Diphtheria toxin: Difference between revisions
Whirlpool4 (talk | contribs) |
Whirlpool4 (talk | contribs) |
||
| Line 107: | Line 107: | ||
# A subunit ADP-ribosylates host EF-2 |
# A subunit ADP-ribosylates host EF-2 |
||
## EF-2 is required for protein synthesis |
## EF-2 is required for protein synthesis |
||
## When inactivated, host cannot make protein and then dies |
|||
The diphtheria toxin has the same mechanism of action as the enzyme [[NAD(+)—diphthamide ADP-ribosyltransferase]] ({{EC number|2.4.2.36}}). It catalyzes the transfer of NAD<sup>+</sup> to a diphthamide residue in eukaryotic [[EEF2|elongation factor-2]] (eEF2), inactivating this protein. It does so by [[ADP ribosylation|ADP-ribosylating]] the unusual [[amino acid]] [[diphthamide]]. In this way, it acts as a [[Translation (genetics)|RNA translational]] inhibitor. The catalysed reaction is as follows: |
|||
: NAD<sup>+</sup> + peptide diphthamide <math>\rightleftharpoons</math> nicotinamide + peptide N-(ADP-D-ribosyl)diphthamide. |
: NAD<sup>+</sup> + peptide diphthamide <math>\rightleftharpoons</math> nicotinamide + peptide N-(ADP-D-ribosyl)diphthamide. |
||
Revision as of 15:12, 2 December 2015
| Diphtheria toxin, C domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
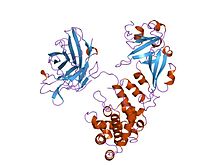 complex of diphtheria toxin and heparin-binding epidermal growth factor | |||||||||
| Identifiers | |||||||||
| Symbol | Diphtheria_C | ||||||||
| Pfam | PF02763 | ||||||||
| Pfam clan | CL0084 | ||||||||
| InterPro | IPR022406 | ||||||||
| SCOP2 | 1ddt / SCOPe / SUPFAM | ||||||||
| TCDB | 1.C.7 | ||||||||
| |||||||||
| Diphtheria toxin, T domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 complex of diphtheria toxin and heparin-binding epidermal growth factor | |||||||||
| Identifiers | |||||||||
| Symbol | Diphtheria_T | ||||||||
| Pfam | PF02764 | ||||||||
| InterPro | IPR022405 | ||||||||
| SCOP2 | 1ddt / SCOPe / SUPFAM | ||||||||
| TCDB | 1.C.7 | ||||||||
| |||||||||
| Diphtheria toxin, R domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 complex of diphtheria toxin and heparin-binding epidermal growth factor | |||||||||
| Identifiers | |||||||||
| Symbol | Diphtheria_R | ||||||||
| Pfam | PF01324 | ||||||||
| InterPro | IPR022404 | ||||||||
| SCOP2 | 1ddt / SCOPe / SUPFAM | ||||||||
| TCDB | 1.C.7 | ||||||||
| |||||||||
| tox diphtheria toxin precursor | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | tox | ||||||
| Entrez | 2650491 | ||||||
| RefSeq (Prot) | NP_938615 | ||||||
| UniProt | Q6NK15 | ||||||
| Other data | |||||||
| EC number | 2.4.2.36 | ||||||
| Chromosome | genome: 0.19 - 0.19 Mb | ||||||
| |||||||
Diphtheria toxin is an exotoxin secreted by Corynebacterium diphtheriae, the pathogenic bacterium that causes diphtheria. Unusually, the toxin gene is encoded by a bacteriophage (a virus that infects bacteria).[1] The toxin causes the disease diphtheria in humans by gaining entry into the cell cytoplasm and inhibiting protein synthesis.[2]
Structure
Diphtheria toxin is a single polypeptide chain of 535 amino acids consisting of two subunits linked by disulfide bridges, known as an A-B toxin. Binding to the cell surface of the B subunit (the less stable of the two subunits) allows the A subunit (the more stable part of the protein) to penetrate the host cell.[3]
The crystal structure of the diphtheria toxin homodimer has been determined to 2.5A resolution. The structure reveals a Y-shaped molecule consisting of 3 domains. Fragment A contains the catalytic C domain, and fragment B consists of the T and R domains[4][4]
- The N-terminal catalytic domain, known as the C domain, has an unusual beta+alpha fold.[5] The C domain blocks protein synthesis by transfer of ADP-ribose from NAD to a diphthamide residue of EF-2.[6][7]
- A central translocation domain, known as the T domain or TM domain. The T domain has a multi-helical globin-like fold with two additional helices at N-termini, but which has no counterpart to the first globin helix. This domain is thought to unfold in the membrane.[8] pH-induced conformational change in the T domain triggers insertion into the endosomal membrane and facilitates the transfer of the C domain into the cytoplasm.[6][7]
- A C-terminal receptor-binding domain, known as the R domain. This domain has a beta-sandwich fold consisting of nine strands in two sheets with Greek-key topology; it is a subclass of immunoglobin-like fold.[5] The R domain binds to cell surface receptor, permitting the toxin to enter the cell by receptor mediated endocytosis.[6][7]
Mechanism

- Processing
- Leader region is cleaved during secretion
- Proteolytic nicking separates A and B subunits, which remain joined by disulfide bonds until they reach the cytosol
- Binds to heparin-binding epidermal growth factor precursor (HB-EGF)
- Endocytosis by host cell
- Acidification inside the endosome induces translocation of A subunit into the cytosol
- Disulfide bonds are broken
- B subunit remains in the endosome as a pore
- A subunit ADP-ribosylates host EF-2
- EF-2 is required for protein synthesis
- When inactivated, host cannot make protein and then dies
The diphtheria toxin has the same mechanism of action as the enzyme NAD(+)—diphthamide ADP-ribosyltransferase (EC 2.4.2.36). It catalyzes the transfer of NAD+ to a diphthamide residue in eukaryotic elongation factor-2 (eEF2), inactivating this protein. It does so by ADP-ribosylating the unusual amino acid diphthamide. In this way, it acts as a RNA translational inhibitor. The catalysed reaction is as follows:
- NAD+ + peptide diphthamide nicotinamide + peptide N-(ADP-D-ribosyl)diphthamide.
Diphtheria toxin has also been associated with the development of myocarditis. Myocarditis secondary to diphtheria toxin is considered one of the biggest risks to non-immunized children.
The exotoxin A of Pseudomonas aeruginosa uses a similar mechanism of action.
Lethal dose
Diphtheria toxin is extraordinarily potent.[3] The lethal dose for humans is about 0.1 μg of toxin per kg of bodyweight. A massive release of toxin into the body will likely cause lethal necrosis of the heart and liver.[9]
History
Diphtheria toxin was discovered in 1890 by Emil Adolf von Behring. In 1951, Freeman found that the toxin gene was not encoded on the bacterial chromosome, but by a lysogenic phage infecting all toxigenic strains.[10][11][12]
Clinical use
The drug denileukin diftitox uses diphtheria toxin as an antineoplastic agent. Resimmune™ is an immunotoxin which is in Clinical Trials in Cutaneous T cell lymphoma patients. It uses diphtheria toxin (truncated by the cell binding domain) coupled to anti-CD3ε Ab (UCHT1).
References
- ^ TABLE 1. Bacterial virulence properties altered by bacteriophages from Patrick L. Wagner, Matthew K. Waldor (August 2002). "Bacteriophage Control of Bacterial Virulence". Infection and Immunity. 70 (8): 3985–3993. doi:10.1128/IAI.70.8.3985-3993.2002. PMC 128183. PMID 12117903.
- ^ Bell CE, Eisenberg D (1996). "Crystal structure of diphtheria toxin bound to nicotinamide adenine dinucleotide". Biochemistry. 35 (4): 1137–1149. doi:10.1021/bi9520848. PMID 8573568.
- ^ a b Murphy JR (1996). "Corynebacterium Diphtheriae: Diphtheria Toxin Production". In Baron S; et al. (eds.). Medical microbiology (4 ed.). Galveston, Texas: Univ. of Texas Medical Branch. ISBN 0-9631172-1-1.
- ^ a b Choe S, Bennett MJ, Fujii G, Curmi PM, Kantardjieff KA, Collier RJ, Eisenberg D (May 1992). "The crystal structure of diphtheria toxin". Nature. 357 (6375): 216–22. doi:10.1038/357216a0. PMID 1589020.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Bell CE, Eisenberg D (January 1997). "Crystal structure of nucleotide-free diphtheria toxin". Biochemistry. 36 (3): 481–8. doi:10.1021/bi962214s. PMID 9012663.
- ^ a b c Bennett MJ, Eisenberg D (September 1994). "Refined structure of monomeric diphtheria toxin at 2.3 A resolution". Protein Sci. 3 (9): 1464–75. doi:10.1002/pro.5560030912. PMC 2142954. PMID 7833808.
- ^ a b c Bell CE, Eisenberg D (January 1996). "Crystal structure of diphtheria toxin bound to nicotinamide adenine dinucleotide". Biochemistry. 35 (4): 1137–49. doi:10.1021/bi9520848. PMID 8573568.
- ^ Bennett MJ, Choe S, Eisenberg D (September 1994). "Refined structure of dimeric diphtheria toxin at 2.0 A resolution". Protein Sci. 3 (9): 1444–63. doi:10.1002/pro.5560030911. PMC 2142933. PMID 7833807.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pappenheimer A (1977). "Diphtheria toxin". Annu Rev Biochem. 46 (1): 69–94. doi:10.1146/annurev.bi.46.070177.000441. PMID 20040.
- ^ Freeman VJ (June 1951). "Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae". J. Bacteriol. 61 (6): 675–88. PMC 386063. PMID 14850426.
- ^ Freeman VJ, Morse IU (March 1952). "Further observations on the change to virulence of bacteriophage-infected avirulent strains of Corynebacterium diphtheria". J. Bacteriol. 63 (3): 407–14. PMC 169283. PMID 14927573.
- ^ Diphtheria from Todar's Online Textbook of Bacteriology, Kenneth Todar 2009. Accessed 08 September 2010.
External links
- Diphtheria+Toxin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- How Diphtheria Toxin Works - Animation

