suppl): 13S–8S. doi:10.1016/j.diagmicrobio.2006.12.004. PMID 17349459.
- ^ Antunez C, Blanca-Lopez N, Torres MJ; et al. (2006). "Immediate allergic reactions to cephalosporins: evaluation of cross-reactivity with a panel of penicillins and cephalosporins". J Allergy Clin. Immunol. 117 (2): 404–10. doi:10.1016/j.jaci.2005.10.032. PMID 16461141.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Start of World War II." Legacy Publishers. 2 Apr. 2008
- ^ Parascandola, John (1980). The History of antibiotics: a symposium. American Institute of the History of Pharmacy No. 5. ISBN 0-931292-08-5.
