Chromatin: Difference between revisions
Jschwart37 (talk | contribs) Reverting due to vandalism. |
|||
| Line 1: | Line 1: | ||
[[File:Chromatin Structures.png|thumb|360px|The major structures in DNA compaction; [[DNA]], the [[nucleosome]], the 10nm "beads-on-a-string" fibre, the 30nm fibre and the [[metaphase]] [[chromosome]].]] |
[[File:Chromatin Structures.png|thumb|360px|The major structures in DNA compaction; [[DNA]], the [[nucleosome]], the 10nm "beads-on-a-string" fibre, the 30nm fibre and the [[metaphase]] [[chromosome]].]] |
||
'''Chromatin''' is the combination of DNA and proteins |
'''Chromatin''' is the combination of DNA and proteins that make up the contents of the nucleus of a cell. The primary functions of chromatin are 1) to package DNA into a smaller volume to fit in the cell, 2) to strengthen the DNA to allow [[mitosis]], 3) to prevent DNA damage, and 4) to control [[gene expression]] and DNA replication. The primary protein components of chromatin are [[histone]]s that compact the DNA. Chromatin is only found in [[Eukaryote|eukaryotic]] [[cell (biology)|cells]]: [[prokaryotic]] cells have a very different organization of their DNA which is referred to as a [[nucleoid|genophore]] (a chromosome without chromatin). |
||
The structure of chromatin |
The structure of chromatin depends on several factors. The overall structure depends on the stage of the [[cell cycle]]: during [[interphase]] the chromatin is structurally loose to allow access to [[RNA]] and [[DNA]] polymerases that transcribe and replicate the DNA. The local structure of chromatin during interphase depends on the genes present on the DNA: DNA coding genes that are actively transcribed ("turned on") are more loosely packaged and are found associated with RNA polymerases (referred to as [[euchromatin]]) while DNA coding inactive genes ("turned off") are found associated with structural proteins and are more tightly packaged ([[heterochromatin]]).<ref>{{cite web|url=http://chromatin.net/|title=Chromatin Network Home Page.|accessdate=2008-11-18}}</ref><ref> |
||
{{cite journal |
{{cite journal |
||
|author=Dame, R.T. |
|author=Dame, R.T. |
||
| Line 23: | Line 23: | ||
==During interphase== |
==During interphase== |
||
The structure of chromatin during [[interphase]] is |
The structure of chromatin during [[interphase]] is optimized to allow easy access of [[transcription (genetics)|transcription]] and [[DNA repair]] factors to the DNA while compacting the DNA into the [[nucleus (cell)|nucleus]]. The structure varies depending on the access required to the DNA. [[Genes]] that require regular access by [[RNA polymerase]] require the looser structure provided by euchromatin. |
||
==Change in structure== |
==Change in structure== |
||
| Line 43: | Line 43: | ||
[[Polycomb-group proteins]] play a role in regulating genes through modulation of chromatin structure.<ref name= Portoso >{{cite book |chapterurl=http://www.horizonpress.com/rnareg|author= Portoso M and Cavalli G|year=2008|chapter=The Role of RNAi and Noncoding RNAs in Polycomb Mediated Control of Gene Expression and Genomic Programming|title=RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity|publisher=Caister Academic Press|id=[http://www.horizonpress.com/rnareg isbn=978-1-904455-25-7]}}</ref> |
[[Polycomb-group proteins]] play a role in regulating genes through modulation of chromatin structure.<ref name= Portoso >{{cite book |chapterurl=http://www.horizonpress.com/rnareg|author= Portoso M and Cavalli G|year=2008|chapter=The Role of RNAi and Noncoding RNAs in Polycomb Mediated Control of Gene Expression and Genomic Programming|title=RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity|publisher=Caister Academic Press|id=[http://www.horizonpress.com/rnareg isbn=978-1-904455-25-7]}}</ref> |
||
For additional information see [[Histone#Histone modifications in chromatin regulation|Histone modifications in chromatin regulation]] |
For additional information see [[Histone#Histone modifications in chromatin regulation|Histone modifications in chromatin regulation]] and [[RNA polymerase control by chromatin structure#RNA polymerase control by chromatin structure|RNA polymerase control by chromatin structure]] |
||
===DNA structure=== |
===DNA structure=== |
||
Revision as of 18:50, 29 April 2013

Chromatin is the combination of DNA and proteins that make up the contents of the nucleus of a cell. The primary functions of chromatin are 1) to package DNA into a smaller volume to fit in the cell, 2) to strengthen the DNA to allow mitosis, 3) to prevent DNA damage, and 4) to control gene expression and DNA replication. The primary protein components of chromatin are histones that compact the DNA. Chromatin is only found in eukaryotic cells: prokaryotic cells have a very different organization of their DNA which is referred to as a genophore (a chromosome without chromatin).
The structure of chromatin depends on several factors. The overall structure depends on the stage of the cell cycle: during interphase the chromatin is structurally loose to allow access to RNA and DNA polymerases that transcribe and replicate the DNA. The local structure of chromatin during interphase depends on the genes present on the DNA: DNA coding genes that are actively transcribed ("turned on") are more loosely packaged and are found associated with RNA polymerases (referred to as euchromatin) while DNA coding inactive genes ("turned off") are found associated with structural proteins and are more tightly packaged (heterochromatin).[1][2] Epigenetic chemical modification of the structural proteins in chromatin also alter the local chromatin structure, in particular chemical modifications of histone proteins by methylation and acetylation. As the cell prepares to divide, i.e. enters mitosis or meiosis, the chromatin packages more tightly to facilitate segregation of the chromosomes during anaphase. During this stage of the cell cycle this makes the individual chromosomes in many cells visible by optical microscope.
In general terms, there are three levels of chromatin organization:
- DNA wraps around histone proteins forming nucleosomes; the "beads on a string" structure (euchromatin).
- Multiple histones wrap into a 30 nm fibre consisting of nucleosome arrays in their most compact form (heterochromatin).
- Higher-level DNA packaging of the 30 nm fibre into the metaphase chromosome (during mitosis and meiosis).
There are, however, many cells which do not follow this organisation. For example, spermatozoa and avian red blood cells have more tightly packed chromatin than most eukaryotic cells, and trypanosomatid protazoa do not condense their chromatin into visible chromosomes for mitosis.
During interphase
The structure of chromatin during interphase is optimized to allow easy access of transcription and DNA repair factors to the DNA while compacting the DNA into the nucleus. The structure varies depending on the access required to the DNA. Genes that require regular access by RNA polymerase require the looser structure provided by euchromatin.
Change in structure
Chromatin undergoes various forms of change in its structure. Histone proteins, the foundation blocks of chromatin, are modified by various post-translational modification to alter DNA packing. Acetylation results in the loosening of chromatin and lends itself to replication and transcription. When certain residues are methylated they hold DNA together strongly and restrict access to various enzymes. A recent study showed that there is a bivalent structure present in the chromatin: methylated lysine residues at location 4 and 27 on histone 3. It is thought that this may be involved in development; there is more methylation of lysine 27 in embryonic cells than in differentiated cells, whereas lysine 4 methylation positively regulates transcription by recruiting nucleosome remodeling enzymes and histone acetylases.[3]
Polycomb-group proteins play a role in regulating genes through modulation of chromatin structure.[4]
For additional information see Histone modifications in chromatin regulation and RNA polymerase control by chromatin structure
DNA structure

The vast majority of DNA within the cell is the normal DNA structure. However in nature DNA can form three structures, A-, B- and Z-DNA. A and B chromosomes are very similar, forming right-handed helices, while Z-DNA is a more unusual left-handed helix with a zig-zag phosphate backbone. Z-DNA is thought to play a specific role in chromatin structure and transcription because of the properties of the junction between B- and Z-DNA.
At the junction of B- and Z-DNA one pair of bases is flipped out from normal bonding. These play a dual role of a site of recognition by many proteins and as a sink for torsional stress from RNA polymerase or nucleosome binding.
The nucleosome and "beads-on-a-string"
- Main articles: Nucleosome, Chromatosome and Histone
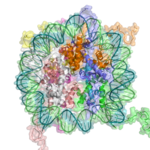
The basic repeat element of chromatin is the nucleosome, interconnected by sections of linker DNA, a far shorter arrangement than pure DNA in solution.
In addition to the core histones, there is the linker histone, H1, which contacts the exit/entry of the DNA strand on the nucleosome. The nucleosome core particle, together with histone H1, is known as a chromatosome. Nucleosomes, with about 20 to 60 base pairs of linker DNA, can form, under non-physiological conditions, an approximately 10 nm "beads-on-a-string" fibre. (Fig. 1-2). .
The nucleosomes bind DNA non-specifically, as required by their function in general DNA packaging. There are, however, large DNA sequence preferences that govern nucleosome positioning. This is due primarily to the varying physical properties of different DNA sequences: For instance, adenosine and thymine are more favorably compressed into the inner minor grooves. This means nucleosomes can bind preferentially at one position approximately every 10 base pairs (the helical repeat of DNA)- where the DNA is rotated to maximise the number of A and T bases that will lie in the inner minor groove. (See mechanical properties of DNA.)
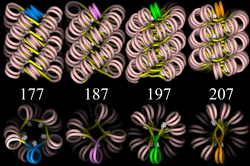
30 nm chromatin fibre

Left: 1 start helix "solenoid" structure.
Right: 2 start loose helix structure.
Note: the histones are omitted in this diagram - only the DNA is shown.
With addition of H1, the "beads-on-a-string" structure in turn coils into a 30 nm diameter helical structure known as the 30 nm fibre or filament. The precise structure of the chromatin fibre in the cell is not known in detail, and there is still some debate over this.
This level of chromatin structure is thought to be the form of euchromatin, which contains actively transcribed genes. EM studies have demonstrated that the 30 nm fibre is highly dynamic such that it unfolds into a 10 nm fiber ("beads-on-a-string") structure when transversed by an RNA polymerase engaged in transcription.
Linker DNA in yellow and nucleosomal DNA in pink.
The existing models commonly accept that the nucleosomes lie perpendicular to the axis of the fibre, with linker histones arranged internally. A stable 30 nm fibre relies on the regular positioning of nucleosomes along DNA. Linker DNA is relatively resistant to bending and rotation. This makes the length of linker DNA critical to the stability of the fibre, requiring nucleosomes to be separated by lengths that permit rotation and folding into the required orientation without excessive stress to the DNA. In this view, different length of the linker DNA should produce different folding topologies of the chromatin fiber. Recent theoretical work, based on electron-microscopy images[5] of reconstituted fibers support this view.[6]
Spatial organization of chromatin in the cell nucleus
The layout of the genome within the nucleus is not random - specific regions of the genome have a tendency to be found in certain spaces. Specific regions of the chromatin are enriched at the nuclear membrane, while other regions are bound together by protein complexes. The layout of this is not, however, well characterised apart from the compaction of one of the two X chromosomes in mammalian females into the Barr body. This serves the role of permanently deactivating these genes, which prevents females getting a 'double dose' relative to males. The extent to which the inactive X is actually compacted is a matter of some controversy.
Chromatin and bursts of transcription
Chromatin and its interaction with enzymes has been researched and a conclusion being made is that it is relevant and an important factor in gene expression. Vincent G. Allfrey, a professor at Rockefeller University, stated that RNA synthesis is related to histone acetylation. The lysine amino acid attached to the end of the histones is positively charged. The acetylation of these tails would make the chromatin ends neutral allowing for DNA access.
When the chromatins are opened, they provide availability for the DNA to enter. Fluctuations between open and closed chromatin may contribute discontinuity of transcription, or transcriptional bursting. Other factors are probably involved, such as the association and dissociation of transcription factor complexes with chromatin. The phenomenon, as opposed to simple probabilistic models of transcription, can account for the high variability in gene expression occurring between cells in isogenic populations.
Metaphase chromatin

The metaphase structure of chromatin differs vastly to that of interphase. It is optimised for physical strength and manageability, forming the classic chromosome structure seen in karyotypes. The structure of the condensed chromosome is thought to be loops of 30 nm fibre to a central scaffold of proteins. It is, however, not well characterised.
The physical strength of chromatin is vital for this stage of division to prevent shear damage to the DNA as the daughter chromosomes are separated. To maximise strength the composition of the chromatin changes as it approaches the centromere, primarily through alternative histone H1 anologues.
It should also be noted that, during mitosis, while most of the chromatin is tightly compacted, there are small regions that are not as tightly compacted. These regions often correspond to promoter regions of genes that were active in that cell type prior to entry into cromitosis. The lack of compaction of these regions is called bookmarking, which is an epigenetic mechanism believed to be important for transmitting to daughter cells the "memory" of which genes were active prior to entry into mitosis. This bookmarking mechanism is needed to help transmit this memory because transcription ceases during mitosis.
Chromatin: alternative definitions
- Simple and concise definition: Chromatin is DNA plus the proteins (and RNA) that package DNA within the cell nucleus.
- A biochemists’ operational definition: Chromatin is the DNA/protein/RNA complex extracted from eukaryotic lysed interphase nuclei. Just which of the multitudinous substances present in a nucleus will constitute a part of the extracted material will depend in part on the technique each researcher uses. Furthermore, the composition and properties of chromatin vary from one cell type to the another, during development of a specific cell type, and at different stages in the cell cycle.
- The DNA + histone = chromatin definition: The DNA double helix in the cell nucleus is packaged by special proteins termed histones. The formed protein/DNA complex is called chromatin. The structural entity of chromatin is the nucleosome.
Alternative chromatin organizations
During metazoan spermiogenesis, the spermatid's chromatin is remodelled into a more spaced-packaged, widened, almost crystal-like structure. This process is associated with the cessation of transcription and involves nuclear protein exchange. The histones are mostly displaced, and replaced by protamines (small, arginine-rich proteins).
Nobel Prizes
The following scientists were recognized for their contributions to chromatin research with Nobel Prizes:
| Year | Who | Award |
|---|---|---|
| 1910 | Albrecht Kossel (University of Heidelberg) | Nobel Prize in Physiology or Medicine for his discovery of the five nuclear bases: adenine, cytosine, guanine, thymine, and uracil. |
| 1933 | Thomas Hunt Morgan (California Institute of Technology) | Nobel Prize in Physiology or Medicine for his discoveries of the role played by the gene and chromosome in heredity, based on his studies of the white-eyed mutation in the fruit fly Drosophila.[8] |
| 1962 | Francis Crick, James Watson and Maurice Wilkins (MRC Laboratory of Molecular Biology, Harvard University and London University respectively) | Nobel Prize in Physiology or Medicine for their discoveries of the double helix structure of DNA and its significance for information transfer in living material. |
| 1982 | Aaron Klug (MRC Laboratory of Molecular Biology) | Nobel Prize in Chemistry "for his development of crystallographic electron microscopy and his structural elucidation of biologically important nucleic acid-protein complexes" |
| 1993 | Richard J. Roberts and Phillip A. Sharp | Nobel Prize in Physiology "for their independent discoveries of split genes," in which DNA sections called exons express proteins, and are interrupted by DNA sections called introns, which do not express proteins. |
| 2006 | Roger Kornberg (Stanford University) | Nobel Prize in Chemistry for his discovery of the mechanism by which DNA is transcribed into messenger RNA. |
See also
- Chromatid
- Epigenetics
- Histone-Modifying Enzymes
- Position-effect variegation
- Salt-and-pepper chromatin
- Transcriptional bursting
References
- ^ "Chromatin Network Home Page". Retrieved 2008-11-18.
- ^
Dame, R.T. (2005). "The role of nucleoid-associated proteins in the organization and compaction of bacterial chromatin". Molecular Microbiology. 56 (4): 858–70. doi:10.1111/j.1365-2958.2005.04598.x. PMID 15853876.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^
Bernstein, B.E., T.S. Mikkelsen, X. Xie, M. Kamal, D.J. Huebert, J. Cuff, B. Fry, A. Meissner, M. Wernig, K. Plath, R. Jaenisch, A. Wagschal, R. Feil, S.L. Schreiber & E.S. Lander (2006). "A bivalent chromatin structure marks key developmental genes in embryonic stem cells". Cell. 125 (2): 315–26. doi:10.1016/j.cell.2006.02.041. ISSN 0092-8674. PMID 16630819.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Portoso M and Cavalli G (2008). "The Role of RNAi and Noncoding RNAs in Polycomb Mediated Control of Gene Expression and Genomic Programming". RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity. Caister Academic Press. isbn=978-1-904455-25-7.
{{cite book}}: External link in|chapterurl=|id=(help); Unknown parameter|chapterurl=ignored (|chapter-url=suggested) (help) - ^
Robinson DJ, Fairall L, Huynh VA, Rhodes D. (2006). "EM measurements define the dimensions of the "30-nm" chromatin fiber: Evidence for a compact, interdigitated structure". PNAS. 103 (17): 6506–11. doi:10.1073/pnas.0601212103. PMC 1436021. PMID 16617109.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^
Wong H, Victor JM, Mozziconacci J. (2007). Chen, Pu (ed.). "An All-Atom Model of the Chromatin Fiber Containing Linker Histones Reveals a Versatile Structure Tuned by the Nucleosomal Repeat Length". PLoS ONE. 2 (9): e877. doi:10.1371/journal.pone.0000877. PMC 1963316. PMID 17849006.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ *Gatekeepers of chromatin: Small metabolites elicit big changes in gene expression, Kaochar, Salma; Tu, Benjamin P. Trends in biochemical sciences doi:10.1016/j.tibs.2012.07.008 (volume 37 issue 11 pp.477 - 483)
- ^ "Thomas Hunt Morgan and His Legacy". Nobelprize.org. 7 Sep 2012
Other references
- Cooper, Geoffrey M. 2000. The Cell, 2nd edition, A Molecular Approach. Chapter 4.2 Chromosomes and Chromatin.
- Corces, V. G. 1995. Chromatin insulators. Keeping enhancers under control. Nature 376:462-463.
- Cremer, T. 1985. Von der Zellenlehre zur Chromosomentheorie: Naturwissenschaftliche Erkenntnis und Theorienwechsel in der frühen Zell- und Vererbungsforschung, Veröffentlichungen aus der Forschungsstelle für Theoretische Pathologie der Heidelberger Akademie der Wissenschaften. Springer-Vlg., Berlin, Heidelberg.
- Elgin, S. C. R. (ed.). 1995. Chromatin Structure and Gene Expression, vol. 9. IRL Press, Oxford, New York, Tokyo.
- Gerasimova, T. I., and V. G. Corces. 1996. Boundary and insulator elements in chromosomes. Current Op. Genet. and Dev. 6:185-192.
- Gerasimova, T. I., and V. G. Corces. 1998. Polycomb and Trithorax group proteins mediate the function of a chromatin insulator. Cell 92:511-521.
- Gerasimova, T. I., and V. G. Corces. 2001. CHROMATIN INSULATORS AND BOUNDARIES: Effects on Transcription and Nuclear Organization. Annu Rev Genet 35:193-208.
- Gerasimova, T. I., K. Byrd, and V. G. Corces. 2000. A chromatin insulator determines the nuclear localization of DNA [In Process Citation]. Mol Cell 6:1025-35.
- Ha, S. C., K. Lowenhaupt, A. Rich, Y. G. Kim, and K. K. Kim. 2005. Crystal structure of a junction between B-DNA and Z-DNA reveals two extruded bases. Nature 437:1183-6.
- Pollard, T., and W. Earnshaw. 2002. Cell Biology. Saunders.
- Saumweber, H. 1987. Arrangement of Chromosomes in Interphase Cell Nuclei, p. 223-234. In W. Hennig (ed.), Structure and Function of Eucaryotic Chromosomes, vol. 14. Springer-Verlag, Berlin, Heidelberg.
- Sinden, R. R. 2005. Molecular biology: DNA twists and flips. Nature 437:1097-8.
- Van Holde KE. 1989. Chromatin. New York: Springer-Verlag. ISBN 0-387-96694-3.
- Van Holde, K., J. Zlatanova, G. Arents, and E. Moudrianakis. 1995. Elements of chromatin structure: histones, nucleosomes, and fibres, p. 1-26. In S. C. R. Elgin (ed.), Chromatin structure and gene expression. IRL Press at Oxford University Press, Oxford.
External links
- Chromatin, Histones & Cathepsin; PMAP The Proteolysis Map-animation
- Recent chromatin publications and news
- Protocol for in vitro Chromatin Assembly
- ENCODE threads Explorer Chromatin patterns at transcription factor binding sites. Nature (journal)
