Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
July 7
I'm looking for how this magic trick works. A magician takes a dollar bill from the audience and makes it appear inside a fruit (orange/lemon). This youtube video demonstrates how it works: http://www.youtube.com/watch?v=cQBrXCcfDyc But I've seen many magicians ask the audience to sign the bill and making it appear in the orange rather than just having a different bill appear inside. Do you know any information on how this one works? I can't seem to find the information anywhere. 69.230.55.21 (talk) 00:19, 7 July 2010 (UTC)
- At a guess they probably switch the dollar bill they put in the fruit with the one the audience gave to them at some stage after opening it Nil Einne (talk) 01:04, 7 July 2010 (UTC)
Of course, it is Sleight of hand or हाथ की सफाई as they call it in India. It is an ancient art. Nowadays tricksters tell you that is nothing but art, in India now that is even required by law, though that is not strictly required by law but Rationalist Society people get you otherwise Jon Ascton (talk) 01:13, 7 July 2010 (UTC)
- Or the "random person from the audience" is in cahoots with the performer. 218.25.32.210 (talk) 01:19, 7 July 2010 (UTC)
- ok thanks for the info, but can you give me specific steps in performing this trick? I know that this can be performed with a "random person" not a confederate of the magician. 69.230.55.21 (talk) 01:21, 7 July 2010 (UTC)
- Yes, there are always more than one way to do a trick. And a clever magician never repeats a trick before the same audience Jon Ascton (talk) 02:24, 7 July 2010 (UTC)
- Hey! I just saw the video (after writting my above comments). The magician simply, honestly gives away the trick - that's amazingly easy ! Did'nt ya get it, man ? What happened is this : Before you do the trick you take a dollar, note its serial number on a paper. Then you roll up the note and stick it neatly in an orange, do it in such a way that it does'nt look as if someone's done something to the fruit. Now you are ready for the trick. You ask a guy to come up and give you a dollar, he will give his own dollar of course with it's different serial, but that does'nt bother you because you don't really write it anywhere, but only pretend you do so ! by moving a pen on the paper on which you have already written the number of the dollar which is readily stuffed in the orange. Now you roll the guy's dollar and put the handkerchief on it, this is where you slip the dollar in your other hand or somewhere else he can't see it. He thinks it is there in the handkerchief, but it is some piece of paper rolled in the handkerchief's hem ! Now you unfurl the handkerchief and the dollar "vanishes" (it is already soemwhere else, what the dumb bastard was feeling was the paper in the hem !) Now put the handkerchief out of picture and cut open the orange which has your original dollar whose serial number is safely recoreded on the paper ! Got it ? Cool ! Jon Ascton (talk) 03:08, 7 July 2010 (UTC)
- I used to dabble a lot with magic tricks when my kid was younger. My favorite fruit trick is to hand several members of the audience a banana pulled from an entire bunch I have brought with me. I ask them to inspect their bananas carefully - then without me being anywhere nearby, to peel them. They are all amazed to find that the inside of their banana is neatly sliced into a half dozen pieces - and even after that, they can look at the skin, eat the banana and not see how the trick was done. It's nice because there are so many bananas that I couldn't possibly have that many confederates - and everyone in even a moderately large audience is sitting close enough to one of the bananas to see the trick happen in front of their eyes.
- I'll let you figure out how I do that one! SteveBaker (talk) 04:31, 7 July 2010 (UTC)
- Hm...I think it's got to do with the art of slicing the inside without damaging the skin ! No ? That can be accomplished either with a sharp needle or a needle and a thread. Right ? Jon Ascton (talk) 04:58, 7 July 2010 (UTC)
- Yep you use a needle and thread. Pick bananas that are sufficiently ripe to have a few brown speckles and use the needle to pull the thread from one brown spot to another around the banana going back in through the exact same pinhole you came out of. When you've gone all around the banana, come back out of the same hole you started at and then just pull on the thread to slice through the flesh, leaving the skin intact - except for the smallest of pinholes. Then, soaking the entire bunch of bananas in water makes the skin expand slightly, closing the pinholes to the point where they are pretty much invisible. If possible, place your pinholes along the 'seams' of the banana skin so that tearing the banana open helps to destroy the evidence even further. It takes a half hour to prepare an entire bunch of bananas - I like to leave them on the kitchen countertop at work on April 1st. SteveBaker (talk) 03:11, 8 July 2010 (UTC)
- Hm...I think it's got to do with the art of slicing the inside without damaging the skin ! No ? That can be accomplished either with a sharp needle or a needle and a thread. Right ? Jon Ascton (talk) 04:58, 7 July 2010 (UTC)
- I think you're somewhat missing the point. The OP is aware the video shows how it works for that case. What they want is to know how it works when the illusionist shows a bill that was signed by a member of the audience, so you can't just use a different bill. As you and I have said, it would likely be some sort of sleight of hand, swapping the bill inside the fruit for the signed one (alternativing signing the one inside, but this seems far less likely given that the signature could easily be recognised as a fake) but the OP wants more. Nil Einne (talk) 05:18, 7 July 2010 (UTC)
- Oops, Nil, you are right, I missed that point ! How stupid of me ! Terribly sorry indeed ! Oh, the OP knows how he did that, my ! I was taking so much pains to tell what he already knew, perhaps better than me ! Sorry, OP man. I overlooked that part - the magician does not explain the whole trick - but leaves a vital loophole - the signature thing. Oh my ! So this is a new way to show magic. You do an easy trick. Explain it is such a way that an asshole like me thinks that all is explained, but when he thinks over he learns there is something he can not get through. Well, there should be a special term for this kind of thing, no ? Yup, Nil man, its good old slieght of hand of course... Jon Ascton (talk) 07:15, 7 July 2010 (UTC)
- ...and what do ya think about the SteveBaker's banana ? Like my solution, eh ?
- For some reason this trick doesn't show up in my trusty copy of "Cyclopedia of Magic" which is usually my go-to source for when I'm curious about such things.
- One way to do it, would be to swap the bills when they're still rolled up. (ie: Cut open the orange, show that it contains a rolled up bill, then use sleight of hand to swap them while you were in the process of unrolling them.) This would probably require the magician to be the one to remove the bill from the orange, which wouldn't have the same effect as letting the spectator do it. APL (talk) 15:21, 7 July 2010 (UTC)
side mirror
what type of glue/ resin do they use to attach a side mirror on a car? i mean attach the actual glass part to the metal part (honda) —Preceding unsigned comment added by Alexsmith44 (talk • contribs) 00:27, 7 July 2010 (UTC)
- Are you sure it's glued on? I would have assumed it was bolted on. ←Baseball Bugs What's up, Doc? carrots→ 00:35, 7 July 2010 (UTC)
i think its glued on. mine broke today and there were no bolts —Preceding unsigned comment added by Alexsmith44 (talk • contribs) 00:37, 7 July 2010 (UTC)
- Glued onto what? The pivot mechanism? ←Baseball Bugs What's up, Doc? carrots→ 00:40, 7 July 2010 (UTC)
only the glass part broke not the metal part —Preceding unsigned comment added by Alexsmith44 (talk • contribs) 00:50, 7 July 2010 (UTC)
- I don't follow. The glass part has to be attached to a pivot mechanism of some kind so it can be adjusted. ←Baseball Bugs What's up, Doc? carrots→ 00:53, 7 July 2010 (UTC)
- No need to interrogate the guy. His question is clear enough. The glass shattered and has to be re-glued to the piece that the mirror itself attaches to. If you don't understand the mechanism, you don't have to answer.
- There was a discussion on this very topic here.
- Check out Steve's post at the end. If he's right, It looks like what you want is some rubbery adhesive pads specially designed for this purpose. (These two allegedly educational YouTube videos back him up on this : [1][2]) APL (talk) 02:00, 7 July 2010 (UTC)
- No need to get snippy. I was asking these questions out of curiosity. Believe it or not, I'm not an expert on everything. :) However, if it were me, I would take it to the dealership and let them figure it out and explain it to me. ←Baseball Bugs What's up, Doc? carrots→ 11:06, 7 July 2010 (UTC)
do they use a formaldehyde resin in the factory to attach it?--Alexsmith44 (talk) 02:08, 7 July 2010 (UTC)
- I don't know - but in the previous discussion on this topic, the person said that when they bought the replacement mirror, it came with double-sided sticky foam pads to mount it with. He was skeptical that this would work and glued the mirror on instead - finding that it broke within a very short time later. I deduce that the pads have some role in isolating the mirror from vibration and that when you buy your replacement mirror, you should definitely use them if that's how they come from the auto-parts store.
- I asked the previous person this too (but didn't get a response): Did you look to see whether there was signs of glue or sticky pads on the shards of broken mirror glass? That would be a clue at least. SteveBaker (talk) 04:18, 7 July 2010 (UTC)
- To reiterate what I mentioned in the aforementioned previous discussion, FWIW, on the several occasions I've had to replace a broken side mirror glass, the replacement glass came with a strong adhesive layer over the whole of the rear surface (covered by a peel-off paper layer): separate glue or adhesive pads was/were unnnecessary. Major UK car component stockists such as Halfords stock a large range of replacement mirrors specific to individual car makes and models, which are generally considerably cheaper than replacements supplied directly by the manufacturers themselves. If Alexsmith44 is not in the UK this information may of course be useless 87.81.230.195 (talk) 09:06, 7 July 2010 (UTC)
Animal Eyes

Why do eyes of animal light up thus when photographed ? And why the same does not happen with humans ? Jon Ascton (talk) 01:07, 7 July 2010 (UTC)
- They probably have much better night vision than we do. I'm sure there's an article about that phenomenon somewhere. Very noticeable with cats, for example. ←Baseball Bugs What's up, Doc? carrots→ 01:10, 7 July 2010 (UTC)
- I don't think that's got something to do with that. Cows are not known for their sight, they can't even discern colours Jon Ascton (talk) 01:16, 7 July 2010 (UTC)
- Bingo. I looked up night vision and it led me to Tapetum lucidum, a layer of tissue in the eyes of many animals but not in humans. ←Baseball Bugs What's up, Doc? carrots→ 01:13, 7 July 2010 (UTC)
- Um...the same does happen with humans. See red-eye effect. Under the right conditions (low light, high sensor sensitivity, subject looking directly at lens), I've seen human eyes glow much brighter than those cow eyes. --Bowlhover (talk) 01:29, 7 July 2010 (UTC)
- You don't get that "mirror" effect with humans that you do with animals that have that layer in their retinas. ←Baseball Bugs What's up, Doc? carrots→ 01:32, 7 July 2010 (UTC)
- It's of note that red-eye effect and eyeshine are actually two different effects. And notably, red-eye is just seen in photos. Eyeshine you can actually see in nature (shine a flashlight on your dog at night, for example). And note that the cow's photo is in daylight. --Mr.98 (talk) 11:17, 7 July 2010 (UTC)
- Goa'uld? -- 58.147.52.199 (talk) 13:33, 7 July 2010 (UTC)
- That cow has been to some kind of supermax space prison with Vin Diesel. Googlemeister (talk) 14:16, 7 July 2010 (UTC)
Dragonfly

Any idea what this is? --The High Fin Sperm Whale 03:59, 7 July 2010 (UTC)
- Should we assume that you took that photo in British Columbia since your user page points out that you live there? Dismas|(talk) 04:02, 7 July 2010 (UTC)
- Yes, you can also assume that since the title says it's in Langley and it has Coordinates. --The High Fin Sperm Whale 04:08, 7 July 2010 (UTC)
- If we can't figure it out, you may want to contact the person behind this site. For some reason, their "Gallery" link doesn't actually contain a gallery. Dismas|(talk) 05:17, 7 July 2010 (UTC)
- Yes, you can also assume that since the title says it's in Langley and it has Coordinates. --The High Fin Sperm Whale 04:08, 7 July 2010 (UTC)
- Looks like a skimmer to me, family Libellulidae. Possibly male Plathemis lydia, not sure at all. --Dr Dima (talk) 06:45, 7 July 2010 (UTC)
An open tube is placed into a container of water
An open tube is placed into a container of water and a vibrating tuning fork placed over the mouth of the tube. As the tube is raised so a greater length of the tube is out of the water, resonance is heard. This occurs when the the distance from the top of the tube to the water level is 12 cm, and again at 50 cm. Determine the frequency of the tuning fork.
The naïve approach would simply evaluate but this is not correct. hElp?--Alphador (talk) 07:01, 7 July 2010 (UTC)
- λ=50cm−12cm? Bo Jacoby (talk) 09:49, 7 July 2010 (UTC).
- No, that's λ/2 because the second harmonic occurs at l = 3λ/4 => λ = 0.78 m => f = 441 Hz (a likely correct answer). The reason why 717 Hz is wrong is because of end correction. MER-C 09:58, 7 July 2010 (UTC)
North American bugs
If I find some random strange looking bug in North America, what are the chances that this bug is unknown to biologists? I know that globally the majority of insects have not been cataloged. But is this true in North America as well? Ariel. (talk) 07:04, 7 July 2010 (UTC)
- This will depend on where you find it. I know from my own country that bugs and plants are surveyed much better in the vicinity of a major university and in locations that are easily accessible. The chance of it being unknown is also not necessarily larger if it is "strange looking". It is the case with many plants and fungi that they are overlooked by biologists because they are very similar to other species. This could be the case with insects as well. 80.202.238.149 (talk) 10:01, 7 July 2010 (UTC)
Source of stream goes both ways?
The source of the Lawrence Brook is at 40°22′33″N 74°32′32″W / 40.37583°N 74.54222°W. Google Maps shows this coordinate along a stream. One direction is the Lawrence Brook and the other direction is the Devils Brook. Is it a spring that goes both ways? Or are the streams interconnected? Or is the map mistaken and the streams aren't connected at all? Thank you. --Chemicalinterest (talk) 10:59, 7 July 2010 (UTC)
- I am not familiar with that stream, but I know of two rivers that have the same source in a marshy area, and on the map they look interconnected. 92.15.27.146 (talk) 20:20, 7 July 2010 (UTC)
- Thanks for answering. --Chemicalinterest (talk) 20:42, 7 July 2010 (UTC)
Is it fair to say that the 4th dimension is not time?
When I was younger, it was my understanding that time was the fourth dimension. But this isn't correct is it? I checked out wiki's article on the 4th dimension, and it made no mention of time in it. Just wanted to make sure that time and the 4th dimension are two different things. 148.168.127.10 (talk) 14:48, 7 July 2010 (UTC)
- It is mostly a matter of semantics. The debate is a question of what gets to be called the "fourth dimension". Should it be time? Should it be a spacial dimension? Much of modern physics is based on a concept of more than five dimensions (I believe 10 is the current best guess). So, is time the fourth dimension or fifth dimension or sixth dimension? Does it matter? Again, it is a debate that will likely never be resolved. -- kainaw™ 14:51, 7 July 2010 (UTC)
- There are indeed physical theories with more than 4 dimensions but they are all speculative. Sticking with what is considered solid knowlege, space-time has three spatial dimensions and one temporal one, so it seems fair to call time the fourth dimension. Dauto (talk) 17:33, 7 July 2010 (UTC)
- To clarify something: the "ordinality" of the dimensions is meaningless. That is, there is no particular significance to which dimension you consider "first", which you consider "second", and so forth. While we conventionally refer to time as "the fourth dimension" because we're already used to considering the other three, I put forth that it's more correct to say merely that "time is one of the four dimensions". Note that this can be extended to the higher-dimensional theories, too; I'm just using 4D so as not to write out all the other possibilities. — Lomn 18:03, 7 July 2010 (UTC)
- There isn't even a set of dimensions that one could assign an order to. You can't point in three specific directions and say that those are the three dimensions of space. Or rather, you can, but there are many different ways of doing it and no one way is more correct than all the others. Even the division of spacetime into "space" and "time" dimensions is somewhat ambiguous because they mix together. -- BenRG (talk) 19:22, 7 July 2010 (UTC)
- There are varying conventions. A lot of authors put time as either the first or the zeroth dimension. --Tango (talk) 21:23, 7 July 2010 (UTC)
- To clarify something: the "ordinality" of the dimensions is meaningless. That is, there is no particular significance to which dimension you consider "first", which you consider "second", and so forth. While we conventionally refer to time as "the fourth dimension" because we're already used to considering the other three, I put forth that it's more correct to say merely that "time is one of the four dimensions". Note that this can be extended to the higher-dimensional theories, too; I'm just using 4D so as not to write out all the other possibilities. — Lomn 18:03, 7 July 2010 (UTC)
So the first three dimensions that we are used to are all spacial dimensions. But time is not a spacial dimension correct? The tesserect exists in a spacial dimension right? 148.168.127.10 (talk) 18:17, 7 July 2010 (UTC)
- That's right. Time is not a spatial dimension and a tesseract needs four spatial dimensions, not three of space and one of time. -- BenRG (talk) 19:22, 7 July 2010 (UTC)
- If you want to talk about arbitrary assignments in both space and time, note that spacetime can be represented as a metric tensor defining how the dimensions interrelate. In particular, the standard basis of flat spacetime is the simplest, yet still arbitrary, representation of this vector, as we could just as easily choose a basis that mixes in some time components with each of the spatial dimensions. It would be ugly, but it would work just the same. You should also note that in General Relativity, the presence of a gravitational field causes the metric used to change, effectively changing the shape, and thus the metric representation, of spacetime. In conclusion, there is some arbitrariness, but it's not totally haphazard how we choose our dimensions. SamuelRiv (talk) 20:13, 7 July 2010 (UTC)
Imaginary time can be considered to be a fourth spatial dimension. Count Iblis (talk) 22:33, 7 July 2010 (UTC)
- It's entirely arbitary. We could have called time "the first dimension" and the three obvious spatial axes "second, third and fourth". But to me it seems bad to lump such an obviously different measure in with the other three. Basically, it's a (slight) mathematical convenience in some sorts of calculations - beyond that, time just isn't similar enough to the three spatial axes to be usefully treated as a fourth "coordinate". Of course, when we talk about three spatial axes, we don't generally have a particularly good idea of what they are. We tend to think of "left/right", "forwards/backwards" and "up/down" - but you could equally choose "azimuth angle", "elevation angle" and "range" as our three spatial 'dimensions'. We tend not to do that because it's a pain to calculate with and it implies a definite origin...but it's really just as valid. SteveBaker (talk) 23:15, 7 July 2010 (UTC)
- Steve Baker claims "Basically, it's a (slight) mathematical convenience in some sorts of calculations - beyond that, time just isn't similar enough to the three spatial axes to be usefully treated as a fourth "coordinate".
- Steve Baker is WRONG. Dauto (talk) 12:22, 8 July 2010 (UTC)
- Comment (disagree) for a lot of us still using Euclidean space, calling 'time' the fourth dimension, or equating it as equivalent to the three spacial dimensions we use is either a conceit or entirely wrong. 87.102.42.55 (talk) 13:21, 8 July 2010 (UTC)
- Sure, but our universe's space ain't euclidian and that's exactly the point. Dauto (talk) 13:40, 8 July 2010 (UTC)
- Please remember to link to something that agrees with your opinion, as is expected on reference desk answers. Thanks.87.102.42.55 (talk) 13:49, 8 July 2010 (UTC)
- There. Dauto (talk) 13:59, 8 July 2010 (UTC)
- See also this. Dauto (talk) 14:06, 8 July 2010 (UTC)
- You obviously know what you are talking about, so why don't you make a proper effort to communincate it. Your hidden link to spacetime is an article about the model not physical reality, your hidden link to general relativity gives no indication where in that article can be found the relavent answers to the question. Although I could find it stated that the symmetry of 'spacetime' is different, I couldn't find out why you think it is physically true.87.102.42.55 (talk) 14:15, 8 July 2010 (UTC)
- See Tests of general relativity for some of the more important experiments and observations that overwhelmingly confirms the reality of spacetime warping (non-euclidian spacetime) in acordance with the predictions of general relativity. Dauto (talk) 14:27, 8 July 2010 (UTC)
- Of course - space is warped and time is warped (and so is mass) - nobody is denying that. However rolling all of those warpages in to a single mathematical object and calling it "spacetime" doesn't mean that space and time are merely aspects of the same thing. They very clearly aren't. For example, it's very easy to find things that are not time-reversible (entropy, for example) - but it's almost impossible to find things that are not space-reversible. You can pick up an object and rotate it through all three spatial dimensions - but you can't rotate it about the time axis. A physical object can have a limited extent in X, Y and Z (imagine a 3 meter cube) - but it can't have a limited extent in time without violating the conservation laws by popping into existence from nowhere, existing for 3 seconds, then popping out of existence again. Spacetime is a mathematical convenience - a handy shorthand, no doubt - but trying to cram what time is into the 'space' mold - or vice-versa isn't a generally workable thing. For a moving object, velocities (dx/dt, dy/dt, dz/dt) are all constrained by the speed of light - but directions (dx/dy, dy/dz and so forth) can take on literally any value without difficulty - time is special and different in that regard.
- Time and space are very different things that just happen to share a few common behaviors (like the way they are warped by gravity). Conflating mathematical and descriptive convenience with physical reality is a dumb idea. Sure, in YOUR discipline, it's handy to imagine space and time as a single four-dimensional system...heck, I do computer graphics for a living and sometimes it's convenient for me to work in 6D space (X,Y,Z,Red,Green,Blue) - or sometimes 15 dimensional space with surface orientation adding nine more dimensions. Doing that sometimes produces some nice mathematical insights and shortcuts - but that's not to say that color space and physical space and 'orientation space' are "the same thing" - it's just temporarily useful to treat them as if they were. (eg Take two smoothly colored triangles that share a common edge: ABC and ACD. Can you replace them with the two triangles ABD and BCD and have the result look the same? Yes - but only if ABCD is 'planar' in 6D space!) There are yet higher numbers of dimensions which are mathematically interesting too: Some people like to work in configuration space where every single property of every single fundamental particle is a 'dimension' and you have an insanely large number of dimensions where the entire universe can be represented by a single point and the trajectory of that point as a function of time is all of physics. This seems crazy - but the network protocol for a game I'm writing works like that (although I'm obviously not going down to the level of fundamental particles)! SteveBaker (talk) 21:11, 8 July 2010 (UTC)
- See Tests of general relativity for some of the more important experiments and observations that overwhelmingly confirms the reality of spacetime warping (non-euclidian spacetime) in acordance with the predictions of general relativity. Dauto (talk) 14:27, 8 July 2010 (UTC)
- You obviously know what you are talking about, so why don't you make a proper effort to communincate it. Your hidden link to spacetime is an article about the model not physical reality, your hidden link to general relativity gives no indication where in that article can be found the relavent answers to the question. Although I could find it stated that the symmetry of 'spacetime' is different, I couldn't find out why you think it is physically true.87.102.42.55 (talk) 14:15, 8 July 2010 (UTC)
- Please remember to link to something that agrees with your opinion, as is expected on reference desk answers. Thanks.87.102.42.55 (talk) 13:49, 8 July 2010 (UTC)
- Sure, but our universe's space ain't euclidian and that's exactly the point. Dauto (talk) 13:40, 8 July 2010 (UTC)
- Comment (disagree) for a lot of us still using Euclidean space, calling 'time' the fourth dimension, or equating it as equivalent to the three spacial dimensions we use is either a conceit or entirely wrong. 87.102.42.55 (talk) 13:21, 8 July 2010 (UTC)
- Steve, you are clearly a very smart person and very knowlegable as well but you also clearly don't know enough about relativity (you know less than what you think) and should probabily refrain from answering questions about that subject. I'm sorry to say but you are dead wrong. Space and time are indeed different apects of a single thing. This is not just a mathematical convenience. In your example where you use a 6D space that includes color and regular space the two different things never mix. Color always remains color and space always remains space. That is indeed just a mathematical convenience. But in relativity space and time do mix (despite your claims to the contrary). that mixing is what is behind lorentz contraction and time dilation. What is time for one observer may be space for a different observer and vice-versa. That's the crucial point that I'm trying to make, spacetime is a physical reality. The warping of space and time by gravity also mixes them. For instance, inside a black hole the radial direction becomes time-like with the future direction pointing inwards. That's why nothing can lieve a black hole. In order to exit the hole something would have to move back in time which is impossible. Sure, you made many points that show that time and space are clearly different from each other but, despite your efforts, you have not shown that they are not different aspects of asingle thing. Dauto (talk) 03:35, 9 July 2010 (UTC)
- In context, I took Steve's remark to be an objection to the old convention, which is indeed a mathematical convenience in a few situations, but overall not a particularly helpful way of thinking about spacetime. It tries to make a semi-Riemannian manifold look formally like an ordinary Riemannian manifold, which it just isn't. --Trovatore (talk) 10:34, 9 July 2010 (UTC)
- Steve, you are clearly a very smart person and very knowlegable as well but you also clearly don't know enough about relativity (you know less than what you think) and should probabily refrain from answering questions about that subject. I'm sorry to say but you are dead wrong. Space and time are indeed different apects of a single thing. This is not just a mathematical convenience. In your example where you use a 6D space that includes color and regular space the two different things never mix. Color always remains color and space always remains space. That is indeed just a mathematical convenience. But in relativity space and time do mix (despite your claims to the contrary). that mixing is what is behind lorentz contraction and time dilation. What is time for one observer may be space for a different observer and vice-versa. That's the crucial point that I'm trying to make, spacetime is a physical reality. The warping of space and time by gravity also mixes them. For instance, inside a black hole the radial direction becomes time-like with the future direction pointing inwards. That's why nothing can lieve a black hole. In order to exit the hole something would have to move back in time which is impossible. Sure, you made many points that show that time and space are clearly different from each other but, despite your efforts, you have not shown that they are not different aspects of asingle thing. Dauto (talk) 03:35, 9 July 2010 (UTC)
- I don't think that's what he meant. May be he should clarify his objection. Dauto (talk) 17:32, 9 July 2010 (UTC)
- Dauto - except time mixes with (flat) space with an opposite metric component. Basically (for those watching), the "distance" between event A and event B is the spatial separation minus the time separation (relativistically scaled). That's what Steve means - they are fundamentally different. SamuelRiv (talk) 09:37, 9 July 2010 (UTC)
- Yes, obviously time and space are different. We don't need any math to know that. But they are also different aspects of the same thing. They are dimensions of a single manifold known as spacetime continuum. Dauto (talk) 10:27, 9 July 2010 (UTC)
- Okay, so we agree. This whole thread is about the math, though, since it's basically addressing what we mean by the fourth dimension, and why it's different from the spatial dimensions. They are the same in that we can make arbitrary rotations of the basis vector, and they are different in that such rotations are not actually arbitrary - there is a bias for time standing alone. SamuelRiv (talk) 19:13, 9 July 2010 (UTC)
- Not quite. It is true that spatial rotations always mix two space dimensions and never mix the time with a apatial dimension. On the other hand, a lorentz transformation (due to relative movement between two reference frames) always mix a spatial dimention with time. The lorentz transformation is the equivalent of a rotation involving the time axis. Dauto (talk) 02:53, 10 July 2010 (UTC)
How do they use fusion reactors to make electricity?
The conclusion of the thread above about someone being locked in a fusion reactor was that he'd be fine and wouldn't be killed by heat. So how do they use a fusion reactor to make electricity? I thought they'd use a steam turbine like they do with almost every other method of generating electricity, but it doesn't seem sufficient for that. What do they do?--92.251.137.196 (talk) 15:53, 7 July 2010 (UTC)
- The glib answer is that they don't use any existing fusion reactor to generate electricity. Existing experimental fusion reactors are designed to test the principles and demonstrate plasma confinement, rather than to supply the grid with electricity. (I can demonstrate the use of hydrocarbon combustion to generate surplus energy by lighting a candle, but I won't be able to spin a large turbine that way.) Once reactors are built which can sustain high average power output – tens or hundreds of megawatts, at least – for hours or days at a time (rather than seconds or fractions of a second) then the surplus heat will be used for power generation, and you'll see reactors coupled to heat exchangers, steam turbines, and generators.
- (As an aside, I would also take the assertions above about the dangers of the interior of an operating fusion reactor with a grain of salt, as they rely on a large number of guesses and few proper sources. One link notes that the JET reactor has sustained a fusion output of 5 MW for 5 seconds, with a heating input power of about 20 MW. That's a non-trivial amount of power floating around — 25 MW for five seconds will do more than warm you slightly....) TenOfAllTrades(talk) 16:19, 7 July 2010 (UTC)
- The basic mechanism is that nuclear fusion reactions occur in the plasma. These emit gamma rays, which travel out of the plasma and strike the interior of the reactor vessel, which is lined with blocks of an absorbent material (perhaps vanadium or carbon fibre). The gamma rays are absorbed, which warms the blocks. Around the outside of the blocks (outside the reactor torus itself) are wrapped cooling coils filled with a fluid (say water). The fluid warms, expands, and this expansion causes it to turn a turbine. The turbine in turn turns an electrical generator, which creates the electrical current. -- Finlay McWalter • Talk 16:32, 7 July 2010 (UTC)
- I goofed somewhat: depending on the fusion reaction, most of the energy is from fusion neutrons rather than gammas. -- Finlay McWalter • Talk 20:58, 7 July 2010 (UTC)
- Hmm why wouldn't a person inside a reactor also heat up?--92.251.236.7 (talk) 23:12, 7 July 2010 (UTC)
- It's perhaps worth noting that all of the discussions of humans being cooked at the center of the reactor are focused on JET, which cannot produce electricity. ITER, which could hypothetically produce more energy than it takes to start the reaction, is a much larger beast and presumably has different plasma temperatures and densities. --Mr.98 (talk) 00:56, 8 July 2010 (UTC)
- Hmm why wouldn't a person inside a reactor also heat up?--92.251.236.7 (talk) 23:12, 7 July 2010 (UTC)
- I goofed somewhat: depending on the fusion reaction, most of the energy is from fusion neutrons rather than gammas. -- Finlay McWalter • Talk 20:58, 7 July 2010 (UTC)
- Fusion_power#Subsystems describes a couple of the different ways you could turn fusion power into electricity. --Mr.98 (talk) 22:11, 7 July 2010 (UTC)
- According to our fusion power article, the rate at which neutrons deposit energy in the plasma-facing components of a full-scale fusion reactor will be around 10 MW/m2. For comparison, the surface power density of direct sunlight at the surface of the Earth is around 100 W/m2. So, roughly speaking, the neutrons releasd by the fusion reaction heat the walls of the reaction chamber (and anything within it) with an intensity that is 100,000 times greater than the noon-day sun. Gandalf61 (talk) 11:01, 8 July 2010 (UTC)
Cookie/cake mixes
I was just wondering how when for example Betty Crocker creates a cookie or cake mix in powder form, I see some of the ingredients are or were liquid like corn syrup or milk or oil, even molasses- become in powder form, how can you change a liquid like that into powder or flour form? —Preceding unsigned comment added by 71.137.252.54 (talk) 17:28, 7 July 2010 (UTC)
- The simple method is dehydration. That is why you have to add water to the mix. There are many methods of dehydration. It is impossible to know specifically which method the cake mix used to become a powder unless the manufacturer feels like exposing the process. -- kainaw™ 17:31, 7 July 2010 (UTC)
- Yes, although oil can't be changed into a powder form, and typically for recipes that use very much, it has to be added by the user. Looie496 (talk) 17:49, 7 July 2010 (UTC)
Actinidain allergies
What % of the US population is allergic to Actinidain? Googlemeister (talk) 20:40, 7 July 2010 (UTC)
- Based on a bit of Google-Scholaring, allergies to kiwifruit are relatively common but I couldn't spot any actual numbers. A paper from 1998, PMID 9564807, identified actinidin (the spelling varies) as the major allergen in kiwifruit, but another paper from 2007, PMID 17845415, found that it is not, at least in the UK, and that the major allergen is a different protein, with levels of allergy to actinidin being minimal. So it seems that the story is not completely clear, but that the incidence of allergy in the USA is likely to be quite low. Looie496 (talk) 21:00, 7 July 2010 (UTC)
- (Conflict: your Google-fu is stronger than mine!) Actinidain is one of the allergens for those with kiwifruit allergies. I wasn't able to find any exact figures for kiwi fruit allergies, but according to [3] and [4] and [5], in the UK (which isn't all that different from the US), allergies to kiwi fruit are less common than other severe food allergies, but are common enough to be labelled "significant". This study from 2007, however, found that an unidentified 38 kDa protein was the major allergen recognized by 59% of the sample population, and not actinidain. Hope that helps. – ClockworkSoul 21:02, 7 July 2010 (UTC)
beetles
Hello Science desk
Tonight I saw several large brownish beetles flying around, their wings making a dull buzzing sound. I've never seen these things before. The time was 9:30pm GMT in the UK. What species might they have been? Thanks 82.43.90.93 (talk) 20:56, 7 July 2010 (UTC)
- Sounds like June Bugs Phyllophaga (genus), but I don't recall if those live in the UK? Googlemeister (talk) 21:06, 7 July 2010 (UTC)
- Might be the formerly rare but recently burgeoning Cockchafer. Probably wouldn't be hard to catch one of the critters and compare it to the pictures. I'm fairly sure all such large beetles in the UK are harmless! 87.81.230.195 (talk) 21:12, 7 July 2010 (UTC)
- The pics look exactly the same as the one I saw. Thanks :) 82.43.90.93 (talk) 21:31, 7 July 2010 (UTC)
Plastic Recycling - Expanding from 1-7 from 1-2 only
For years places typically were only accepting plastic resin codes 1 & 2 for recycling, but now many places in Florida, including my own home town, are beginning to accept 1-7. Is there now a market for the other plastics, and are they actually recycling *all* of the codes, or is it likely that they're only recycling some of the codes but saying 1-7 to avoid the confusion that would occur say if they said 1, 2, 4, and 6? I already know about articles like Plastic recycling. PCHS-NJROTC (Messages) 20:59, 7 July 2010 (UTC)
- All the plastics listed in Resin identification code can be recycled. They all have monetary value too; from what I've heard/read about the same price as the equivalent weight of oil (not sure what type of oil?) - so it's likely that they want and will recycle all the types.77.86.6.186 (talk) 21:45, 7 July 2010 (UTC)
How hot is hot water?
I don't see a setting on my water heater. I looked at Water heating#Safety but didn't get much help.
I've lived alone for 11 years and since I don't know how to reset it, the setting must have been the same. A plumber replaced a pipe last winter and I'm not aware that he changed anything. I did ask if he thought the water heater should be replaced (though it is making noises like a rodent now when it loads up or starts heating, whatever it's doing). He said no.
It is true, though, that ever since that pipe was replaced the water seems slightly hotter. Before, I could comfortably wet my hands (briefly) to wash them without turning on the cold if I had done something really disgusting, and I could wash dishes without gloves. Now, it seems too hot.
This is important in case I want to replace the water heater.Vchimpanzee · talk · contributions · 21:20, 7 July 2010 (UTC)
- The new pipe could easily be shorter and/or better insulated. Either or those would increase the temperature as it comes out of the tap. You don't seem to have asked a question, though (other than the one in the header, to which there is obviously no meaningful answer). What is it you want to know? --Tango (talk) 21:28, 7 July 2010 (UTC)
- There will be some sort of temperature control on the heating (to prevent it boiling etc) - but it may or may not be user-adjustable.
- However, what and where it is depends on the type of heating you've got - ie is it an immersion heater, integrated gas central heating, wall mounted gas or electrical water heater ?? For increased likelyhood of a workable answer the make and model of the heater will be useful.77.86.6.186 (talk) 21:40, 7 July 2010 (UTC)
- Okay, I was really looking for a general answer about how hot water can be and still be comfortable briefly. Now that I think of it, this thing must have an owner's manual somehwre in this house. It is electric and a cylinder in a closet; that much I know.Vchimpanzee · talk · contributions · 21:50, 7 July 2010 (UTC)
- Sounds like an immersion heater. All the immersion heaters I've had have been non-adjustable (ie, they're set in the factory). Domestic hot water is usually 60–70 ºC (140–155 ºF) when it comes out the tap. Physchim62 (talk) 22:43, 7 July 2010 (UTC)
- According to my mum electric immersion heaters can be adjusted with a screwdriver .. eg don't try this without disconnecting the electricity/know what you're doing etc - I think some versions had a screwdriver adjustable part that could be accessed without removing the top.
- I was told that at 50degrees C water causes reflexive pain that makes it impossible to hold your hand in it. Below that it's hot but bearable.. According to http://www.tap-water-burn.com/ the pain threshold is 106-108 F (that's about 41 degrees C). According to this http://www.bre.co.uk/pdf/WaterNews4.pdf the pain threshold is 46.7C or 116F77.86.6.186 (talk) 22:46, 7 July 2010 (UTC)
- Sounds like an immersion heater. All the immersion heaters I've had have been non-adjustable (ie, they're set in the factory). Domestic hot water is usually 60–70 ºC (140–155 ºF) when it comes out the tap. Physchim62 (talk) 22:43, 7 July 2010 (UTC)
- Okay, I was really looking for a general answer about how hot water can be and still be comfortable briefly. Now that I think of it, this thing must have an owner's manual somehwre in this house. It is electric and a cylinder in a closet; that much I know.Vchimpanzee · talk · contributions · 21:50, 7 July 2010 (UTC)
- By the way the standard (in the UK at least) is 60C, which is well above the pain threshold - in every house I've ever lived water from the hot tap on its own was always too hot to touch..77.86.6.186 (talk) 22:55, 7 July 2010 (UTC)
- In NZ 60 ºC is recommended for the hot water cylinder. No lower because of the risk of bacterial growth particularly Legionnaires disease but no higher because it's considered just a waste of energy.
- However water from sanitary fixtures provided for personal hygiene i.e. sinks for washing hands, taps in the bathroom, the shower etc (the kitchen isn't included obviously) is not supposed (according to the building code) to be any higher then 55 ºC and lower (45-50) is recommended for homes with young children (45 is required for schools and stuff) [6] [7]. Modern systems usually include a tempering valve (which mixes cold water) for this reason (depending on how this is setup it may or may not affect the kitchen, laundry etc).
- The temperatures are chosen more for safety then being able to comfortably use the water. At 55 ºC it takes on average 10 seconds for a full thickness burn of a child (22 seconds for an adult), at 50 ºC 40 seconds for a child (5 minutes for an adult), I think 45 ºC is considered fairly safe even for a child for long periods of exposure [8] [9] [10] Nil Einne (talk) 23:10, 7 July 2010 (UTC)
- By the way the standard (in the UK at least) is 60C, which is well above the pain threshold - in every house I've ever lived water from the hot tap on its own was always too hot to touch..77.86.6.186 (talk) 22:55, 7 July 2010 (UTC)
In the UK the recommended standard temperature for hot water has been increased, to avoid Legionarres Disease lurking in the pipes. If it is the same where Vchimpanzee is, then the plumber may have adjusted the thermostat to increase the temperature. 92.29.125.22 (talk) 08:42, 8 July 2010 (UTC)
- I doubt that the plumber actually did anything. The pipe may have caused the change because it's a fairly minor change. I'm going with 77.86.6.186, and if I need a new water heater anytime soon, I'll mention those figures. I stayed in a motel where the hot water never reached the point of being uncomfortable.Vchimpanzee · talk · contributions · 17:23, 8 July 2010 (UTC)
- If you told us the brand and model of your water heater, someone may be able to find out where the thermostat is and if it is adjustable. 92.24.188.89 (talk) 18:35, 8 July 2010 (UTC)
- I'm somewhat surprised by that. I can understand requiring a 60 ºC storage temperature in the cylinder as with NZ. But requiring/recommending 60 ºC coming out from the pipes seems pointless. Since the water isn't continously flowing and isn't continously heated, unless you have very good insulation on the pipes it seems unlikely the water will stay at 60 ºC in the pipes for long, in fact it could easily drop to within the danger range within an hour or so I would guess if you don't have any insulation on the pipes. So if you are worried about Legionarres Disease in the pipes a better bet may be to flush out the pipes regularly. According to some source I read at 50 ºC it takes between 5-6 hours to kill Legionarres Disease so it doesn't even seem occasionally flushing the pipes with 50 ºC is likely to be much more effective in killing anything that is in them then flushing at a lower temperature (it obviously will depend somewhat how long the water stays that high and how often you flush out the pipes). I've looked for some refs, and the best I can find is [11] [12] and various similar refs which suggest a 50 ºC minimum delivery temperature is required in the UK which still surprises me but isn't as extreme as 60 ºC. [13] in fact recognises that the temperature is not going to stay at 50 ºC in the pipes and simply requires it be 50 ºC within one minute (although does at least say the pipework containing water below 50 ºC should be kept to a minumum), which adds to my confusion. Of course since there doesn't seem to be a maximum many may end up with a close to 60 ºC delivery given the 60 ºC required storage and the fact they probably won't bother installing a tempering valve but I haven't yet found any refs mentioning a 60 ºC delivery is required or recommended in the UK. As an aside, I also found [14] which disputes whether the 60 ºC storage is really necessary or 50 ºC is enough. However issues like stratification and thermostat inaccuracy could be one of the reasons why 60 ºC is chosen as 50 ºC even if it is enough doesn't give much leeway for error. Nil Einne (talk) 20:16, 8 July 2010 (UTC)
- The U.S. Department of Energy says that 120º F (so 49º C) is a useful and efficient temperature.[15] 75.41.110.200 (talk) 22:25, 8 July 2010 (UTC)
Finger squashing objects
I look at a clock face with one eye closed, holding my index finger upright in front of my face so it appears at the right side of the clock, out of focus. As I move my finger to the left, in front of the clock face, the clock face appears to "squash" or compress a bit while remaining completely in view before it starts to disappear. Why does it appear to "squash#"?--92.251.236.7 (talk) 23:49, 7 July 2010 (UTC)
- I'm not sure I get what you mean, i'm trying it out but not really seeing any kind of effect.. how far is the clock and how far is your finger from your face? Arm out stretched or near your face? Vespine (talk) 01:45, 8 July 2010 (UTC)
- Even just looking at the text on the computed screen, if a finger is held very near the eye and moved across the text, the letters move a tiny bit, in the blur next to the finger, before they are covered. They also seem slightly sharper. As the finger moves left and right, the letters nearest the finger move a tiny bit in the opposite direction, as if there were a lens. It may be an effect akin to a pinhole image. What would ray tracing of the image imply? Edison (talk) 02:16, 8 July 2010 (UTC)
- I don't entirely understand what the original poster means, but it sounds like the phenomenon they're describing is one arising from the pupil not being a pinhole. That is, most of the time we can treat the pupil as being a point hole, but if some object (like the finger) is held near the eye, but the eye is focussed on a distant object like the clock) then some lightpaths from retinal cells will intersect the finger and some will not, producing a blurred image. -- Finlay McWalter • Talk 02:20, 8 July 2010 (UTC)
- Well the clock face (or text in Edison's case) actually appears sharper and clearer when "squashed".--92.251.228.73 (talk) 12:25, 8 July 2010 (UTC)
- I don't entirely understand what the original poster means, but it sounds like the phenomenon they're describing is one arising from the pupil not being a pinhole. That is, most of the time we can treat the pupil as being a point hole, but if some object (like the finger) is held near the eye, but the eye is focussed on a distant object like the clock) then some lightpaths from retinal cells will intersect the finger and some will not, producing a blurred image. -- Finlay McWalter • Talk 02:20, 8 July 2010 (UTC)
- Even just looking at the text on the computed screen, if a finger is held very near the eye and moved across the text, the letters move a tiny bit, in the blur next to the finger, before they are covered. They also seem slightly sharper. As the finger moves left and right, the letters nearest the finger move a tiny bit in the opposite direction, as if there were a lens. It may be an effect akin to a pinhole image. What would ray tracing of the image imply? Edison (talk) 02:16, 8 July 2010 (UTC)
- Light does bend a little bit around object. that phenomenon is called diffraction.Dauto (talk) 11:57, 8 July 2010 (UTC)
- Your finger is probably acting as a partial pinhole lens. Hopefully the physics of it will be explained at pinhole camera. See also Pinhole occluder and pinhole glasses. 92.24.188.89 (talk) 18:38, 8 July 2010 (UTC)
- For anyone who wants a clarification as to the OP's question, a video of head-crushing by Kids In The Hall is quite illustrative. SamuelRiv (talk) 09:41, 9 July 2010 (UTC)
July 8
WTF is that on your ear? AKA fleshy protuberance on the targus?
Here in China I often see people walking around with - for lack of a better word - ``growths`` just in front of their ears - basically just ahead of the targus. Sometimes they're quite small, perhaps 2~3mm tall. Other times they can exceed 1cm in length. Never in my life have I seen these outside of China. I suspect that's because other cultures remove them for cosmetic reasons? In any case, I am VERY curious what causes these growths? what they're called? etc... 218.25.32.210 (talk) 05:21, 8 July 2010 (UTC)
I think the word is tragus 86.4.183.90 (talk) 07:01, 8 July 2010 (UTC)
- Is it a Preauricular skin tag? I've noticed them in China, too. Maybe it's more common to remove them at birth in the west? —Preceding unsigned comment added by 128.12.174.253 (talk) 06:10, 8 July 2010 (UTC)
I have heard that Lego blocks can be used to make even functionable robots that's with motors and all, how i's done ? Jon Ascton (talk) 10:42, 8 July 2010 (UTC)
- You would need additional parts such as motors and bearings and hinges. --Chemicalinterest (talk) 11:17, 8 July 2010 (UTC)
- By using the Lego Technics ®/ Lego Mindstorms® kits. CS Miller (talk) 11:37, 8 July 2010 (UTC)
- Yes! Lego Mindstorms sets make that really simple. You get motors, gears, wheels, a small computer module, some switches and (in some sets) rotation sensors and light sensors. The computer can be programmed remotely from your PC using either a C-like programming language or in a 'drag and drop' environment where you build things that are like flow charts. People have built some rather impressive robots and both NASA and the "FIRST Lego League" run competitions of various kinds for Lego robots. There is a thriving user community of 'AFOLs' (Adult Fans Of Lego) - of which I confess to being one - they too have occasional challenges (things like: "Build a robot to stack empty coke cans - the biggest stack wins - send in a video of your robot stacking cans - the tallest stack wins"). The Mindstorms sets cost a couple of hundred dollars - and contain enough to do some reasonably complex projects - but it definitely helps to have a larger stock of more traditional Lego and Lego Technics to give you a wider range of component choices. There is also a pretty good used market for such things when parents belatedly discover that their 'little darling' isn't a gifted with computers as they thought! SteveBaker (talk) 18:30, 8 July 2010 (UTC)
- Are there any YouTube videos available of these impressive feats please? 92.24.188.89 (talk) 18:49, 8 July 2010 (UTC)
- Searching http://www.google.co.uk/search?q=Lego%20Mindstorms&um=1&ie=UTF-8&tbo=u&tbs=vid:1&source=og&sa=N&hl=en&tab=wv seems to work.
- This seems suitably AWESOME!!! http://www.youtube.com/watch?v=eaRcWB3jwMo (got a bit carried away).87.102.42.55 (talk) 20:00, 8 July 2010 (UTC)
- Are there any YouTube videos available of these impressive feats please? 92.24.188.89 (talk) 18:49, 8 July 2010 (UTC)
KBr (Potassium Bromide)
Potassium Bromide was called in medical education?--אנונימי גבר (talk) 13:28, 8 July 2010 (UTC)
- Do you mean Kalium bromide or Kalii bromidum [16] aka Kalium bromatum or Bromide of Potash ? 87.102.42.55 (talk) 13:56, 8 July 2010 (UTC)
- Are you asking "What was it called in medical education?". --Chemicalinterest (talk) 15:22, 8 July 2010 (UTC)
"Shadow Biosphere" life on earth with arsenic DNA backbone?
Last night on the program "Through the Wormhole with Morgan Freeman," this researcher found some cells in Mono Lake that could survive in an environment with arsenic levels thousands of times higher than what most life could stand. The woman sheepishly (to my ears) said it's possible the DNA backbone of the cells in question have arsenic in place of phosphorus, but she just left it at that. Isn't there a way to verify that? (P.S. I read the timesonline article sourced in the Mono Lake article about this exact scientist of whom I am speaking. I'm just surprised it doesn't seem they can verify the composition of a DNA molecule there in their petri dish. I thought we could do that these days.) 20.137.18.50 (talk) 13:53, 8 July 2010 (UTC)
- from search of "DNA Arsenate" see [17] quote
The only stumbling block to the idea is that arsenic-based DNA tends to break down quickly. "You don't want to build your DNA out of a compound with a half-life in the order of a couple of minutes," points out Steve Benner of the Foundation For Applied Molecular Evolution in Gainesville, Florida
- Yes arsenic can be detected accurately in molecules - but with only a few percent substitution it could be difficult.87.102.42.55 (talk) 14:06, 8 July 2010 (UTC)
is it present? The skeletal structure looks funny .... does it reflect real life? John Riemann Soong (talk) 15:44, 8 July 2010 (UTC)


- The Haworth projection grossly exaggerates the angular difference between 'equatorial' and 'axial' bonds on the sugar structure.
- The ball and stick picture is more accurate - the 6ring is standard (chair) formation, the 5 ring is standard formation too (envolope)
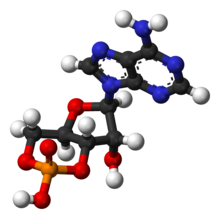
more accurate 3d image - If the question was about the ball and stick image - I think the angle chosen and blue heterocyclic structure makes the image look slighly odd- but it's not - note the two sets of rings are at right angles to each other - so the image has been projected at a slightly 'non-aesthetic' angle.87.102.42.55 (talk) 16:41, 8 July 2010 (UTC)
stimulation of exocytosis of nanoparticles by administration of 8-Br-cAMP
8-Br-cAMP is a lipophilic source of intracellular cAMP that can be added to solution. If I added some to epithelial cells (HeLa, lung cancer, etc.), should I expect gold particles trapped in vesicles to be exocytosed faster? Or would it do nothing if they hadn't reached the ER or a lysosome yet? John Riemann Soong (talk) 16:03, 8 July 2010 (UTC)
I have a point-and-shoot digital camera
It came with panasonic alkaline batteries. They used up in a day or two. OK. Then I changed them, and put brand new zinc chloride batteries, it shows "battery exhausted" even though they are brand new. Why ? Or should one only use alkaline batteries ? What's wrong ? Jon Ascton (talk) 16:17, 8 July 2010 (UTC)
- Take an ohmmeter across the terminals in your camera. Is the resistance unusually low? 20.137.18.50 (talk) 16:38, 8 July 2010 (UTC)
- Some ohmmeters use a high enough voltage that they would damage some electronic devices. I would instead measure the current drawn by the device from the usual battery, and perhaps the voltage the battery is putting out under the same circumstances. Care is needed to make sure the battery polarity is correct, the meter is connected with the correct polarity if an analog one, and the battery is not getting shorted out when such a test is done. Sometimes I have made a current probe by inserting as an insulator a piece of paper or very thin plastic between the battery terminal and the battery contact, or between two batteries, with a thin piece of metal on either side of the insulator, which get connected to the current input of the meter. Edison (talk) 14:57, 9 July 2010 (UTC)
- No, man the zinc chloride bat are BRAND NEW, just tore the wrapper ! Jon Ascton (talk) 16:49, 8 July 2010 (UTC)
- Try some alkaline batteries again. If it works, there's your answer. If it doesn't, you've either got yourself a physical fault with the camera (see above with ohmmeter) or a software/hardware fault which would need to be inspected by the manufacturer. Presumably it's still under at least a years warranty? Regards, --—Cyclonenim | Chat 16:53, 8 July 2010 (UTC)
- Take an ohmmeter across the terminals in your camera. Is the resistance unusually low? 20.137.18.50 (talk) 16:38, 8 July 2010 (UTC)
- FWIW, I once bought a package of alkaline batteries and one of them was "dead" straight out of the package. -- Coneslayer (talk) 16:54, 8 July 2010 (UTC)
- Some zinc batteries will rapidly lose voltage under load - giving a false 'battery dead' reading. This happens usually when the zinc part of the battery is the case of the battery .. batteries using finely divided zinc with more surface area are less susceptable to this effect. This page [18] seems to say that zinc chloride cells also use the zinc can construction.
- Still a good idea to test the battery in something else (like a torch or motor).87.102.42.55 (talk) 17:03, 8 July 2010 (UTC)
- Check the instructions.. but I'm fairly sure that for digital cameras alkaline batteries (or better) are always recommended.87.102.42.55 (talk) 17:10, 8 July 2010 (UTC)
- Zinc chloride or Zinc carbon cells are usually prone leaking since the can disintegrates as the battery discharges. This page http://michaelbluejay.com/batteries/ concludes Absolute crap. Do not buy I've got to agree with that. - alkaline batteries are so cheap nowadays and according to the data on that site give more energy per currency unit.87.102.42.55 (talk) 17:16, 8 July 2010 (UTC)
- By the way if you can tell us what state you are in I'm sure someone will be able to give a good place to buy the most cost effective batteries.87.102.42.55 (talk) 17:22, 8 July 2010 (UTC)
- My digital camera will only work with alkaline batteries, and not with zinc chloride. I imagine the ZC ones do not provide enough power. 92.24.188.89 (talk) 18:32, 8 July 2010 (UTC)
- The battery voltage depends on the battery chemistry; alkalines have a slightly different voltage than NiMH rechargeables, and I suppose zinc chloride is slightly different as well. Also, digital cameras (unlike flashlights) draw a lot of current while taking photos and much less at other times, and different chemistries respond differently to that. All digital cameras that use AA batteries are designed to work with NiMH and alkaline, but I don't know about zinc. I'd advise buying some NiMH rechargeables, since they're the cheapest in terms of cost per photo, and also more convenient since they will give you far more photos per charge. -- BenRG (talk) 00:42, 9 July 2010 (UTC)


- Ok, Now we have brought home a pack of rechargeable Nippo batteries. I am pasting a picture Are these OK for use in my Nikon Coolpix L20 ?
- Note that they are 1.2 v not 1.5 v Jon Ascton (talk) 16:11, 10 July 2010 (UTC)
- Why did you buy them before finding out if they would work? Googling your camera shows it needs alkaline batteries, so rechargeable alkaline batteries will work but the battery life, especially after several recharges, will be worse than with standard alkaline AA batteries, but who cares, because you can recharge them. Regards, --—Cyclonenim | Chat 17:31, 10 July 2010 (UTC)
- Note alkaline ≠ AA. The camera needs AA batteries, but they don't have to be alkaline, and these aren't. The package says "Ni-Cd rechargeable". According to this spec page, the Coolpix L20 supports "Alkaline, NiMH, Oxyride or Lithium" batteries. NiCd isn't mentioned, but that might be only because it's obsolete (replaced by the superior NiMH). I don't necessarily trust these batteries because I haven't heard of the brand and I was under the impression that nobody made NiCd batteries any more. Also, there's wide variation in rechargeable battery capacity (quoted capacities range from 900 to 2700 mAh at least) and wide variation in charger speed (from <1 hour to many hours—the one you bought says 8 hours), so you should check those specs before you buy. -- BenRG (talk) 19:25, 10 July 2010 (UTC)
- Why did you buy them before finding out if they would work? Googling your camera shows it needs alkaline batteries, so rechargeable alkaline batteries will work but the battery life, especially after several recharges, will be worse than with standard alkaline AA batteries, but who cares, because you can recharge them. Regards, --—Cyclonenim | Chat 17:31, 10 July 2010 (UTC)
- Is it possible that there may be any setting in Camera's menu about which battery to use ? —Preceding unsigned comment added by Jon Ascton (talk • contribs) 22:55, 10 July 2010 (UTC)
- According to this page there is. -- BenRG (talk) 01:06, 11 July 2010 (UTC)
- Is it possible that there may be any setting in Camera's menu about which battery to use ? —Preceding unsigned comment added by Jon Ascton (talk • contribs) 22:55, 10 July 2010 (UTC)
Burning polythene
What is the offensive and acrid gas resulting from the burning of polythene bags? Ethylene is just C2H4 after all. Androstachys (talk) 17:19, 8 July 2010 (UTC)
- Partially oxidised decomposition products ?? Are you sure it's polyethene - this might interest you http://www.boedeker.com/burntest.htm also see google books .. burn smells 87.102.42.55 (talk) 20:08, 8 July 2010 (UTC)
Polyethylene is a polymer....it's not the same as ethylene. Polyethylene has a lot of sigma bonds. Plus don't forget the presence of plasticisers and stabilisers. John Riemann Soong (talk) 21:58, 8 July 2010 (UTC)
- AFAIK the stench is due to pyrolysis and partial oxidation products: low-MW hydrocarbons, aldehydes, ketones, organic acids, that sort of stuff. (Pretty much the same stuff that makes diesel exhaust stink like a sonuvabitch.) FWiW 67.170.215.166 (talk) 03:01, 9 July 2010 (UTC)
- If you are getting an acrid gas perhaps you are burning polyvinyl chloride and making hydrogen chloride gas. Graeme Bartlett (talk) 11:10, 9 July 2010 (UTC)
Killer swan mom part 2

Ok, the cygnets hatched out June 12 or 13 and are already larger than the fattest mallard. Their parents are captive swans living in a half of a pond about the size of a soccer field (the other half houses another pair). There's also a pair of nesting common terns (the chicks will apparently fledge in a couple of days), and some poor mallards (of course these are wild, not captive) ... This female swan does not even look at the terns, but for some reason she kills the ducklings. They hide most of the day behind a rock ledge, but as soon as they venture in the open, the swan mom forgets her litter and charges at the ducklings. She reduced the older mallard litter to just one survivor, the other (younger) bunch still holds, but not for long. The swan daddy does not really care, he's obsessed with another male...
Question: do the females behave just as bad in the wild, or it's the price of captivity and tight space? East of Borschov 17:37, 8 July 2010 (UTC)
- The BBC's Springwatch programme this year featured a swan's brood being completely wiped out, mostly by such behaviour from other swans, so I would guess it's wild behaviour.--TammyMoet (talk) 20:11, 8 July 2010 (UTC)
- Swan attack (video) Cuddlyable3 (talk) 00:08, 10 July 2010 (UTC)
The effect of life on light shining through the atmosphere
Now that some crude images of extra-solar planets have been made, would the presence of life make any difference to the light that would pass through the atmosphere of a planet as it passes in front of its star? Could light reflected from the surface of the planet also be detected? 92.24.188.89 (talk) 18:47, 8 July 2010 (UTC)
- Yes, it is generally believed that living organisms will influence the atmosphere of a planet in ways that could be observed my studying the spectrum of light that has passed through the atmosphere (whether at the limb of the planet as it transits the star, or by reflection off the surface). Oxygen, for example, is not thought to persist well in planetary atmospheres, and must be replenished like it is by plants on earth. See, for example, Terrestrial Planet Finder: Detecting signs of life. -- Coneslayer (talk) 19:08, 8 July 2010 (UTC)
- Some astronomers are already trying to do that - so it's certainly not unreasonable. There have been several pronouncements about odd chemical imbalances in the atmospheres of Mars and some of the more interesting moons. For example, it was announced that the anomalous amounts of Methane in the atmosphere of Mars could only be explained by there being active microbial life there...until someone suggested that "serpentinization" could be responsible...and now we're not so sure again. The lesson to be learned here is that if we were to find a planet with a lot of oxygen (say), you can bet that "there must be life there!" will be the first conclusion - and then within a year or two of that, "well...we've thought of this other mechanism...so maybe not". What makes it difficult is that when you don't know the composition of the rocks - or almost anything else about the planet other than it's mass and orbital parameters - it's very tough to come up with a definitive answer. SteveBaker (talk) 21:40, 8 July 2010 (UTC)
I wonder how you would seperate the 'signal' of the light shining through the atmosphere of an extra-solar planet from the overwhelming 'noise' of the starlight? 92.24.181.157 (talk) 10:55, 9 July 2010 (UTC)
- Conceptually it's not that hard... obtain "baseline" spectra of the star when the planet is not transiting, and then take spectra during the transit and look for "new" absorption features (in excess of the overall decrease of light from the planet's opacity) corresponding to gasses of interest in the atmosphere. In practice, though, it's a challenging measurement, because the absorption features will be quite weak. You have to be very careful about understanding your instrumentation, along with any temporal variations in the equipment, Earth's atmosphere (if using a terrestrial telescope), or the star's light. We are getting there, though. See HD 189733 b; footnote 18 has an arXiv link where you can get a PDF of a paper with Spitzer Space Telescope spectra that are said to show water and methane in the planet's atmosphere. This is a Jupiter-sized planet, though... a small terrestrial planet will be considerably harder. (But I'm 33 years old, and when I was an undergraduate, the discovery of extrasolar planets at all was the Big New Thing. We keep moving forward, and we'll get there.) -- Coneslayer (talk) 17:39, 9 July 2010 (UTC)
- It is possible to detect the spectra of certain greenhouse gases in the atmosphere of a planet, for example methane which could be produced by life. See Extrasolar planet#Temperature and composition. ~AH1(TCU) 14:55, 10 July 2010 (UTC)
mythbusters nitro ram recoil.
In a mythbusters episode I saw they used a nitro ram to power a fist that knocked a dummy around, but the pipe that held the fist was just on a stand. So in theory someone could hold the pipe with the fist in and fire it off. So where does the equal and opposite force go that projects the first forward so fast. Does it work something like a recoiless rocket launcher, is there an apposing force? or is the recoil just applied elsewhere?
Thanks guys —Preceding unsigned comment added by 86.129.209.180 (talk) 20:43, 8 July 2010 (UTC)
- There is always an opposing force. I'm not familiar offhand with the specific episode, but from your description it sounds like the device is powered by pressurized gas. In that case, the gas can't escape out the fist end of the device, instead escaping out the back -- that is, a rocket. The one force is on the (closed) fist end (propelling the device, per Newton's second law), the other on the (open) exhaust end. Whether the system is recoilless is incidental to the way the forces conceptually work; either way it's a rocket-type system. — Lomn 21:01, 8 July 2010 (UTC)
- A ram isn't a rocket. A ram is usually more like a gun - the gas expands behind the ram forcing it out the front and the gas then follows it out. --Tango (talk) 21:04, 8 July 2010 (UTC)
- The stand was probably just strong enough to withstand the recoil, so it is the Earth that actually recoils. A person can also throw a punch without falling over. --Tango (talk) 21:04, 8 July 2010 (UTC)
acoustic levitation and spiderman

If objects can be levitated by soundwaves, then is it at least in theory possible to make an acoustic pulse weapon that can knock people down or objects, kind of like how shocker does it in spiderman. Or is there some kind of acoustic cut off point, or limit it can reach in terms of impulse .
thanks and sorry the question is kind of stupid. I was just wondering is all. —Preceding unsigned comment added by 86.129.209.180 (talk) 21:32, 8 July 2010 (UTC)
- I think the frog you're thinking of (yes, I know you're thinking about a frog) is the one pictured, which is being subjected to magnetic levitation. Comet Tuttle (talk) 21:41, 8 July 2010 (UTC)
No talking about acoustic levitation.
Thanks —Preceding unsigned comment added by 86.129.209.180 (talk) 21:50, 8 July 2010 (UTC)
- Can objects be levitated by ordinary soundwaves? It seems unlikely. Sound consists of alternating bands of high and low pressure - the air itself doesn't move bodily outwards from the source - it merely moves back and forth with the motion propagating outwards by no part of the air moving by very much without moving back again. If an object were to get a little 'push' as the sound wave compresses air up next to it - then a half-cycle later, the low pressure part of the wave would suck it right back again...so at best, you could only make your target vibrate (which might be enough to 'kill' it - but not enough to move it physically).
- The "acoustic levitation" trick relies on non-linearities in these properties of the air at extremely high pressures - I think it's more likely that you'd vibrate them to death before you knocked them over. Our article on acoustic levitation says that there is a practical limit of a kilogram or so that can be levitated in this way - but that's not enough to knock over a person.
- There are toys (called things like Air bazooka) which send a single large pulse of compression wave outwards...and giant versions of those like this are able to knocks over stuff like empty soda cans - but that's not like a continuous sound wave, material actually travels along the path of the compression - you can see this happen when people use such machines to shoot smoke rings. It's generally a bad idea to try to learn physics from spiderman comics! SteveBaker (talk) 21:56, 8 July 2010 (UTC)
- I've heard there's a variant of the Brown note that makes you lose your balance and actually exists. I might be wrong about that second part. 67.172.112.226 (talk) 22:06, 8 July 2010 (UTC)
Of course it's possible to make an acoustic pulse that will knock people down: just set off a suitably sized explosion. If you want to do it with a non-explosive device, that'd be harder. --Anonymous, 16:30 UTC, July 9, 2010.
- No - an explosion isn't like sound. It's large amounts of gaseous products from the rapid combustion of the explosive - that bodily moves outwards. Sound is a back-and-forth motion - which is why this is a problematic suggestion. At any given point along the path of a sound wave, the pressure alternates higher and lower than the 'ambient' air pressure. Which exerts alternating outwards and inwards forces on whatever it impacts. With an explosion, a few kilogrammes of gas (which is a LOT) move outwards - bodily in an effort to equalize the pressures everywhere. The pressure at any given point goes up as the shock wave goes by - and then gradually falls back to ambient. It never gets below the ambient air pressure so it never 'sucks' the object it hits back towards the source...but that's precisely what DOES happen with sound - and ordinarily the outward 'push' is immediately and perfectly counteracted by the inward 'suck'. Explosions only 'push'. The physical motion of that gas at great speed is what imparts the net force onto the object - and the force is ONLY away from the source. The only way for 'sound' to do this is for some very extreme thing to happen where the sound is so spectacularly loud sound that the air pressure drops to zero in the low pressure parts (and can therefore drop no further) - or the high pressure parts hit some non-linear effect within the gas (like it liquifies or something). That produces asymmetry between high and low parts of the sound wave which really can produce a net outward force. But that's crazy loud sound! SteveBaker (talk) 03:44, 10 July 2010 (UTC)
- Good point. However, you're talking about the blast and I was thinking of the shock wave, which is felt much farther away. I'm not sure to what extent, if any, it involves back-and-forth motions like ordinary sound, but I think the fact that it propagates through the air as a wave qualifies it as sound even if it does move supersonically. --Anon, 05:13, July 10, 2010.
Forest terminology — non-coniferous?
Can't for the life of me remember if there is a term to describe forests consisting only of leafy trees, as opposed to those containing pines, furs, and so on. Is there such a term that I can use in contrast to coniferous? Spent the last hour or so reading about trees to no avail. BigNate37(T) 22:07, 8 July 2010 (UTC)
- Deciduous or hardwood. Not that all deciduous trees are hardwood, and not all hardwood is hard (eg balsa) CS Miller (talk) 22:12, 8 July 2010 (UTC)
- Perfect, thank you. BigNate37(T) 22:19, 8 July 2010 (UTC)
- And not all hardwoods are deciduous. Googlemeister (talk) 13:25, 9 July 2010 (UTC)
- Broad-leaved might be a better bet. Alansplodge (talk) 23:18, 9 July 2010 (UTC)
- And not all hardwoods are deciduous. Googlemeister (talk) 13:25, 9 July 2010 (UTC)
dehydrated yogurt
will dehydrated yogurt spoil quickly if left sealed in a package on the shelf and not refrigerated? —Preceding unsigned comment added by 71.137.244.115 (talk) 22:20, 8 July 2010 (UTC)
- Please clarify what you mean by "dehydrated yoghurt". That could mean half a dozen things, and I don't feel like trying to give all the possible answers (even if I could!). Looie496 (talk) 22:50, 8 July 2010 (UTC)
- Dehydrated yoghurt is probably similar to dehydrated milk that keeps indefinitely in powdered form without refrigeration, and can be carried as part of a trail snack. Cuddlyable3 (talk) 10:24, 9 July 2010 (UTC)
Evolution and entropy
Why isn't the accumulation of beneficial mutations a violation of entropy? Is it because we assume that accumulations of neutral and disadvantageous mutations also occurs, but that the former is silent and the latter causes those involved to perish, leaving only the beneficial mutation accumulators to seem as though they are violating this concept? DRosenbach (Talk | Contribs) 23:56, 8 July 2010 (UTC)
- I don't know whether they help, but we have articles on entropy and life and negentropy. ---Sluzzelin talk 00:04, 9 July 2010 (UTC)
- (edit conflict - the article negentropy is what I'm describing here roughly) I'm not really answering the evolution/dna part of your question - but stating that (all) living creatures are machines that 'violate entropy' that is they internally decrease or maintain their entropy whilst converting other material (eg food) to products that have much higher entropy.
- In the same way a machine that sorts M&Ms or Smarties into different colours, then destroys all colours except blue doesn't violate entropy (even though the entropy of the sweets has decreased by increased ordering) - since the machine requires a source of energy to operate (that energy being converted ultimately to heat - and thus to increased entropy).87.102.42.55 (talk) 00:08, 9 July 2010 (UTC)
- (after some really weird Wiki bugs) Ultimately the life on Earth is driven by the energy we get from the Sun, so the global entropy of the Solar System is probably not decreasing :) . That being said, a relation between thermodynamic entropy and life has been researched on and off for about 100 years now. We have some articles on Entropy and life, but they are little more than stubs. I haven't found a good book on the subject yet, either. --Dr Dima (talk) 00:13, 9 July 2010 (UTC)
- The second law of thermodynamics only applies to closed systems. Earth's biosphere is not a closed system, because of the influx of energy from the sun. Even if it were, the accumulation of mutations wouldn't automatically violate the second law, because it only requires that the total entropy of a system increase, not that the entropy of every part individually increase. Looie496 (talk) 00:51, 9 July 2010 (UTC)
- (ec) You have a mistaken understanding of the Second law of thermodynamics. It says merely that entropy increases over time in closed systems. "Closed systems" is important here—it means that additional energy is not entering into the system. You can lower the amount of entropy in an open system. I do it all the time when I clean my office, moving things from chaos towards order. It just requires energy. In the case of my room, I bring the energy in through a chain that ultimately traces itself back to the Sun. Life is itself a giant lowering of entropy—it is organization, self-organization. It requires huge amounts of energy to do this. It doesn't violate the second law of thermodynamics, though, because the system is not closed. In the case of genetics, the entropy is kept at a reasonable level because of natural selection itself—it weeds out the total chaos, the total duds, and channels that "energy" (which is now getting extremely metaphorical) towards the beneficial genes. --Mr.98 (talk) 00:53, 9 July 2010 (UTC)
- Victor Stenger's book, God the Failed Hypothesis has a good treatment of this subject as it applies to life and the whole universe.. Since after you get past "life", why should there be planets and suns and galaxies at all if everything started from nothing. I'm not starting the argument, i'm just saying his book explains it with science and all quite well. Vespine (talk) 01:35, 9 July 2010 (UTC)
- This and many other fallacious arguments espoused by proponents of creationism (and its dressed-up cousins) are touched on in our article on intelligent design. This page provides a thorough explanation of the flaws in the 'evolution violates the laws of thermodynamics!' argument of intelligent design proponents. TenOfAllTrades(talk) 01:53, 9 July 2010 (UTC)
- Also, I think it's debatable whether or not life, or the evolution thereof, would necessarily increase entropy at all. I think increased amounts of life certainly will, and more complex machines will, but how is a human more complex of a machine than a cow? Surely the cow has more moving parts - look at all that chewin' and digestin'! SamuelRiv (talk) 09:46, 9 July 2010 (UTC)
- After a certain threshold from an entropy point of view I doubt it matters. The difference between a human-sized mass of bacteria and a human being is not so great when it comes to entropy, I don't think. --Mr.98 (talk) 22:21, 9 July 2010 (UTC)
- Also, I think it's debatable whether or not life, or the evolution thereof, would necessarily increase entropy at all. I think increased amounts of life certainly will, and more complex machines will, but how is a human more complex of a machine than a cow? Surely the cow has more moving parts - look at all that chewin' and digestin'! SamuelRiv (talk) 09:46, 9 July 2010 (UTC)
- I would suggest that evolutionary radiation - the diversification of a small number of species into many - represents an example of an increase in entropy consistent (more precisely, inherent to) evolution. Looked at this way, evolution is perfectly Second Law compliant! – ClockworkSoul 22:49, 9 July 2010 (UTC)
Entropy applies to 'closed systems' - the earth isn't a closed system because we get sunlight coming in - which is nice low-entropy stuff - and it winds up as low grade infra-red radiation - which is high-entropy stuff. Think of it like this - you have a room with a robot and a whole bunch of children's building blocks scattered all over everywhere. If you supply power to the robot, it can pick up the blocks and stack them neatly - which reduces their entropy by removing chaos and adding 'order'. But wait! That can only happen if you supply external power to the 'system' by plugging in the robot - it's not a 'closed system'. If you don't plug in the robot, nothing happens and entropy doesn't decrease. However, when the robot is plugged in, somewhere a power station is converting organized, low entropy coal into chaotic high entropy CO2 in order to make the electricity to run the robot. The amount of entropy created by the power station is much more than the entropy removed by the robot. In a 'closed system' comprising (1) a pile of coal, (2) a power station, (3) a robot and (4) the building blocks, the coal gains entropy at a higher rate than the building blocks lose it - so the TOTAL entropy of the closed system increases over time - just as the laws of thermodynamics tell us they must. With animals (rabbits maybe), the local lowering of entropy in their bodies is more than compensated for by the way they consume low entropy foods like carrots maybe and produce high entropy heat and poop. The carrots, in turn, took high entropy CO2, water and dirt and 'organized it' into low-entropy carrot - but only at the cost of turning low entropy sunlight into high entropy infrared. Just as with the robot and the power station, we have low entropy sunlight turned into high entropy IR faster than the carrots and the rabbits can keep their low entropy existence intact. So it's safe to say that the small amount of local lowering of entropy due to evolution is dramatically outweighed by the additional entropy that process created. SteveBaker (talk) 03:36, 10 July 2010 (UTC)
July 9
Human flight to mars or venus
What's the shortest time it would take a spaceship carrying astronauts to reach Mars or Venus, assuming both of those planets were at their maximum distance from Earth? Are there any propulsion systems in development that could speed up the journey? And no need to question why they would be going to Venus, I'd just like to know the travel time. Thanks in advance!--92.251.187.65 (talk) 00:01, 9 July 2010 (UTC)
- There is no shortest time, except the time it would take light to make the trip. You can always shorten the time by expending more energy. There is a lowest-energy trip -- would that help you? (And why in the world would you assume the planets are at their maximum distance?) Looie496 (talk) 00:56, 9 July 2010 (UTC)
- At maximum distance for Mars means 400 million km "as the crow flies". That's ignoring having to avoid the sun, while at its closest it's only 55 million km. Apollo 11 reached a max speed of around 800m/s. Not sure how much faster an interplanetary craft could go. Voyager is going at about 16km/s, so i imagine somewhere between those two values, but probably closer to the lower end. Vespine (talk) 01:12, 9 July 2010 (UTC)
- Only 800 m/s? Man, that's slow for a spaceship -- even some airplanes can go faster than this! 67.170.215.166 (talk) 03:10, 9 July 2010 (UTC)
- 800 m/s is wrong. Apollo 13 (which was the fastest of the Apollo missions) topped out at around 40,000 kph, which is around 11,000 m/s. The others were slower, but by a factor of ~10% or less. — Lomn 14:24, 9 July 2010 (UTC)
- Only 800 m/s? Man, that's slow for a spaceship -- even some airplanes can go faster than this! 67.170.215.166 (talk) 03:10, 9 July 2010 (UTC)
- It depends. It sounds like the OP is meaning humans and a trip starting from rest relative to earth. Unless we find some way [19] to counteract the effect of acceleration on the human body inside the space ship and this seems a rather difficult thing to do, you're going to have limited acceleration so particularly for a short trip like from earth to Mars/Venus the speed of light isn't really the limit for human spaceflight since even with unlimited energy and a wondership you still need to accelerate/decelerate and you need to do it slow enough that you don't kill the human inside (and of course you can never reach the speed of light anyway even if you used 10^10^10^10^10^10G acceleration). For such a short trip, the acceleration/deceleration is really the limit. While admitedly the OP just said to reach Mars/Venus so you could argue there's no need for deceleration, I suspect the OP want's to actually stop on or near Mars/Venus rather then either slam into them of fly past at a high velocity so I'm presuming we do need deceleration. It's common to assume a 1G acceleration in these sort of wondership scenarios usually on trips to stars although humans could likely survive higher even on such long trips. You can use this [20] (or plenty of other calculators) to help with calculations based on a 1G acceleration. Liquid breathing#Space travel suggests a potentially practical method for 15-20G perhaps higher so if you want to be generous perhaps take 20G. Of course practically we can't even manage close to 1G constant acceleration and there are so many technical challenges to achieving that that it's a moot point. Nil Einne (talk) 05:55, 9 July 2010 (UTC)
- At maximum distance for Mars means 400 million km "as the crow flies". That's ignoring having to avoid the sun, while at its closest it's only 55 million km. Apollo 11 reached a max speed of around 800m/s. Not sure how much faster an interplanetary craft could go. Voyager is going at about 16km/s, so i imagine somewhere between those two values, but probably closer to the lower end. Vespine (talk) 01:12, 9 July 2010 (UTC)
- It's worth noting that nobody's planning to make a worst-case trip from Earth to Venus, Mars, or anywhere for the foreseeable future. Instead, we'd use Hohmann transfer orbits, which are achievably fuel-efficient. Travel times vary depending on how long you want to stay at the destination -- anywhere from three to ten months is a possible Earth-Mars transfer time. Venus would be faster (and the launch windows more frequent), but as nobody is discussing a near-term manned mission to Venus, I'm not aware of literature on trip times. However, JPL's site discusses the mechanics of interplanetary travel and includes some commentary on possible future use of ion drives. — Lomn 01:27, 9 July 2010 (UTC)
- So basically only someone who works for Nasa could know how long a "worst-case" trip would take?--178.167.149.82 (talk) 13:43, 9 July 2010 (UTC)
- Plenty of people could calculate how long it would take. The point is, no one in the industry cares about calculating it, because no one in the industry is going to plan a worst-case trip. In all probability, with our current tech, a worst-case launch is simply not doable. That's why we have launch windows for missions of all types. — Lomn 14:22, 9 July 2010 (UTC)
- Hohmann transfer orbits are great for unmanned probes. Manned missions would probably use something quicker. They wouldn't do the trip while the planets were on opposite sides of the sun, though; that would be crazy. --Tango (talk) 21:10, 9 July 2010 (UTC)
- So basically only someone who works for Nasa could know how long a "worst-case" trip would take?--178.167.149.82 (talk) 13:43, 9 July 2010 (UTC)
According to the introduction, Navajo Sandstone is a large formation of sandstone found in Utah, northern Arizona, and northwestern Colorado. No map is shown, and the statement isn't sourced. Is this really true, or is "northwestern" an error for "southwestern"? After all, the only portion of Colorado that borders Arizona is the southwestern corner. Nyttend (talk) 00:31, 9 July 2010 (UTC)
- This USGS webpage gives the areal extent of the formation as northern Arizona, northwestern Colorado, Nevada and Utah, remember that later events can chop up an originally continuous distribution. I'll add the link to the article page. Mikenorton (talk) 08:45, 9 July 2010 (UTC)
- There is no discontinuity. Almost the entire western border of Colorado is shared with Utah, where the bulk of the Navajo Sandstone lies. Looie496 (talk) 17:40, 9 July 2010 (UTC)
Self-inflating Ball
My dog has a rubber playground ball that he loves to chase around and chew on. One day, he bit a hole in the rubber and it deflated (Although, this didn't seem to bother him at all!). Strangely enough, over the next couple of hours while the ball was left alone, it began to re-inflate! This seems to happen every time that he squeezes the air out. Why does this occur? Perhaps there is some difference in the air pressure on the inside and outside of the ball? I'd appreciate any ideas as to what's going on! Thanks! Stripey the crab (talk) 00:46, 9 July 2010 (UTC)
- Possible: The ball is a sponge - the air is squeeze out using force, the rubber holds its shape and wants to return to spherical, slow ingress of air prevents the sponge rapidly returning to its normal shape - it happens slowly.87.102.42.55 (talk) 00:56, 9 July 2010 (UTC)
- It may be that the rubber's "natural" shape is the full ball shape. If you think about the deflated state, the surface of the ball is very folded, and the rubber may exert a force against that which overcomes gravity pulling the top in. Rckrone (talk) 01:56, 9 July 2010 (UTC)
- Is it a hollow ball or a solid one?87.102.42.55 (talk) 02:05, 9 July 2010 (UTC)
- How does a dog deflate a solid ball? Cuddlyable3 (talk) 10:07, 9 July 2010 (UTC)
- The ball is completely hollow and made of a flat sheet of rubber. It's like a small kickball. It has that distinctive crosshatching pattern of squares. —Preceding unsigned comment added by Stripey the crab (talk • contribs) 12:48, 9 July 2010 (UTC)
- By the ball being a fine sponge, which is solid, not hollow ;) --Chemicalinterest (talk) 10:41, 9 July 2010 (UTC)
- It's not a sponge. If you completely empty any ball of air and leave it alone for a while, it will re-inflate to a certain extent although it will remain very soft. Probably because the rubber or leather can't retain a squashed shape.--178.167.144.225 (talk) 15:46, 9 July 2010 (UTC)
Wind turbine blades

Wind turbine blades appear to be usually long and thin (in both other directions) - I was wondering what reasons there are why shorter, wider blades are never seen? Indeed Wind_turbine_design#Turbine_size says :"For a given survivable wind speed, the mass of a turbine is approximately proportional to the cube of its blade-length. Wind power intercepted by the turbine is proportional to the square of its blade-length" - a shorter blade would seem to be easier to support and thus lighter for a given cross sectional area, as well as having a lower rotational inertia. Are aesthetics a consideration causing long thin blades?, or other factors I haven't considered? (ie I wondering about something more like File:HydroelectricTurbineRunner.png but made of lightweight composites instead of cast metal, and with the ability to vary the pitch, and not quite so stubby either..) 87.102.42.55 (talk) 02:42, 9 July 2010 (UTC)
- The reason for long narrow blades is aerodynamics -- an airfoil with a high aspect ratio makes better use of the pressure difference between its two surfaces by minimizing the induced drag caused by vortices at the wingtip, therefore it's more efficient. Same reason why helicopters and autogyros use long narrow blades for their rotors. (This, incidentally, is my last post here for a while, since I am boycotting Wikipedia because of its extreme left-wing, cosmopolitan, pro-Soviet, pro-Third World, and anti-American/anti-Israel bias.) Clear skies to you, and farewell. 67.170.215.166 (talk) 03:19, 9 July 2010 (UTC)
- This is off-topic - but in the interests of truth, I should point out that the previous poster has been blocked from editing Wikipedia for making abusive personal attacks on other editors. So this is not so much a 'moral standpoint' boycott as it is a 'shut out for breaking our rules' situation. SteveBaker (talk) 13:32, 9 July 2010 (UTC)
- Looking over your edit history reveals a majority of posts -- technical/scientific in nature -- which were very valuable and constructive, and a minority of posts -- political in nature -- which were inflammatory or worse. I hope that some day you'll find some modus vivendi that allows you to continue the helpful posts and leave out the obvious political disagreement you have with many Wikipedians. --Sean 16:30, 9 July 2010 (UTC)
- We have a cosmopolitan bias? I thought the whole idea was to be accessible to the world, and not just you know, Americans. John Riemann Soong (talk) 16:13, 9 July 2010 (UTC)
- (rewritten once) I didn't really understand how induced drag is less for longer blades - the article says it related to the angle of attack, which makes sense. Yet it will also be proportional to the speed of the moving object.Mmmh but induced drag just pushes the blades back, not like an airplane where it affects flight..I'm guessing that induced drag due to reactive forces is not a big issue for a stationary object (excluding the strength of the support)
- How does the drag due to wingtip vortices compare to the frictional drag? 87.102.42.55 (talk) 03:40, 9 July 2010 (UTC)
- see Wingtip_vortices#Cause_and_effects. as I understand it (which may be badly) drag-inducing wingtip vortices only affect the end of the lift-generating surface, so a long, thin blade produces - proportionally - a lot of unaffected lift area with respect to drag area. but then, I'm just a left-wing, cosmopolitan, anti-American liberal, so what do I know?
 --Ludwigs2 04:26, 9 July 2010 (UTC)
--Ludwigs2 04:26, 9 July 2010 (UTC)
- I don't see why they would be only at the tip - I get the explanation of them reducing lift (by air flowing back to increase the top surface wing pressure) - but why not all along the trailing edge ?? I don't get the airspeed explanation for why the trailing edge doesn't have the same effect - isn't the airspeed similar at the tip. I did understand the bit about shorter chords being better. (Maybe wingtip vortices are a red-herring?). I'll think about the chord effect.87.102.42.55 (talk) 04:52, 9 July 2010 (UTC)
- see Wingtip_vortices#Cause_and_effects. as I understand it (which may be badly) drag-inducing wingtip vortices only affect the end of the lift-generating surface, so a long, thin blade produces - proportionally - a lot of unaffected lift area with respect to drag area. but then, I'm just a left-wing, cosmopolitan, anti-American liberal, so what do I know?
- I'll say this and hopefully find an online source later - a couple physicists and engineers at my university were very interested in a slow-rotating wide-short-blade wind turbine concept - one of the key selling points, apparently, was its inability to kill birds. They must have run into some subtle technical problems along the way - bad lubrication, poor wind deflection, etc - that killed the concept, though I'll have to double-check. SamuelRiv (talk) 09:57, 9 July 2010 (UTC)
If 3 blades is good, would not more blades be better? Cuddlyable3 (talk) 10:05, 9 July 2010 (UTC)
- Not necessarily. Adding a fourth blade will increase the weight of the turbine, which means it is harder to keep it spinning. Now this would probably be overcome by the additional force of another blade (though your tower now needs to be stronger from the additional force), but then you also have a higher cost to buy the additional blade, and the added complexity of the system (one more thing to possibly break). I would be utterly shocked if the company that produces these turbines did not do some kind of cost/benefit analysis and optimization on these turbines and found that the additional power a fourth blade would add did not overcome the added costs and complexities. Googlemeister (talk) 13:16, 9 July 2010 (UTC)
- Three blades keep the wind pressure more evenly distributed. Note that normally, the wind speed increases with altitude. In a four-blade rotor, you always have the same surface distribution above and below the point of rotation. Hence there always is a stronger push on the upper half of the rotor than on the lower (surface is the same, but average wind speed is higher). This creates a torque that has to be borne by the bearings, something that is, apparently, very undesirable. With the configuration of a three-bladed rotor, the effect is less pronounced. In the Y configuration (two blades up), there is a larger surface area above the axis, but the blades are less high, and the lever is much smaller. In the inverted-Y configuration, the longer lever on the upper blade and the higher reach balances the larger area below. As a result, there is less torque, and the rotor runs more evenly. --Stephan Schulz (talk) 13:34, 9 July 2010 (UTC)
- Vertical wind speed gradient loads the bearings with near-constant torque with a 4-blade rotor or a torque that oscillates at 3x the spin rate with a 3-blade rotor. If I were a bearing I'd prefer the 4-blade rotor. Cuddlyable3 (talk) 19:30, 9 July 2010 (UTC)
- I can't even imagine being a bearing ;-). But as I understand it (i.e. not very well), it's the magnitude of the torque that matters, not the frequency. --Stephan Schulz (talk) 20:02, 9 July 2010 (UTC)
- Vertical wind speed gradient loads the bearings with near-constant torque with a 4-blade rotor or a torque that oscillates at 3x the spin rate with a 3-blade rotor. If I were a bearing I'd prefer the 4-blade rotor. Cuddlyable3 (talk) 19:30, 9 July 2010 (UTC)
In aircraft wing terms, lift is proportional to area. A windmill blade is just a sideways wing. For a given weight of rotor, you can have a fixed amount of area and choose to have more or fewer blades, wider or narrower blades - but the total area-to-weight ratio is pretty much a constant. So why longer/thinner blades? Well, that tip-vortex thing is the main issue. If you think about an airplane wing (because it's easier) you know that the air pressure under the wing is higher than on top - that's what keeps the plane in the air. But at the tip of the wing, there is nothing to stop the high pressure air underneath from sneaking up around the wingtip and into the low pressure region on top. This 'wastes' lift by lowering the pressure beneath and increasing it above - and the swirling motion caused by that air sneaking around where it's not wanted causes a spiral motion to get started in the air that swirls out behind the wingtip as a vortex. Making that air swirl around takes energy that's clearly being lost from someplace and therefore wasted. Having a narrow tip cuts down on that effect. Hence, a long, narrow 'wing' or 'blade' is more efficient. However, in aircraft, there are other trade-offs with stability, controllability, etc that mitigate that effect. Jet fighters that need to turn on a dime with high g-forces can't have long thin wings because they'd snap off! But in things like the U2 'spy plane', gliders ('sailplanes') and helicopters - and also windmills, the benefits are all in the direction of long-and-thin and against short-and-fat.
You could have more blades - but more blades means more 'tips' and that's functionally just like having fatter blades - for a given weight and the same total area - four blades have more 'tip width' than three. You could go down to two blades - but (I'm told) that causes problems with vibration and wears out the bearings on the windmill faster than three. Also, there is a point with long-thin blades where the height of the tower needed to keep the blades above ground level becomes a problem. If you go from three blades to two - and keep the same total blade area and the same blade width, you'd need a 50% taller tower - which is structurally difficult and costly. Having said that, I have seen two-bladed windmills - so this isn't such a cut-and-dried choice.
In the end, all engineering is about compromise - and it seems that three blades is the best compromise in this situation.
SteveBaker (talk) 13:48, 9 July 2010 (UTC)
- Just to highlight the main point in what Steve said - it's the sideways slippage of air around the tip of the airfoil (not the direct slippage of air under the edge of the airfoil) that causes problems. --Ludwigs2 17:24, 9 July 2010 (UTC)
veneer
do they use formaldehyde resin or contact cement to glue the Wood veneer on a a particle board or MDF desk? —Preceding unsigned comment added by Alexsmith44 (talk • contribs) 02:44, 9 July 2010 (UTC)
- A similar question came up before Wikipedia:Reference_desk/Archives/Science/2010_May_20#particle_board_desk. - I'm sure it depends on whether it's a wood veneer or a plastic effect wood veneer.
- For a real wood veneer this site recommends the same things you would use to glue wood to wood [21] I'm not sure if this applys to commercially produced wood on MDF veneers.87.102.42.55 (talk) 02:57, 9 July 2010 (UTC)
- This product http://www.titebond.com/ProductLineTB.asp?prodcat=4&prodline=19 is a PVA glue, this seems the most common, though I can also find references to people using epoxy, and urea/formaldehyde resins, as well as hot melt adhesives.87.102.42.55 (talk) 03:06, 9 July 2010 (UTC)
- There are also real wood veneers produced with the glue already attatched, not sure what sort of glue they use for these.87.102.42.55 (talk) 03:09, 9 July 2010 (UTC)
its a real wood veneer on a mdf desk by Ashley furniture. im just curious how its attached. —Preceding unsigned comment added by Alexsmith44 (talk • contribs) 03:17, 9 July 2010 (UTC)
- Could you contact them and ask http://www.ashleyfurniture.com/ I'm sure they won't mind answering a customer's question. There's a contact link at the bottom of their home page. (Normally for home-diy I'd guess PVA , but for a commercial product it's also likely that they use a heat bonded glue) 87.102.42.55 (talk) 03:44, 9 July 2010 (UTC)
whats a heat bonded glue? —Preceding unsigned comment added by Alexsmith44 (talk • contribs) 04:02, 9 July 2010 (UTC)
- Like the glue they use in Glue guns - it melts and becomes sticky. For small scale verneers there's an example here of using it with a household iron http://www.joewoodworker.com/veneering/iron-on-veneering.htm . This is the stuff they might buy for factory use http://www.bostik-amer.com/products/ardal-t7631 it's just Ethylene-vinyl acetate also known as EVA glue. 87.102.42.55 (talk) 04:09, 9 July 2010 (UTC)
so they dont use formaldehyde resin ? —Preceding unsigned comment added by Alexsmith44 (talk • contribs) 04:25, 9 July 2010 (UTC)
- I don't think there is a single answer. Certainly you can stick plastic laminates to particle board using a Contact adhesive (our article calls out exactly that as an application). Contact adhesive is basically just natural or synthetic rubber dissolved into some kind of solvent - it's not a formaldehyde resin. On the other hand, we know that there was a big kerfuffle about the trailers that FEMA supplied to the victims of hurricane Katerina that had used formaldehyde resin for this job - and that subsequent outgassing from the counter tops produced dangerous levels of formaldehyde inside the confined spaces of the trailers. Hence, there must be multiple different adhesive technologies used by the furniture and kitchen cabinet industries in different applications. Which particular technology is used where is clearly an important matter. If you are doing this yourself, then you should probably use contact adhesive in a well-ventilated area where there are no sources of ignition (both of those things are really important for contact adhesives - especially when you're using it in huge quantities to stick large areas of veneer/laminate). Be sure to follow the instructions carefully - contact adhesive has to be applied correctly and left to dry for a while BEFORE you bring the two surfaces together. Also, the stuff 'grabs' like crazy - so you have to align the two surfaces perfectly the first time you bring them together - there is no 'wiggle room' once they are in contact. For real wood veneers - use any kind of 'white' wood glue. SteveBaker (talk) 13:25, 9 July 2010 (UTC)
Dehydrated vs. freeze-dried
What is the difference between dehydrated food and freeze-dried? Is there a different process? —Preceding unsigned comment added by 71.137.244.115 (talk) 04:57, 9 July 2010 (UTC)
- Wouldn't dehydration be anything that dehydrates or dries the food (like in the sun, hot air, freeze drying, whatever), but Freeze-drying be the specific process that requires freezing (well be the specific process outlined in the article)? Nil Einne (talk) 06:22, 9 July 2010 (UTC)
Immiscible liquids
Suppose you have two immiscible liquids in a beaker, with boiling points separated by 20 degrees or so. Obviously, one is going to float on top of the other. If you gradually heat the beaker and its contents, a book I have claims that at a temperature lower than both boiling points, the mixture will start to boil from the interface. The reasoning is that both liquids would contribute to the vapor pressure of a bubble nucleated on the interface, so that's where the first boiling would occur. The book also claims that this boiling from the interface would occur until one of the liquids is gone, at which point the temperature starts to rise until the other liquid's boiling point is reached.
Is any of this true? I've never observed boiling from an interface before. --99.237.234.104 (talk) 08:24, 9 July 2010 (UTC)
- Liquids being immiscible does not mean that their mixed vapours do not have a common pressure. A bubble at the interface will have the higher of the two liquids' vapour pressures. That prevents the other liquid supplying any vapour to the bubble. Cuddlyable3 (talk) 09:54, 9 July 2010 (UTC)
- It's not only true, but it also has important practical applications: see our article on steam distillation. Physchim62 (talk) 10:16, 9 July 2010 (UTC)
- Cuddlyable3 got it wrong. Cuddlyable3 (talk) 11:25, 9 July 2010 (UTC)
- Steam distillation involves two WELL-AGITATED immiscible liquids. So well agitated that both liquids are roughly evenly distributed. I'm asking about the exact opposite: the case where one fluid floats on top of the other, with no intermixing at all. --99.237.234.104 (talk) 01:31, 10 July 2010 (UTC)
- Cuddlyable3 got it wrong. Cuddlyable3 (talk) 11:25, 9 July 2010 (UTC)
- It's not only true, but it also has important practical applications: see our article on steam distillation. Physchim62 (talk) 10:16, 9 July 2010 (UTC)
Magnetism
Two long parallel current carrying wires attract each other.Currents are in same direction.Due to magnetic force,they will come towards each other which will increase their kinetic energy.Does that mean magnetic force carry out a work as change in kinetic energy is equal to total work done? —Preceding unsigned comment added by Shreshtha Vibhu (talk • contribs) 09:29, 9 July 2010 (UTC)
- Yes. Force multiplied by distance (over which force is applied) equals work. Someguy1221 (talk) 09:37, 9 July 2010 (UTC)
- If the currents are in superconducting wires (so we can ignore resistance) where does the energy to do the work come from? Cuddlyable3 (talk) 09:57, 9 July 2010 (UTC)
- If you actually use the system to do work (that is, you let one of the current-carrying wires move), you will generate an electromotive force in the other wire which opposes the movement: this is Lenz's law. So, to keep the current flowing, you have to counteract that electromotive force from whatever power source you are using. Physchim62 (talk) 10:14, 9 July 2010 (UTC)
- The wires are superconducting loops that maintain a current with no external source. Will any two such loops if allowed to approach one another lose all their currents? Cuddlyable3 (talk) 10:50, 9 July 2010 (UTC)
- Not all their currents, but presumably the motion will induce currents in the opposite direction, in effect reducing the original currents. How does one calculate the energy stored in a superconducting loop? Dbfirs 16:38, 9 July 2010 (UTC)
- WHAAOE: Superconducting magnetic energy storage -- Coneslayer (talk) 16:50, 9 July 2010 (UTC) ... ... Thanks. Dbfirs 10:01, 10 July 2010 (UTC)
- The article doesn't explain what happens when two superconducting coils with current I in each attract and are allowed to approach each other until they become one coil. What is the current in that coil? Cuddlyable3 (talk) 19:17, 9 July 2010 (UTC)
- As they approach they loose energy, and when you pull them apart (which will take effort AKA energy) you generate electricity inside them. Although it's not totally reversible, since as they loose energy the magnetic force goes down, so it's easier to pull them apart the second time. Ariel. (talk) 23:33, 9 July 2010 (UTC)
- The article doesn't explain what happens when two superconducting coils with current I in each attract and are allowed to approach each other until they become one coil. What is the current in that coil? Cuddlyable3 (talk) 19:17, 9 July 2010 (UTC)
- WHAAOE: Superconducting magnetic energy storage -- Coneslayer (talk) 16:50, 9 July 2010 (UTC) ... ... Thanks. Dbfirs 10:01, 10 July 2010 (UTC)
- Not all their currents, but presumably the motion will induce currents in the opposite direction, in effect reducing the original currents. How does one calculate the energy stored in a superconducting loop? Dbfirs 16:38, 9 July 2010 (UTC)
- The wires are superconducting loops that maintain a current with no external source. Will any two such loops if allowed to approach one another lose all their currents? Cuddlyable3 (talk) 10:50, 9 July 2010 (UTC)
- If you actually use the system to do work (that is, you let one of the current-carrying wires move), you will generate an electromotive force in the other wire which opposes the movement: this is Lenz's law. So, to keep the current flowing, you have to counteract that electromotive force from whatever power source you are using. Physchim62 (talk) 10:14, 9 July 2010 (UTC)
- If the currents are in superconducting wires (so we can ignore resistance) where does the energy to do the work come from? Cuddlyable3 (talk) 09:57, 9 July 2010 (UTC)
- Nonsense. As you described, that would be a reversible process. Dauto (talk) 00:53, 10 July 2010 (UTC)
Cyanide death
Hi, This is to resolve a bet with a friend..does poisoning by cyanide consumption lead to a quick and painless death? —Preceding unsigned comment added by 122.160.164.64 (talk) 11:23, 9 July 2010 (UTC)
- Please read Cyanide poisoning. --Chemicalinterest (talk) 11:28, 9 July 2010 (UTC)
- (ec)I tidied the layout of your question and hope you don't mind. The Cyanide poisoning article describes symptoms of non-fatal cyanide intake. Fatal intake quickly induces coma and is a popular method of suicide, suitable for the whole family. Please do not encourage your friend to try this at home. Cuddlyable3 (talk) 11:40, 9 July 2010 (UTC)
- Not the editing other people's comments again; another 3-page discussion on WT:RD. (Rolls eyes). --Chemicalinterest (talk) 11:46, 9 July 2010 (UTC)
- It's OK. Cuddlyable3 only removed leading spaces. It's OK to fix formatting errors that disrupt the flow of the page - and having the question displayed as 'preformatted' text made it disappear off the right side of the page. The prohibition is only against changing the actual text. No debate required this time. SteveBaker (talk) 13:10, 9 July 2010 (UTC)
- I took the time to individually delete every letter in the comment preceding this one (by SteveBaker), and put them in one by one. Clearly not a single letter is the same one as that deleted, but merely another one of the same value. Am I within bounds or outside of it for having done this action? 84.153.202.156 (talk) 14:50, 9 July 2010 (UTC)
- It's OK. Cuddlyable3 only removed leading spaces. It's OK to fix formatting errors that disrupt the flow of the page - and having the question displayed as 'preformatted' text made it disappear off the right side of the page. The prohibition is only against changing the actual text. No debate required this time. SteveBaker (talk) 13:10, 9 July 2010 (UTC)
- Not the editing other people's comments again; another 3-page discussion on WT:RD. (Rolls eyes). --Chemicalinterest (talk) 11:46, 9 July 2010 (UTC)
- (ec)I tidied the layout of your question and hope you don't mind. The Cyanide poisoning article describes symptoms of non-fatal cyanide intake. Fatal intake quickly induces coma and is a popular method of suicide, suitable for the whole family. Please do not encourage your friend to try this at home. Cuddlyable3 (talk) 11:40, 9 July 2010 (UTC)
- How are we to know if you really did that since apparently nothing has changed? hydnjo (talk) 17:31, 9 July 2010 (UTC)
- It's the old Comment of Theseus paradox. -- Coneslayer (talk) 17:46, 9 July 2010 (UTC)
- How are we to know if you really did that since apparently nothing has changed? hydnjo (talk) 17:31, 9 July 2010 (UTC)
- DID NOT! --Chemicalinterest (talk) 17:43, 9 July 2010 (UTC)
- Ain't a paradox, just check Special:Contributions/84.153.202.156! There is no record of doing that. --Chemicalinterest (talk) 19:16, 9 July 2010 (UTC)
- The answer appears to be that it is quick (a matter of minutes) and perhaps slightly uncomfortable (at low doses you feel cold and short of breath before you lose consciousness; I don't know how much overlap there is between experiencing these feelings and the dose being fatal). 81.131.27.38 (talk) 20:12, 9 July 2010 (UTC)
- Perhaps it depends what you mean by "consumption". By all accounts the victims of gas chambers did not die either quickly or painlessly. AndrewWTaylor (talk) 20:25, 9 July 2010 (UTC)
- Cyanide poisoning isn't always effective, either. See Kim Hyon Hui. ~AH1(TCU) 14:46, 10 July 2010 (UTC)
Zucchini
What is that awefully sticky substance on a freshly peeled zucchini and does it exist on any other produce that I'm just not aware of or can't think of right now? DRosenbach (Talk | Contribs) 11:34, 9 July 2010 (UTC)
- I've never heard of anyone peeling a courgette before - it must be almost as rare as peeling a grape. 92.24.181.157 (talk) 12:37, 9 July 2010 (UTC)
- I don't think peeling them is that uncommon in the US, particularly in larger specimens which can develop a thick skin. (Heck, maybe our zucchini are thicker-skinned than your courgettes owing to differences in climate or varieties planted.) -- Coneslayer (talk) 12:42, 9 July 2010 (UTC)
- Now that I think about it I never remember peeling a zucchini in all the years we grew and ate them from our family garden (in the USA)... It's almost always cooked, being not as tasty when raw as it's cousin the cucumber. --144.191.148.3 (talk) 15:24, 9 July 2010 (UTC)
- I don't think peeling them is that uncommon in the US, particularly in larger specimens which can develop a thick skin. (Heck, maybe our zucchini are thicker-skinned than your courgettes owing to differences in climate or varieties planted.) -- Coneslayer (talk) 12:42, 9 July 2010 (UTC)
- You don't mean on the peel itself, do you? Because they often put food-grade wax on the peel to prevent dehydration and it can be quite sticky. --Sean 16:16, 9 July 2010 (UTC)
- I think you're talking about zucchini sap aren't you. When most vegetables are peeled there is a slight oozing of juice, and the zucchini sap is a bit thick and adhesive. It will do you no harm. 86.4.183.90 (talk) 17:04, 9 July 2010 (UTC)
- Sticky? I've always found that peeled zucchini are very slippery: I peel zucchini, sweet potatoes, potatoes, carrots, apples, and rutabaga with various degrees of frequency, but only zucchini slip out of my hands while I'm holding them partially peeled.—msh210℠ 18:09, 9 July 2010 (UTC)
Well, yes -- at first zucchinis are very slippery, but if you leave the film on your fingers it becomes very sticky after a minute or so (very astute of you, by the way!). DRosenbach (Talk | Contribs) 03:22, 11 July 2010 (UTC)
homosexuality
G'morning, everyone. I read somewhere that Homosexuality is an inherited (i.e., genetic) trait. How is this possible? Wouldn't it have been eliminated from the gene pool (for obvious reasons) and then we would have no more homosexuals if this were the case? But there is a large LGBT minority in the world. 76.199.146.154 (talk) 16:23, 9 July 2010 (UTC)
- Some people believe that homosexuality is 100% inherited. Some people believe that it is 100% a learned behavior. I believe that most people believe that some is inherited and some is learned. -- kainaw™ 16:32, 9 July 2010 (UTC)
- See Biology and sexual orientation, particularly at Biology_and_sexual_orientation#Sexual_orientation_and_evolution, for different theories. ---Sluzzelin talk 16:38, 9 July 2010 (UTC)
- BTW, even if homosexuality were a simple Mendelian trait where people with the recessive phenotype were 100% homosexual and the others were 100% heterosexual, that wouldn't mean the homosexual population would die out. You could have heterosexual "carriers" for the homosexuality allele who would reproduce and propagate it, sometimes resulting in offspring with the homosexual phenotype. -- Coneslayer (talk) 16:44, 9 July 2010 (UTC)
- And this is particularly true if the same gene that was recessive for homosexuality also produced another, reproduction-favorable trait (for example, good looks) and was dominant for that. Say if the gene for homosexuality is H and the allele for heterosexuality is h: then an HH individual is gay and probably won't reproduce, but an Hh (heterozygous) individual is attractive and not gay, and will have more children than average. In real life this sort of effect has helped the gene for sickle-cell anemia to persist; the disease is detrimental but heterozygous individuals are more resistant to malaria than others, hence more likely (in some countries) to survive and have children. --Anonymous, 16:51 UTC, July 9, 2010.
- Related concept of some use: kin selection. Having, say, one male who works with the women exclusively (just to use a very crude and problematic example) could be of overall benefit to the survival of the family, so a gene pool that produced someone like that 10% of the time could be of benefit even if the person with that particular expression of the gene does not reproduce. --Mr.98 (talk) 22:16, 9 July 2010 (UTC)
- And this is particularly true if the same gene that was recessive for homosexuality also produced another, reproduction-favorable trait (for example, good looks) and was dominant for that. Say if the gene for homosexuality is H and the allele for heterosexuality is h: then an HH individual is gay and probably won't reproduce, but an Hh (heterozygous) individual is attractive and not gay, and will have more children than average. In real life this sort of effect has helped the gene for sickle-cell anemia to persist; the disease is detrimental but heterozygous individuals are more resistant to malaria than others, hence more likely (in some countries) to survive and have children. --Anonymous, 16:51 UTC, July 9, 2010.
- Note that there are many genetic traits that either have no apparent survival advantage, or an apparent disadvantage, and yet they persist in the gene pool. --Sean 16:47, 9 July 2010 (UTC)
Being inherited is not the same thing as being genetic. Suppose a given trait is seen in people who have both of a pair of genes, A and B, and not in any other people. The trait is purely genetic, but since the two genes propagate independently, it is only inherited to a small degree. This isn't just a technical point, it's the key issue: even of scientists who believe that homosexuality has a genetic component, none of them believe that there is a single gene that is individually responsible for it. Looie496 (talk) 17:33, 9 July 2010 (UTC)
This entire thread assumes that gay people never have children, which simply isn't the case. Especially in older times or in countries that discriminated against gay people, maintaining a heterosexual lifestyle, had families etc just because that's what "society" expected 82.43.90.93 (talk) 17:38, 9 July 2010 (UTC)
- I don't think any of us are assuming that gay people never have children in the real world. We're pointing out that even if the genetic basis for homosexuality were much simpler and stronger than it really is, the premise of the question (that it would die out) would not hold. -- Coneslayer (talk) 17:49, 9 July 2010 (UTC)
- It also assumes that just because a gene sequence is a dead-end (something that kills you or otherwise disables your reproductive system before you can reproduce) it would be quickly extinguished from the population; several genetic diseases would like to disagree with that thesis. In fact, that leads me to a question; is there a specific list of genetic diseases that are dead-end in this way? Things like Kleinfelters, Cystic Fibrosis, and Turners that severely inhibit reproduction come to mind. --144.191.148.3 (talk) 17:51, 9 July 2010 (UTC)
I read someplace that homosexuality was some the result of different devlopment in the womb?--92.251.220.50 (talk) 18:14, 9 July 2010 (UTC)
- That's one of many hypotheses but it holds no more weight than any of the others. This debate is very much undecided and no 'cause' of homosexuality has been pinpointed. Regards, --—Cyclonenim | Chat 18:50, 9 July 2010 (UTC)
- Also different hormone triggers, etc. Note that all those "in-the-womb" causes would be considered Nurture, as opposed to inherited causes, which are Nature, but not learned behavior. So in your original question, OP, you present what is called a false dichotomy - it is a wonderful illustration, because you are smart and everyone here is smart, but most of first posters seem to fall into this trap of arguing within the dichotomy. Sorry, just an interesting side note. Anyway, it just says that, as with blindness, left/right handedness, and certain allergies, there are "inherent" effects that aren't genetic (as with left-handedness, not necessarily bad effects). SamuelRiv (talk) 19:28, 9 July 2010 (UTC)
- You can read about xenoestrogens at http://www.gobeyondorganic.com/Weekly-News-Tips/feminizing-of-america-part-i.html
- and http://www.gobeyondorganic.com/Weekly-News-Tips/feminizing-of-america-part-ii.html. —Wavelength (talk) 23:03, 9 July 2010 (UTC)
The thing about evolutionary processes is that they don't require that (for example) no gay people ever have children in order to have that gene vanish from the gene pool given enough time. Consider animals like fish or amphibians that happen to fall into a cave system where there is no daylight. They always, without fail, lose their eyesight within a surprisingly small number of generations. You wouldn't think that there was any measurable advantage to being blind in such a system. But even the ever-so-slight benefit of needing slightly less nutrients to maintain those eyes and being slightly less vulnerable to injury and infection is enough to drive the entire population completely blind within perhaps a hundred generations. So it only takes a miniscule disadvantage to eliminate a gene in short order. So if there is a 'gay gene' (and I've seen no hard evidence of that), then it would have to confer some significant advantages to 'carriers' of the gene.
If this were a single gene (so no carriers - you either have the gene and are gay - or you don't) then consider what happens if even 80% of gay people overcame their sexual preference and had one child who inherits their gene. In the second generation, there would be only 0.8 times as many gays as in the first generation, then 0.8x0.8 in the third generation...and 0.8N in the N'th generation. Once 0.8N times the size of the original population gets anywhere close to 1 person - the gene will disappear completely with a 20% per-generation chance of vanishing. If you started out with a million gay people - the gene would die out within about 62 generations - just 1,200 years. If only 10% of gays had children, they'd be extinct in six generations - 120 years.
We're left with only a few possibilities:
- This is not (predominantly) a genetic condition.
- It's genetic but it's a really complicated consequence of a bunch of different genes - or maybe it's only switched on by some kind of environmental trigger (overpopulation, for example, might be a reasonable guess).
- This is like sickle cell disease: People only turn out gay if they have two copies of the gene and there is some significant benefit to people who only have one copy ('carriers').
- It's a simple single gene condition - but gay people produce surviving children at close to the same rate as straight people.
The last of these seems spectacularly unlikely - their rate of having children simply must be low enough that that 0.8N thing (or whatever the number is) would have comprehensively erased the gene within the last few thousand years. (3) also seems unlikely because we'd surely notice if both parents of gay people were resistant to some disease or otherwise more likely to have (non-gay) children. - so it's one of the first three...but separating 'nature from nurture' is very hard and it may be a long time before we know for sure. I'm not sure I believe (1) either - gay people come from a huge range of backgrounds and in all countries of the world. There just doesn't seem to be a common theme running through the population.
So that leaves us with (2)...it's complicated. A random combination of many genes with some other environmental triggers would be almost impossible to track down - particularly with the 'political correctness' issues surrounding the study of this behavioral pattern. I doubt we'll ever know the true causes of this...but I suspect we'll grow as a species to the point where we actually don't really care. Once we end the discrimination and social stigmas, there will be no need to 'cure' people or even particularly influence the way people behave...so who cares?
SteveBaker (talk) 03:01, 10 July 2010 (UTC)
- First, the options in that list do not have to be disjoint. Secondly, you miss the kin selection part (unless you subsume it under #2). And thirdly, of course, hetero/homo does not seem to be a strict dichotomy, but rather a spectrum, and the need to identify oneself as either/or is very much cultural. For some cultures (like ancient Greece) it's completely normal to have a homosexual relationship for male bonding and raise a family. Being in a prestigious homosexual relationship may actually open up more chances of siring children. Consider the Sacred Band of Thebes - don't you think those young studs would get plenty of laid if they return victoriously from the battlefield, covered in manly sweat and extra virgin olive oil? --Stephan Schulz (talk) 07:02, 10 July 2010 (UTC)
- What some consider the standard family now is quite rare on a historical scale. For most of human existence we have lived in extended family groups with all members contributing in some way to the care and raising of children. And human children require very long periods in that care situation. Gay family members would actually enhance the probability of children reaching reproductive age. HiLo48 (talk) 07:52, 10 July 2010 (UTC)
- You make the simple assumption that male members of the community would be willing to provide that long-term care for the child and increase it's likelihood of reaching puberty. In historical times, men didn't have a caring role (or at least a very minimal one). Care was left to the mother whilst the father was hunter-gatherer. I thought I read somewhere that when children were motherless, they were probably adopted by another mother or left to survive (depending on the age, presumably). Regards, --—Cyclonenim | Chat 11:12, 10 July 2010 (UTC)
- But those males who go hunting tend to do it in groups and share their spoils with the rest of the extended family. Still an advantage to the child. HiLo48 (talk) 03:40, 11 July 2010 (UTC)
- Hardly, males have to leave the child alone to go hunting. How is that an advantage over having a mother to care for them whilst the males are away? That way the child gets protection AND food. Regards, --—Cyclonenim | Chat 10:24, 11 July 2010 (UTC)
- I cannot see your point at all. Of course the men leave the children to go hunting. Most are seeking food for their own children. A homosexual does not have children of his own. His hunting spoils will be shared with children of others. That is a benefit those children have that would not be possible if the homosexual was not there. HiLo48 (talk) 11:24, 11 July 2010 (UTC)
- Sorry I misunderstood you, but I still think your point is flawed. It's likely that the strongest males won over females and had children, and that the others were 'forced' to turn gay for their sexual fulfillment (one of the two key pleasures in prehistoric life, along with food). It seems unlikely that a) a homosexual then would be able to hunt with decent prowess compared to those who had won the females; and b) that those heterosexual males would allow the homosexual males to approach their families and give them food anyway. That could be seen as the homosexual male trying to work his way into a family, and I find it sincerely unlikely that being a homosexual would be an evolutionary advantage in this sense. It's hard for evolution to work indirectly as you described, i.e. with a homosexual helping children not their own. Evolution works to keep your own genetic line going, not those of others. Regards, --—Cyclonenim | Chat 12:30, 11 July 2010 (UTC)
- I cannot see your point at all. Of course the men leave the children to go hunting. Most are seeking food for their own children. A homosexual does not have children of his own. His hunting spoils will be shared with children of others. That is a benefit those children have that would not be possible if the homosexual was not there. HiLo48 (talk) 11:24, 11 July 2010 (UTC)
- Hardly, males have to leave the child alone to go hunting. How is that an advantage over having a mother to care for them whilst the males are away? That way the child gets protection AND food. Regards, --—Cyclonenim | Chat 10:24, 11 July 2010 (UTC)
- But those males who go hunting tend to do it in groups and share their spoils with the rest of the extended family. Still an advantage to the child. HiLo48 (talk) 03:40, 11 July 2010 (UTC)
- You make the simple assumption that male members of the community would be willing to provide that long-term care for the child and increase it's likelihood of reaching puberty. In historical times, men didn't have a caring role (or at least a very minimal one). Care was left to the mother whilst the father was hunter-gatherer. I thought I read somewhere that when children were motherless, they were probably adopted by another mother or left to survive (depending on the age, presumably). Regards, --—Cyclonenim | Chat 11:12, 10 July 2010 (UTC)
- What some consider the standard family now is quite rare on a historical scale. For most of human existence we have lived in extended family groups with all members contributing in some way to the care and raising of children. And human children require very long periods in that care situation. Gay family members would actually enhance the probability of children reaching reproductive age. HiLo48 (talk) 07:52, 10 July 2010 (UTC)
- Homosexuality is slightly correlated to higher IQ. Most likely, some genes that promoted intelligence and creativity may have influenced sexual attraction pathways. Bear in mind, sex is not all about reproduction. Look at our bonobo cousins. Sex is used for social bonding. Thus, homosexuality starts to have some evolutionary fitness.John Riemann Soong (talk) 18:00, 10 July 2010 (UTC)
- DANGER WILL ROBINSON! JRSoong, that assertion requires citation first, a definition of "slightly" second, and a thorough checking of sources to make sure the author isn't a eugenicist third, because that very much resembles the old "penis size vs brain size" trade-off line of the racial eugenicists. IQ correlation is a very problematic thing in general anyway, since IQ itself has a still-debatable heritability factor, and is a quantity defined exclusively on a correlation itself. SamuelRiv (talk) 19:17, 10 July 2010 (UTC)
- [22] it's a blog post, but it's basically interpreting data from the GSS. I haven't crunched the numbers myself but it seems to verify the anecdotal observation that homosexual people do tend to 'seem' smarter on average at least. (or at least among my schoolmates) John Riemann Soong (talk) 20:31, 10 July 2010 (UTC)
- Yeah - that's a crazily 'iffy' assertion...even if it's true. Recall that correlation does not imply causation. Even if there were a 'gay' gene, it might still be a developmental matter that would produce a higher IQ. You can think of all sorts of reasons - to pick a silly one: maybe gay teens don't get invited to so many parties when they are in college - so they have fewer hangovers and study harder. The other problem is the assertion that higher IQ results in a better probability of having children...and for 100% sure, that's not at all the case. There is a strong NEGATIVE correlation between IQ and number of offspring...Idiocracy rules! SteveBaker (talk) 04:30, 11 July 2010 (UTC)
- That is only a very modern phenomenon, and even then only true in some cultures. In low-tech cultures, the number of (surviving) children you have is usually a function of how well you can provide for them, and that may very well be linked to IQ (or at least intelligence - we know that IQ primarily measures the ability to do IQ tests...). --Stephan Schulz (talk) 08:22, 11 July 2010 (UTC)
- ...and if that (and all of the other assumptions that go with it) were true - then homosexuality ought to be in steep decline because the one genetic advantage for 'carriers' (parents of gay people) would have been overtaken by societal change. I don't think that's the case - although with the general population having a (mostly) more accepting view of the gay community, more people are "coming out of the closet" than in the past - so any idea of the numbers involved would be tough to ascertain. SteveBaker (talk) 17:19, 11 July 2010 (UTC)
- That is only a very modern phenomenon, and even then only true in some cultures. In low-tech cultures, the number of (surviving) children you have is usually a function of how well you can provide for them, and that may very well be linked to IQ (or at least intelligence - we know that IQ primarily measures the ability to do IQ tests...). --Stephan Schulz (talk) 08:22, 11 July 2010 (UTC)
- Yeah - that's a crazily 'iffy' assertion...even if it's true. Recall that correlation does not imply causation. Even if there were a 'gay' gene, it might still be a developmental matter that would produce a higher IQ. You can think of all sorts of reasons - to pick a silly one: maybe gay teens don't get invited to so many parties when they are in college - so they have fewer hangovers and study harder. The other problem is the assertion that higher IQ results in a better probability of having children...and for 100% sure, that's not at all the case. There is a strong NEGATIVE correlation between IQ and number of offspring...Idiocracy rules! SteveBaker (talk) 04:30, 11 July 2010 (UTC)
- [22] it's a blog post, but it's basically interpreting data from the GSS. I haven't crunched the numbers myself but it seems to verify the anecdotal observation that homosexual people do tend to 'seem' smarter on average at least. (or at least among my schoolmates) John Riemann Soong (talk) 20:31, 10 July 2010 (UTC)
- DANGER WILL ROBINSON! JRSoong, that assertion requires citation first, a definition of "slightly" second, and a thorough checking of sources to make sure the author isn't a eugenicist third, because that very much resembles the old "penis size vs brain size" trade-off line of the racial eugenicists. IQ correlation is a very problematic thing in general anyway, since IQ itself has a still-debatable heritability factor, and is a quantity defined exclusively on a correlation itself. SamuelRiv (talk) 19:17, 10 July 2010 (UTC)
pheresis rate
Already checked: [23], pheresis, plateletpheresis.
Why do plateletpheresis machines (or at least the newer, electronic ones) go so much faster — that is, collect so many more platelets per minute — toward the end of a draw than toward the beginning? For example, I was on a machine today for 39 minutes, in which it collected 7.0×1011 platelets (estimated), but in the first 28 minutes (72% of the time) it collected only 4.0×1011 (57%) of the platelets — and the discrepancy was even worse earlier in the draw.—msh210℠ 18:02, 9 July 2010 (UTC)
- This is quite simply a guess and I'll readily admit as such, so you're all welcome to shoot me down. Removing platelets will decrease blood viscosity and wouldn't that increase blood pressure? Increased blood pressure would lead to the blood traveling through the machine faster and more platelets would be removed in that time than earlier when your blood pressure was lower. Again, purely a guess. Regards, --—Cyclonenim | Chat 18:43, 9 July 2010 (UTC)
- Actually, that makes no sense. Removing stuff from your blood would make it less viscous and thus slightly lower in pressure. I'm stumped. Regards, --—Cyclonenim | Chat 18:45, 9 July 2010 (UTC)
Best season for avoiding camel spiders?
I am considering a trip tp Jordan that includes a two day stay in the desert. However, I'm arachnofobic and absolutely terrified of camel spiders. Which would be the season during which I am least likely to come across one? —Preceding unsigned comment added by 213.10.229.86 (talk) 20:27, 9 July 2010 (UTC)
If you're worried because of things you've read about them online i'd highly recommend reading the article Solifugae. Contrary to popular belief they aren't as fast, large and dangerous as rumours suggest. That said as a fellow 'phobe' I can certainly see why they'd be a worry regardless! ny156uk (talk) 22:04, 9 July 2010 (UTC)
do people really believe their beliefs?
It seems to me that actual conclusions from religious beliefs, such as the idea that Jesus must have ridden dinosaurs, always come from scientists. In other words, I've never seen a religious person actually use what they believe in the way they use their personal experiences. So, my question is, do they even believe their beliefs in a literal sense? For example, I bet I could elicit a conclusion from a religious person about what must have happened during the nineteenth century, for example if two people they know lived in that time might have met, but I think it would be impossible for me to casually elicit a conclusion based on their religious "beliefs", I don't think they picture the things in the same way. In other words, I am saying that I think even the Pope does not think his beliefs actually happened, as he thinks of his beliefs about nineteenth century Europe. Am I right in this, a-hem, belief? 84.153.230.67 (talk) 20:47, 9 July 2010 (UTC)
- I'm afraid your question doesn't make that much sense to me, and I'm not sure it's a particularly scientific question anyway. If you hold a certain belief, then you 'believe' that belief. The key is in the world itself. If you're asking whether religious people are deluded, that's a hard question to answer. Certainly some people would conclude that they are; others wouldn't. I'm fairly confident that the Pope does truly believe in Catholic/Christian principles and that it's not just some sort of cover story. Feel free to elaborate further if I haven't answered your question. Regards, --—Cyclonenim | Chat 20:56, 9 July 2010 (UTC)
- I think some "believers" genuinely believe and others say they do more out of habit than anything. An example of evidence for that latter would be a Christian that has repented for all their sins yet fears death. If they genuinely believe they will go to heaven, there is no reason to fear death. There are plenty of examples of Christians fearing death (ask any hospital chaplain). The opposite is hard to prove - someone might not genuinely believe what they say they believe but might be a very good actor, so we can't find any definite examples of someone that genuinely believes. I'm confident they exist, though. --Tango (talk) 21:28, 9 July 2010 (UTC)
- I don't think you can infer from a Christian's fear of death that he doesn't really believe in the afterlife. He may not be quite sure where he'll wind up. Trying to figure out if you're really sorry for something you did, especially if you really enjoyed it, is pretty tough. --Trovatore (talk) 21:35, 9 July 2010 (UTC)
Fear may be a biological or psychological activity rather than an intellectual activity. Why should an atheist fear death any more or less than a religious person? Bo Jacoby (talk) 22:13, 9 July 2010 (UTC).
- Are you asking if people believe in their religion in a literal sense or a figurative one? If that was the question, you'll find both. Creationists believe that God created the world five or six thousand years ago in a very short period of time. They, so far as I know, firmly believe that, and will not hear otherwise. Other Christians believe that God created the world, but not such a literal interpretation. You can find similar variances for a lot of scripture. But with my particular beliefs (I am a member of a Christian denomination), my faith does not involve the Creationist theory; whether God created the world in 6 billion years or 5000 years is irrelevant. I believe in the most widely accepted scientific explanations (chunks of rock got together and created a planet etc). Absolute irrefutable proof of either would not change my belief one bit. Therefore, I conclude that we all believe firmly in the things we believe, and even though other people with the same labels (i.e. other Christians or Jewish people etc) believe in other things, we really don't believe in anything that we don't believe in. That is a confusing, but I think, the most accurate answer to the question. Falconusp t c 22:48, 9 July 2010 (UTC)
- This is a really interesting question, and quite a difficult one. The classical concept of belief requires that if a person believes something, they must also believe everything that logically follows from it. But it is quite obvious that real people don't work that way. Philosophers and psychologists have struggled endlessly to find the right way to deal with this; the best way to get into the literature is to key on the concept of irrationality. Looie496 (talk) 23:24, 9 July 2010 (UTC)
- One interesting angle are scientists who believe. 84.153, you might be interested in some of the references listed under studies of scientists' belief in God. ---Sluzzelin talk 23:34, 9 July 2010 (UTC)
- You have to be a little careful about those studies. The problem is that if you are being strictly scientific about it, you cannot say: "God doesn't exist" because there is essentially no way to prove that - you have to say that "God might exist - but we can neither prove nor disprove it". Similarly, even if you believe in God, you shouldn't say "God definitely exists" - because you can't prove it either. Hence, taking things strictly, there should be no atheist scientists - we should all be agnostic no matter what we feel inside because the 'God hypothesis' is unfalsifiable.
- That's why the numbers from these surveys are all over the map. The answer you're going to get depends sensitively on how you ask: If you ask something like "What is your gut feel about this whole "god" thing?" - you'll get a lot of atheists and a reasonable number of religious types - but relatively few agnostics. But if you ask "Does God exist?" - then you should logically get almost all agnostics. For myself, I always say I'm an atheist - even though, strictly speaking, I should be retaining some measure of doubt...that's because nobody ever asks me whether I also believe that pink piano-playing aardvarks live on the dark side of the moon - or whether the Tooth Fairy is real - because if we're speaking strictly - then I have to say that those are both rather more likely than God - so I'm also "Tooth Fairy-agnostic". But religious people always seem much happier when you just say "agnostic" because they kinda think that you're 50/50 on the subject and could easily be pursuaded to become a full-time churchgoer if only they could give you a little encouragement. When (like me), you're more like 99.99999999999999999999999% no-God/0.000000000000000000001% god then the term "agnostic" is a poor one because it doesn't have shades of meaning. These days I say "atheist" because if we drew a line with 0% chance of god existing at one end of the line, 50% in the middle and 100% certain that he/she/it exists on the other end and label those three points 'atheist', 'agnostic' and 'believer' - then my personal position is so very close to the 0% point that it would be nuts to go with any other label. Telling people that I believe that "God might exist" gives an entirely false impression of my position on the subject - so I'm forced to say "God doesn't exist" even though I can't prove it because that delivers a much more accurate impression of my degree of doubt to laypersons.
- So when you consider a bunch of scientists making these kinds of determination about how to express our true understandings, it's not surprising that these surveys are all over the chart. SteveBaker (talk) 04:28, 10 July 2010 (UTC)
- So basically you're a strong agnostic atheist? Regards, --—Cyclonenim | Chat 11:22, 10 July 2010 (UTC)
- I have similar views and describe myself as a weak atheist. It's not particularly precise, but it is accurate and it's good to keep things simple. I usually explain it by analogy: I believe there could be a god in the same way that I believe that when I drop something it might not fall and just levitate. (Dawkins gives a similar analogy with fairies at the bottom of the garden rather than levitation, but I prefer mine since his is more of a restatement of the god question than an analogy - of course his views on gods and fairies are the same, there isn't really a difference between gods and fairies, they are both supernatural entities.) --Tango (talk) 11:53, 10 July 2010 (UTC)
- So basically you're a strong agnostic atheist? Regards, --—Cyclonenim | Chat 11:22, 10 July 2010 (UTC)
- Yep - hence "Tooth-Fairy agnostic". But 'weak' doesn't really cover it either. There are an infinity of possible things that might be true but which are unfalsifiable. God is one of those infinite possibilities. Thus far, not one of those crazy things has proven to be true - so the 'probability' that I assign to God being true is roughly the same as the probability that any one of those other things are true. Although it has to be said that the probability of the Tooth fairy being true seems somewhat higher than God because there are severe logical and thermodynamical problems with a being that can do literally anything and which has no origin. The Tooth fairy could possibly be a naturally evolved flying creature with human appearance who has some biological drive to collect human teeth - this doesn't break any laws of physics, biology, logic. But with an infinite number of seemingly impossible things that one might possibly believe in - it seems odd that you'd pick the one that's least possible! So God-atheist, Tooth Fairy-agnostic describes my position most accurately. SteveBaker (talk) 15:21, 10 July 2010 (UTC)
It's unclear why you find the 19th century significant. The last person who remembered the last of the years 1800 to 1900 died a few years ago but thousands of us have had friends or parents that told of those times. There was photography, sound recording and you can see some of the people on film. Nobody that I know of believes that Jesus rode on a dinosaur. Typical Christian teaching is that Jesus was spiritually active in the creation of everything but did not appear as a human until long after all the dinosaurs were dead, and therefore Jesus had to ride on a donkey. It's not practical to test how strongly people believe what they say they believe, but if you read the article Religion you may notice peoples' different religions depend largely on where they grew up i.e. what their parents believed. That suggests that a belief in a religion (or no religion) is a psychological state that is hard to change. Cuddlyable3 (talk) 23:33, 9 July 2010 (UTC)
- It's certainly surprising that people who profess to believe in any serious kind of god would even consider breaking his/her/it's rules. However, the bible is the most shoplifted book in the world (and for sure, it's the most shoplifted in the deep bible-belt city of Austin Texas where I happen to live. I suppose it's possible that people have to steal the book in order to discover that pesky little "Thou shalt not steal" clause - but I really don't think so. The jails in the US are full of deeply religious murderers, car thieves and so forth - the rate of religious belief amongst criminals is much higher than in the general population! But if you truly, deeply believe that you're going to suffer a literal infinity of time spent in the most unbearable pain imaginable rather than an equally infinite amount of time in the most pleasurable place imaginable - why on earth would you even consider breaking the slightest one of those 10 simple rules? It is truly incredible that in the deepest bible-belt US states, we have the highest tolerance for the death penalty. Whatever happened to being required to forgive people? It is indeed hard to believe that any self-proclaimed religious people actually believe at their core what's being told to them...because in 99% of cases, their behavior certainly doesn't mirror their belief systems. SteveBaker (talk) 01:55, 10 July 2010 (UTC)
- You got that wrong there. People believe that they will go to hell (the place of torment) if they do not accept God's cover (salvation) for the sins they have committed. Religious people can steal just as much, or more (the Inquisition confiscating estates of people they condemned) than a nonbeliever. Major laws in the US were based on the Bible. Like "Murdering is wrong and should be punished". If the Bible is disregarded, then all laws have only the basis of tradition, a weak barrier that can be crashed any time (example the Third Reich). Many religious people do not make an effort to please God; they just sin, get pardoned, sin, get pardoned, sin, get pardoned... like eating junk food and getting heartburn, taking antacids, eating junk food again and getting heartburn, taking antacids... That is not God's plan.
- If you don't support the death penalty, why do you support the "death penalty" of the people who were killed in the crime? The death penalty is based on the Bible, and the only reasons it is still instated is a belief in the Bible or its laws, or tradition. The Muslims who hijacked planes on 9-11 were deeply religious, dying for their cause. It is hard work to become a perfect Christian; it does not come automatically. Most just slip back into their old ways, or they believe that they can buy pardon every time they commit a sin.
- About the dinosaur thing. Jesus might have or might not have rode on a dinosaur; all I know is that it is not notable enough to be included in the Bible.
- Conclusion: People have been given the freedom of choice by God. They can choose to believe in a god, remain neutral, or not believe in a god regardless of the evidence. --Chemicalinterest (talk) 11:08, 10 July 2010 (UTC)
- No, Jesus did not ride a dinosaur. Dinosaurs were extinct, and it isn't mentioned. The two go pretty well together, and people who say otherwise have absolutely no reason to believe he really did. Regards, --—Cyclonenim | Chat 11:15, 10 July 2010 (UTC) That is... in my opinion ;) Regards, --—Cyclonenim | Chat 11:22, 10 July 2010 (UTC)
- Conclusion: People have been given the freedom of choice by God. They can choose to believe in a god, remain neutral, or not believe in a god regardless of the evidence. --Chemicalinterest (talk) 11:08, 10 July 2010 (UTC)
- I'm not sure why we're looking at it solely as a religious question. Look: "beliefs" exist for everything. What did primitive man look like, tens of thousands of years ago? You can conjure up an image. You see it quite clearly and you find it quite compelling. You can imagine what it would look like to walk around in Roman times. You can imagine what the Pyramids must have looked like under construction. You have a whole intricate vision in your head of things you yourself have really very little good knowledge of, and you believe that things happened in a certain way. When someone comes to you and says, "Oh, here is a new and surprising fact about the past!" you say, "huh," and, if you find it compelling (and the source reliable), you integrate it into that belief system of yours. Now whether the "beliefs" held by archaeologists and historians are better founded than those produced by theologians or preachers is a different question (and a good one, to be sure). But the underlying structure of how it works in our heads is probably the same; the difference is how we come up with new beliefs in the first place, what lines of evidence and logic we find permissible, etc. I believe Julius Caesar existed (and have what I consider pretty good reasons to believe this); I believe that Attila the Hun existed; I believe that humans are descended from odd ape-men like Lucy and so on. Just because these beliefs are validated by current archaeologists, historians, and scientists doesn't change the fundamental mental structures that I use to make sense of them, as compared with someone who believes in the miracles of Jesus, or the bizzaro-world Creationist vision of things. If you have a hard time taking seriously the idea that other people may indeed sincerely believe things that you do not believe, I suspect you need to travel a bit more—the world in which you, yourself, live as an individual is unspeakably small when compared against the world as a whole, and what seems perfectly straightforward and "obvious" to someone who lives in one place is totally arbitrary and mysterious to those who live elsewhere. --Mr.98 (talk) 12:18, 10 July 2010 (UTC)
- This question actually gets to the heart of a source of much misunderstanding. People with a belief in God, with 'faith' in God, absolutely feel that God is there. It is a feeling, like loving someone or grief, which seems like an intrinsic part of the world and being human. It can be difficult for people who feel this, particularly if they've felt it as long as they can remember, to really believe that other people don't feel it. That's where you get all the strange 'atheists are just lying' stuff that a few people throw up. But equally, if you've never felt it, or haven't for a long time, it can be hard to believe that people do feel it. And that's where you get the strange stuff about 'Christians lying to themselves' and attempts to logically explain that there is no reason to believe this stuff.
- The old saying is 'For those who believe, everything is proof: for those who don't, nothing is.' And this is so true, because those who believe have this feeling that makes attempts to ridicule their faith seem like someone who has never felt lust ridiculing you for having sex.
- But your original question is slightly confused by including the Pope and certain literalist views. Roman Catholicism has a different view on scripture to the literalist/creationist Christians. Catholicism is much more open to allegorical interpretations, and interpretations that allow for the documents having been written by flawed humans over the course of centuries. So it is indeed possible that the Pope doesn't believe some stuff that you might assume he does, but we can be pretty sure he really believes in God. 86.164.57.20 (talk) 14:16, 10 July 2010 (UTC)
- There is a big difference between religious self-affirmation and religious doubt. While both may be considered "believers", the former generally has positive life effects and the latter negative effects. Apart from "group" experiences in religion such as attending a church, a person may have a much stronger belief if they undergo a religious experience, in which their beliefs are often confirmed or expanded upon. A type of withdrawl from a religious or spiritual experience is often termed the Dark Night of the Soul, which can cause doubt within the believer or a lack of a sense of religious direction. Mother Teresa was a well-known sufferer of DNotS. Also, would it be safe to say that the opposite of agnosticism is gnosticism? ~AH1(TCU) 14:34, 10 July 2010 (UTC)
- Oh, I absolutely agree that it's more complicated than I described, and that's a really good addition. I just figured that sort of thing wouldn't really make sense to someone without understanding that there is this feeling in the first place. 82.24.248.137 (talk) 08:44, 11 July 2010 (UTC)
- There is a big difference between religious self-affirmation and religious doubt. While both may be considered "believers", the former generally has positive life effects and the latter negative effects. Apart from "group" experiences in religion such as attending a church, a person may have a much stronger belief if they undergo a religious experience, in which their beliefs are often confirmed or expanded upon. A type of withdrawl from a religious or spiritual experience is often termed the Dark Night of the Soul, which can cause doubt within the believer or a lack of a sense of religious direction. Mother Teresa was a well-known sufferer of DNotS. Also, would it be safe to say that the opposite of agnosticism is gnosticism? ~AH1(TCU) 14:34, 10 July 2010 (UTC)
- Some readers of this discussion may be interested in http://www.whywebelieve.ca/. -- Wavelength (talk) 21:11, 10 July 2010 (UTC)
- In Breaking The Spell, Daniel Dennett pointed out the almost impossible goal of determining certain religious beliefs. 67.243.7.245 (talk) 14:16, 11 July 2010 (UTC)
The barge hitting the "Duck" boat
In a recent incident, a vintage amphibious "duck" boat carrying a load of 3 dozen or so tourists had engine failure in a river, and 10 minutes later was run over by a barge pushed by a Towboat(or perhaps pulled by a tugboat), resulting in the sinking of the tourist boat and the deaths of some passengers. I have seen many long barge strings going down rivers, with a powerful towboats behind them. A news article said the barge was being "directed" by a "tugboat," which sounds like it the propelling craft might have been in front of the barge. I wonder if they normally have a lookout at the front to inform the skipper if there is a boat or obstruction stopped ahead in the river or channel? Or is the pilot high enough above the water to see over the barge except for some dead zone in front of the barge? How long or over what distance does it normally take a towboat to stop a string of barges? Does a commercial boat carrying dozens of passengers normally have radio communication with other commercial craft on the river, or have a powerful boat horn, or other means of signalling its inability to get out of the way? Would it be the normal practice for passengers on a craft which has suffered engine failure in the middle of a river to be issued life jackets, rather than waiting until seconds before the collision to start grabbing for them? Edison (talk) 23:01, 9 July 2010 (UTC)
- Wikipedia has an article on Duck tour that mentions this and earlier fatal incidents. Cuddlyable3 (talk) 23:42, 9 July 2010 (UTC)
- Really no info there, any more than in the sketchy news articles. Basically, a barge going along a river, with no one able to see a craft or obstruction ahead, and with no capability to stop, and with the certainty that anything in its path which can't dodge will be destroyed, makes as much sense as someone driving a large truck at high speed down the highway with his eyes closed. Edison (talk) 02:27, 10 July 2010 (UTC)
- I sincerely doubt that the barge pilot is at fault here. Barges are huge, slow, and not what one would call nimble in the water. They undoubtably have forward lookouts (the last thing a barge wants to do is run aground on a submerged obstacle), but if a boat positions itself in front of the barge, the best options the lookout and the pilot will have will be jumping up and down and yelling really loudly. to use your analogy, if you're driving down the highway in a Mini Cooper and you slam on your brakes without noticing that there's a semi right behind you, inertia will most surely have its way with you. --Ludwigs2 03:32, 10 July 2010 (UTC)
- Yes, indeed :-( SteveBaker (talk) 03:53, 10 July 2010 (UTC)
- And whoever was driving the semi would get in deep shit for following too close Nil Einne (talk) 04:18, 10 July 2010 (UTC)
- Of course, the rules of the road are different at sea. (And, of course, the semi driver wouldn't be at fault if the Mini driver made a sharp lane change and slammed on his brakes immediately.) There are provisions in maritime law dealing with vessels operating in narrow navigable channels, vessels constrained by draft (that is, vessels that require deep water), and vessels with limited ability to manouevre. There are rules regarding maintaining a lookout, but there are also rules about notifying other vessels about hazardous situations — in the recent incident named above, there is some question about whether the disabled duck boat issued a general warning to other vessels that they were in distress. Given the limited information available to us, we should strictly avoid speculation about legal or moral fault. TenOfAllTrades(talk) 06:22, 10 July 2010 (UTC)
- The difference with the rules of the road are that the guy who is behind a car in front is supposed to allow enough distance to be able to stop - so if someone gets rear-ended, it's clearly the fault of the guy at the back. However, this kind of rule only works well when everyone is organized into nice, neat lanes. On a lake or wide river, people are criss-crossing about all over the place - and the stopping distances for big heavy ships can be measured in miles so leaving enough room to stop isn't possible in many cases. There are cases when no-one is to blame...I think this is probably one of them. The duck pilot unexpectedly lost power so he couldn't get out of the way - the barge captain had no way to avoid them. Sad - but perhaps no fault. SteveBaker (talk) 07:21, 10 July 2010 (UTC)
- Note that there are also "rules of the water", which generally include that commercial traffic has precedence before leisure craft, that sail has precedence before power, and, critically, that whoever has precedence in a potential conflict must maintain course. In this case, I agree with Steve, though. Unless the Duck operator was lazy in ensuring the seaworthiness of the vehicle. --Stephan Schulz (talk) 07:46, 10 July 2010 (UTC)
- The difference with the rules of the road are that the guy who is behind a car in front is supposed to allow enough distance to be able to stop - so if someone gets rear-ended, it's clearly the fault of the guy at the back. However, this kind of rule only works well when everyone is organized into nice, neat lanes. On a lake or wide river, people are criss-crossing about all over the place - and the stopping distances for big heavy ships can be measured in miles so leaving enough room to stop isn't possible in many cases. There are cases when no-one is to blame...I think this is probably one of them. The duck pilot unexpectedly lost power so he couldn't get out of the way - the barge captain had no way to avoid them. Sad - but perhaps no fault. SteveBaker (talk) 07:21, 10 July 2010 (UTC)
- In Ludwig's the driver of the semi would still likely be at fault, particurly since if we are talking about the duck example, it was evidentally there for 10 minutes. But I agree that we shouldn't try to apply the road case to this, that was part of my point, Ludwig's example doesn't really help explain his/her point since in his/her given example the semi driver is generally expected to be able to stop in time without ramming into the Mini in front. This doesn't work very well at the sea for reasons like SB mentioned. Nil Einne (talk) 11:46, 11 July 2010 (UTC)
- The problem for both truck drivers and barge pilots is that even when you DO leave plenty of stopping distance to the vehicle in front, you have no way to prevent someone from crossing into your lane right in front of you and cutting that gap in half (or worse). Your only recourse then is to slow down in order to open up an acceptably wide gap again - but it is in the nature of the beast that this takes time - so for a while, you don't have enough stopping distance even though you obeyed the traffic rules perfectly. On water, with hundred ton barges, the whole thing plays out in agonizingly slow motion (10 minutes in this case) - but the result is exactly the same. If you change lanes in your car and end up cutting into the stopping distance of that truck that's behind you, you are placing both yourself and the truck in danger...and that's what happened with the 'duck'. SteveBaker (talk) 17:09, 11 July 2010 (UTC)
- I may be mistaken but my impression is it's unclear if the barge did try to stop for 10 minutes (while I appreciate it may take longer then 10 minutes for it to stop), the general consensus of the above discussion to me appears to be that it's not normally expected the barges have a lookout capable of seeing something 10 minutes away (which doesn't surprise). Also from what I've read, it's not clear the duck did 'change lanes' so to speak. It may have been in the 'lane' of the barge for 50 minutes for all we know.
- The problem was it broke down in the lane, a rather unfortunate thing and then 10 minutes later it was struck by the barge. The equivalent in the case of a motorway would be if your Mini broke down in the fast lane of the motorway and 1 minute later (for example) a truck hit you. In such a case the truck would likely still be responsible although the Mini driver would also be in trouble if they failed to properly maintain their Mini, failed to signal that their car was broken, or whatever. There may be some edge cases like in extreme fog or something (particularly if the car was indeed failing to signal and/or the signals were broken) although I think they'll be very rare (in a case of fog for example I presume the normal expectation is the truck reduces speed so it can see obstacles with enough time to stop).
- Note that if you change lane into the path of a truck, even if you do so unsafely, if 1 minute later the truck hits you because they were following too closely because they still hadn't adjusted (perhaps because they had a form of road rage), in the vast majority of cases the truck is going to be the one responsible (the driver of the Mini may be had up for an unsafe lane change or whatever but the truck driver still has to adjust their following distance no matter what stupid thing the Mini driver my have done 1 minutes ago). To avoid dispute, I will mention there are bound to be some edge cases where the truck driver won't be responsible because it's not resonable to expect them to adjust speed. For example if the truck brakes are broken, or it is travelling up a hill and can't safely slow down or whatever.
- To put it a different way, it seems to me this 'changing lane' thing is a red herring as we've no indication the barge shouldn't have been there, or suddenly appeared out of no where, rather it appears it was there for a while since it was broken down for at least 10 minutes and may have been there for a lot longer while it was working. In other words, the way I see it this truck equivalency just doesn't work because the rules of the water don't require or expect a barge to have a lookout to see any obstacles 10 minutes away, so even though it had been there for 10 minutes, and may have been in the 'lane' for longer, it not clear they saw it that far out, and it doesn't appear it was expected they should have seen it that far out, and one of the reasons is it's not even clear the barge could have stopped in 10 minutes nor is it clear they should have tried had they even be aware it was broken down 10 minutes in front of them.
- Nil Einne (talk) 17:44, 11 July 2010 (UTC)
- The problem for both truck drivers and barge pilots is that even when you DO leave plenty of stopping distance to the vehicle in front, you have no way to prevent someone from crossing into your lane right in front of you and cutting that gap in half (or worse). Your only recourse then is to slow down in order to open up an acceptably wide gap again - but it is in the nature of the beast that this takes time - so for a while, you don't have enough stopping distance even though you obeyed the traffic rules perfectly. On water, with hundred ton barges, the whole thing plays out in agonizingly slow motion (10 minutes in this case) - but the result is exactly the same. If you change lanes in your car and end up cutting into the stopping distance of that truck that's behind you, you are placing both yourself and the truck in danger...and that's what happened with the 'duck'. SteveBaker (talk) 17:09, 11 July 2010 (UTC)
- Of course, the rules of the road are different at sea. (And, of course, the semi driver wouldn't be at fault if the Mini driver made a sharp lane change and slammed on his brakes immediately.) There are provisions in maritime law dealing with vessels operating in narrow navigable channels, vessels constrained by draft (that is, vessels that require deep water), and vessels with limited ability to manouevre. There are rules regarding maintaining a lookout, but there are also rules about notifying other vessels about hazardous situations — in the recent incident named above, there is some question about whether the disabled duck boat issued a general warning to other vessels that they were in distress. Given the limited information available to us, we should strictly avoid speculation about legal or moral fault. TenOfAllTrades(talk) 06:22, 10 July 2010 (UTC)
- I sincerely doubt that the barge pilot is at fault here. Barges are huge, slow, and not what one would call nimble in the water. They undoubtably have forward lookouts (the last thing a barge wants to do is run aground on a submerged obstacle), but if a boat positions itself in front of the barge, the best options the lookout and the pilot will have will be jumping up and down and yelling really loudly. to use your analogy, if you're driving down the highway in a Mini Cooper and you slam on your brakes without noticing that there's a semi right behind you, inertia will most surely have its way with you. --Ludwigs2 03:32, 10 July 2010 (UTC)
- There should certainly not be any placing of blame here, just a quest for referenced information. Nor should there be a determination of "no fault." I would not apply the highway analogy too closely, but in a highway analogy the Cooper did not "slam on the brakes." It had been disabled in the roadway for ten minutes. In the railroad analogy, a high speed train has a very long stopping distance which often exceeds the distance at which it can see a car stopped on the tracks (the US, unlike UK, has far more grade crossings than roadway/railroad overpasses/underpasses). But modern railroads have an electric block signal system to tell the driver/engineer if there is train traffic on the next section of track. This does not protect a car stopped on the crossing, but it should prevent catastrophies of train hitting train. It is surprising that speeeds of a barge are allowed too great for it to stop before hitting, say another barge or boat large enough to sink the barge. Even at 12 knots, a high speed for a barge string, a 10 minute warning would have allowed a couple of miles for the barge to stop. A tugboat typically has a several thousand horsepower engine. I have seen one rise up in the water like a stunt boat while trying to pull a barge off a mud bank. Has the specific tugboat in question ever been identified? Is there a reference for the stopping distance that a tugboat/barge combination is supposed to maintain on the Delaware River in Philadelphia? Rationally, the allowed speed should be limited by visibility and stopping distance. Rules like this are typically spelled out as 20/20 hindsight gets written into administrative law after the many thousands of marine tragedies in the last 200 years. This concerns me a bit because, in addition to some tourists being killed and a boat sunk in this case, barges often carry dangerous cargos. I well remember my family having to evacuate suddenly once when a batge sank and chemicals on board released chlorine gas. If a barge string stops because of engine trouble, how is the following barge supposed to avoid a similar collision with even worse consequences, if the stopping distance is the claimed unspecified "miles?" Edison (talk) 13:17, 11 July 2010 (UTC)
- Apologies if I took this somewhat offtrack. I came across [24] which goes into some of the complicated factors although it is looking at it primarily from a blame angle. The comments also have some interesting suggestions (although as always take them with a grain of salt) Nil Einne (talk) 18:10, 11 July 2010 (UTC)
July 10
Elevation of the Cajon Pass in San Bernardino County, CA
Wikipedia states the summit elevation to be 4190 Ft and the coordinates to be 34deg 18.7 & 117Deg 28.5.. Google Earth shows this location to be right at the CA 138 junction, and the elevation at 3111 ft.
Is the Wikipedia article incorrect, or is Google Earth just a fun toy, not to be used for data?
Other search hits verify the 4190 elevation! —Preceding unsigned comment added by Chipscom (talk • contribs) 02:35, 10 July 2010 (UTC)
- I think you can trust the elevations shown in Google Earth, although sometimes the surface features are drawn at the wrong spot because of incorrect handling of the satellite photos -- for example you can sometimes see rivers flowing along the walls of canyons, which looks pretty weird. But it is less reliable about putting labels at the right places. Looie496 (talk) 05:02, 10 July 2010 (UTC)
- The reason for rivers flowing up the sides of canyons in Google Earth is that the source elevation data is sampled as some regular interval (say, every 50 meters). If the bottom of a 50m wide canyon is at (say) 15m above sea level - and there are vertical cliffs either side of the canyon bottom that rise to 65m above sea level. The regular 50m sampling might say something like 65m,65m,15m,65m,65m - but the Google Earth software can't tell from those elevation numbers whether there is a 45 degree slope slope from 65m down to 15m and back up again (a V-shaped valley) - or whether there is a vertical cliff-face somewhere between one 50m sample point and the next (a U-shaped valley, perhaps). If the river happens to run somewhere other than through the dead-center of the 50m-wide canyon then the software has no way to know whether it actually runs along the top of the cliff, the bottom of the cliff - or whether there is a 45 degree slope with a river running along halfway up it on a narrow ledge. Now, you and I both know that rivers almost always run along the bottoms of canyons - not the tops or the sides - but remember that Google Earth doesn't necessarily know that there is a river there - all there is to represent it are some pixels in a photograph. A typical muddy river is really hard for software to pick out from the similarly colored dirt that's nearby - especially if there are overhanging trees, rocks and white-water in the river, etc. So the software makes a guess at how to draw the slope - and the river ends up flowing along a 45 degree slope! Looking like a really good place to go water skiing without a boat! (An old joke amongst people like me who have to actually solve these kinds of problems for a living!) SteveBaker (talk) 07:13, 10 July 2010 (UTC)
- Well, no doubt that's part of the story, but from looking at the actual pattern of errors, I suspect a more important factor is that the satellite photos are often taken at an angle, and the software has problems in shifting points correctly to compensate for differences in ground elevation. In other words, my impression is that the software projects the photos onto a flat surface and then warps the surface according to the topographic data, rather than taking the theoretically correct approach of warping the surface first and then projecting the satellite photos onto the warped surface. Looie496 (talk) 16:50, 10 July 2010 (UTC)
- I doubt that. The process of 'orthorectification' (which fixes those problems) is well understood and there is a mountain of free/OpenSourced software produced by various US government agencies that can perform that task. It's really unlikely that Google would be getting that wrong given how easy it is to fix. I work in this very area of computer graphics technology - and the 'sloping rivers' problem is well known - even when orthorectification has been optimally performed. The bottom line is that the terrain photography is almost always provided at a much higher resolution than the elevation data. In effect, the imagery 'samples' the elevation data to determine how it should be drawn - and the Nyquist sampling limit isn't close to being met. So you get all sorts of weird-looking artifacts as a result. SteveBaker (talk) 04:19, 11 July 2010 (UTC)
- Well, no doubt that's part of the story, but from looking at the actual pattern of errors, I suspect a more important factor is that the satellite photos are often taken at an angle, and the software has problems in shifting points correctly to compensate for differences in ground elevation. In other words, my impression is that the software projects the photos onto a flat surface and then warps the surface according to the topographic data, rather than taking the theoretically correct approach of warping the surface first and then projecting the satellite photos onto the warped surface. Looie496 (talk) 16:50, 10 July 2010 (UTC)
- The reason for rivers flowing up the sides of canyons in Google Earth is that the source elevation data is sampled as some regular interval (say, every 50 meters). If the bottom of a 50m wide canyon is at (say) 15m above sea level - and there are vertical cliffs either side of the canyon bottom that rise to 65m above sea level. The regular 50m sampling might say something like 65m,65m,15m,65m,65m - but the Google Earth software can't tell from those elevation numbers whether there is a 45 degree slope slope from 65m down to 15m and back up again (a V-shaped valley) - or whether there is a vertical cliff-face somewhere between one 50m sample point and the next (a U-shaped valley, perhaps). If the river happens to run somewhere other than through the dead-center of the 50m-wide canyon then the software has no way to know whether it actually runs along the top of the cliff, the bottom of the cliff - or whether there is a 45 degree slope with a river running along halfway up it on a narrow ledge. Now, you and I both know that rivers almost always run along the bottoms of canyons - not the tops or the sides - but remember that Google Earth doesn't necessarily know that there is a river there - all there is to represent it are some pixels in a photograph. A typical muddy river is really hard for software to pick out from the similarly colored dirt that's nearby - especially if there are overhanging trees, rocks and white-water in the river, etc. So the software makes a guess at how to draw the slope - and the river ends up flowing along a 45 degree slope! Looking like a really good place to go water skiing without a boat! (An old joke amongst people like me who have to actually solve these kinds of problems for a living!) SteveBaker (talk) 07:13, 10 July 2010 (UTC)
- I think you can trust the elevations shown in Google Earth, although sometimes the surface features are drawn at the wrong spot because of incorrect handling of the satellite photos -- for example you can sometimes see rivers flowing along the walls of canyons, which looks pretty weird. But it is less reliable about putting labels at the right places. Looie496 (talk) 05:02, 10 July 2010 (UTC)
The AAA Road Atlas, 1996 edition, shows on its Los Angeles area map that after the route 138 interchange as you go north on I-15, the road turns right, then more sharply right, and then left in the space of a few miles, and Cajon Pass is marked near the end of the left turn, with an elevation of 4,190 feet. Nearby is Summit Lookout, 4,260 feet.
The National Geographic Road Atlas, 1999 edition, shows a pass symbol at very nearly the same place on the road, but labeled Cajon Summit, 4,259 feet. The American Map Road Atlas, 2006 edition, appears to have used maps from the same source and agrees with this.
The Rand McNally Road Atlas, 2001 edition, renders the road differently, showing a left turn between the two right turns. But it puts the pass symbol in the same place and labels it Cajon Pass, 4,260 feet. Unlike the others, this one also marks the pass on its main Southern California map, but this time the label is Cajon Summit and the elevation is given as 4,257 feet! (The location looks farther south, too, nearer the route 138 interchange, but I assume that's due to the limitations of the map scale.)
In view of all this I am inclined to believe that the AAA atlas has it right with 4,190 feet, while the others are confusing locations on and just off the Interstate, and that the elevation that was found in Google Maps is probably the elevation of the route 138 interchange, not the pass.
--Anonymous, 05:40 UTC, July 10, 2010.
Some old US maps used a different measure of a 'foot' called a 'survey foot' - but I don't think the difference is enough to account for this much error. SteveBaker (talk) 07:13, 10 July 2010 (UTC)
- I've adjusted the coordinates a bit in the article. Anonymous, can you confirm that they now match the location shown in the atlases? Deor (talk) 12:07, 10 July 2010 (UTC)
- Now I'm really confused. The USGS topographic map of the region, which one would expect to be definitive, has the label "Cajon Pass" at a point on California State Route 138 (34°19′32″N 117°25′43″W / 34.3255°N 117.4286°W), at what looks to be an altitude of only a little over 3,800 feet. Perhaps this whole thread should be copied to the article's talk page, so that those interested in the article can work out the best-sourced location and altitude to include in it. Deor (talk) 16:59, 10 July 2010 (UTC)
- As far as I can tell, the railroad tracks occupy the pass's lowest high point (if that makes sense), while the road winds along somewhat higher slopes. The U.S. Geological Survey Geographic Names Information System: Cajon Pass page gives an elevation of 3835 feet, but the GNIS database is not always to be trusted, especially with elevation. This page, about the railroad tracks, says that the pass "crests the summit at 3872 feet", but also that in 1972 the Santa Fe Railway decided to "reconstruct the summit of Cajon Pass". The "summit was lowered by 50 feet to 3822 feet". The section of track is known as the "Big Cut". Pfly (talk) 17:11, 11 July 2010 (UTC)
- Also I just discovered that the article, Cajon Pass, puts its location at 34.349,-117.447, which according to USGS topo maps is not Cajon Pass but "Cajon Summit": USGS topographic map of article coords. Interstate 15 doesn't cross Cajon Pass. Perhaps the confusion arises from the assumption that it does? Perhaps it is common to say I-15 crosses Cajon Pass, but to be precise, the pass is about a mile southeast of I-15.Pfly (talk) 17:20, 11 July 2010 (UTC)
submitting an article for review.
I have tried numerous times to submit an article for review but I can not seem to get it to post. I have reviewed the process on many of the pages available and just can't figure out what I am doing wrong. I have several articles I could submit. I am currently taking online courses towards the acquistion of my Masters Degree in the Science of Higher Education. Each term I am required to submit a course project, all of which I have received an A grade on. I am more than willing to submit these papers to Wiki, however, I must first figure out how to do so. If I could get some assistance, it would be great. Thank you in advance for your help and I hope to get a reply soon. My new term just started and I have a little bit of extra time at the present. Once the term gets into full swing I will not be able to do any extracurriular activities such as submission of articles for Wiki. So the sooner you can reply, the better. —Preceding unsigned comment added by Patter lake (talk • contribs) 02:41, 10 July 2010 (UTC)
- Questions about Wikipedia itself are best directed to the Wikipedia Help Desk - where I'm sure they'll be happy to help you. SteveBaker (talk) 03:50, 10 July 2010 (UTC)
- Try following the instructions on WP:PR --Chemicalinterest (talk) 12:40, 11 July 2010 (UTC)
Night sky on Mars
I've been wondering what the night sky looks like on Mars. My hypothesis is that all the constellations would look the same as here, but that since the axial tilt of Mars is different from that of Earth, the North Star isn't directly above the Martian North Pole, and therefore there is some other point in the Martian Northern Hemisphere about which the stars appear to rotate. Can anyone confirm or deny? +Angr 13:11, 10 July 2010 (UTC)
- Celestia is a good tool to find the night sky on other planets. I'm not sure about the exact axial tilt, but even on Earth, precession plays a role in which star becomes the North Star more than any variations in tilt (22-24°). As for the planets on Mars, they would have different positions across the constellations and different brightnesses than seen on Earth. ~AH1(TCU) 14:25, 10 July 2010 (UTC)
- I should have checked the links before posting. I meant: since Polaris isn't the North Star on Mars, there is some other point in the Martian Northern Hemisphere about which the stars appear to rotate. Likewise the apparent point of rotation in Earth's Southern Hemisphere is different from that in Mars's Southern Hemisphere. I suppose not only the planets but also the Sun would have different positions across the constellations, so that the Martian Zodiac would be different from ours. +Angr 14:55, 10 July 2010 (UTC)
- Well, for the Southern hemisphere of earth, there is no southern "pole star" that's visible to the naked eye. The two stars Acrux and Gacrux in the Southern cross constellation are the closest thing we have - but they aren't as exact as Polaris is for the Northern hemisphere. SteveBaker (talk) 15:08, 10 July 2010 (UTC)
- Okay, but this isn't answering my question. Am I correct in thinking that the constellations would look the same on Mars as on Earth but that their point of apparent rotation would be different? And here's a related question: would the smaller circumference of Mars play a role in the appearance of the night sky? +Angr 15:20, 10 July 2010 (UTC)
- Yes, the constellations would look the same on Mars as they do on Earth, at least with the naked eye (astronomers could take very precise measurements and see very small differences). The point in the sky that they rotate around would be different, although there is no actual (visible) star at that point(see here). Mars' axial tilt is actually quite similar to Earth's (25.2 compared to 23.5, see here, but points in a different direction.
- Okay, but this isn't answering my question. Am I correct in thinking that the constellations would look the same on Mars as on Earth but that their point of apparent rotation would be different? And here's a related question: would the smaller circumference of Mars play a role in the appearance of the night sky? +Angr 15:20, 10 July 2010 (UTC)
- Well, for the Southern hemisphere of earth, there is no southern "pole star" that's visible to the naked eye. The two stars Acrux and Gacrux in the Southern cross constellation are the closest thing we have - but they aren't as exact as Polaris is for the Northern hemisphere. SteveBaker (talk) 15:08, 10 July 2010 (UTC)
- I should have checked the links before posting. I meant: since Polaris isn't the North Star on Mars, there is some other point in the Martian Northern Hemisphere about which the stars appear to rotate. Likewise the apparent point of rotation in Earth's Southern Hemisphere is different from that in Mars's Southern Hemisphere. I suppose not only the planets but also the Sun would have different positions across the constellations, so that the Martian Zodiac would be different from ours. +Angr 14:55, 10 July 2010 (UTC)
24.150.18.30 (talk) 15:39, 10 July 2010 (UTC)
- The reduced diameter of Mars would result in the horizon being closer - which might allow you to see more stars closer to the horizon than here on Earth - but aside from that, the only noticable difference would be the point about which the stars rotate. Constellations would look essentially identical. Earth and Mars are very close together compared to the distance to even the nearest stars. Even out as far as Pluto, you'd need some rather precise measurements with a big telescope to see a measurable difference. SteveBaker (talk) 04:12, 11 July 2010 (UTC)
- According to Astronomy on Mars, "The orientation of Mars's axis is such that its north celestial pole is in Cygnus at R.A. 21h 10m 42s Decl. +52° 53.0′. The top two stars in the Northern Cross, Sadr and Deneb, point to the north celestial pole of Mars. The pole is about halfway between Deneb and Alpha Cephei." The article also mentions that the Martian north celestial pole lies only a few degrees from the Milky Way, which therefore is always visible. Looie496 (talk) 16:40, 10 July 2010 (UTC)
As for the Zodiac question, this is determined by the planet's orbital plane. As it says in the Mars article under "inclination", the orbital plane of Mars is only 1.85° different from that of the Earth (which we call the ecliptic). So the Sun's path through the constellations would be almost the same as we see here. --Anonymous, 17:01 UTC, July 10, 2010.
- One more thing worth noting: All the modern photos from Mars show a hazy pink sky. The sky itself is blue there (product of the atmosphere), but it gets very dusty and windy so there is always a haze,
and that will make nighttime viewing a bit problematic. However, I haven't read up on this too much so this may be completely oversimplistic. SamuelRiv (talk) 19:20, 10 July 2010 (UTC) EDIT from article Extraterrestrial skies: "The sky is thus rather bright during the daytime and stars are not visible" SamuelRiv (talk) 16:31, 11 July 2010 (UTC)
- Thanks to everyone for the informative answers and especially to Looie496 for directing my attention to Astronomy on Mars, a topic I never imagined we would have an article on. That and Extraterrestrial skies are going to make interesting reading I think. (Next we need an article on Astrology on Mars!) +Angr 20:45, 10 July 2010 (UTC)
- WP:WHAAOE (nearly). CS Miller (talk) 21:35, 10 July 2010 (UTC)
- Thanks to everyone for the informative answers and especially to Looie496 for directing my attention to Astronomy on Mars, a topic I never imagined we would have an article on. That and Extraterrestrial skies are going to make interesting reading I think. (Next we need an article on Astrology on Mars!) +Angr 20:45, 10 July 2010 (UTC)
Hollow core concrete slabs

How are hollow core concrete slabs manufactured? —Preceding unsigned comment added by 113.199.149.217 (talk) 14:59, 10 July 2010 (UTC)
- I you're referring to the things in the picture - they just pour concrete into molds. SteveBaker (talk) 15:04, 10 July 2010 (UTC)
- those are blocks not slabs.. eg I think these are meant http://www.concretec.ae/products/Home_Img/DSC00703_2_26_200802_39_164071250.JPG
- According to Hollow-core slab they are extruded.
- For more info try google books and search for "concrete extrusion slab hollow" or similar eg [25] 87.102.85.197 (talk) 15:41, 10 July 2010 (UTC)
- Actually youtube is very useful here eg http://www.youtube.com/watch?v=wEDPtSK1RLw&feature=related there are several videos showing hollow core slab manufacturing from a variety of manufacturers and sources.87.102.85.197 (talk) 18:02, 10 July 2010 (UTC)
DRI and RDA intake
What are the DRI and RDA intake for carbohydrate, protein, total fat, saturated fat, fiber, sodium and calcium? —Preceding unsigned comment added by 74.14.117.187 (talk) 15:12, 10 July 2010 (UTC)
- You can find the Reference Daily Intake at Reference Daily Intake which are based on RDA.
- The DRI's can be found at Dietary Reference Intake
- The figures are for specific conditions (age sex), for the general picture try the external links in those articles. 87.102.85.197 (talk) 15:45, 10 July 2010 (UTC)
Recharging the unrechargeable Issue
Of course, rechargeable batteries are meant to be recharged, but others are dry cell, the type we use once and then simply throw them away. Is it possible that all, cells, even those not build to be recharged, i.e. the ones we use-up and then throw away can be somehow recharged ? Of course it's implausible, but some one thinks it can be done → http://shopping.rediff.com/product/maxis-green-magic-alkaline-battery-charger/10471140 Check it out, boys... Jon Ascton (talk) 18:10, 10 July 2010 (UTC)
- Non-rechargeable batteries drop their available voltage with further use, regardless of how long they are charged for. Furthermore, there is a risk of explosion if they are recharged unsuitably, so I'd advise against it. Regards, --—Cyclonenim | Chat 18:40, 10 July 2010 (UTC)
- All reactions are reversible, but there are many examples were the reverse reaction is not feasible. Recharging alkaline batteries is one such case. When the cell discharges zinc is converted to zinc ions; to recharge one would usually reverse the potential to re-deposit zinc. However since the cell is alkaline the anode reaction is this:
Zn + 4OH- > Zn(OH)42- + 2e- (A)
- The problem is that the zincate ion (Zn(OH)42-) is negatively charged (unlike normal Zn2+ ions), so when the 'anode' is made negative in an attempt to recharge the zincate ion actually moves away from the 'anode' rather than moving towards as it would be if it where Zn2+. This means that the alternative reaction which is basically electrolosis of water occurs:
H2O + e- > 1/2H2 + OH- (B)
- H2 is hydrogen gas - production of this increases the pressure inside the cell hence the warning "Do not attempt to recharge, Danger of leak or explosion" , usually the battery does not explode since there is a pressure releasing valve built in - instead the high pressure can force out the electrolyte which is corrosive and caustic Potassium hydroxide which can damage people and electrical equipment.
- The hydrogen produced may be able to reduce zincate to zinc in fact this isn't likely - not a strong enough reductant - but there is no guarantee that this will happen at the electrode - which means that it may not be available to the battery:
H2 + Zn(OH)42- > Zn + 2OH- + 2H2O (C)
- Similar but less problematic processes happen at the cathode, which can also result in the production of oxygen gas.
- That's why attempting to recharge alkaline batteries is neither realistic nor a good idea.
- (How they work) Devices that attempt to recharge non-rechargable alkaline batteries use on-off pulses of electricity: The on pulse causes reaction B. During the off pulse reaction C can occur which should prevent build up of pressure.87.102.85.197 (talk) 18:54, 10 July 2010 (UTC)
- I remember hearing that those chargers can only be used for about 5 times before the voltage drops so low it is essentially useless. --Chemicalinterest (talk) 20:33, 10 July 2010 (UTC)
Medically pure tin
How can I make pure tin? I want to make it convert into the alpha form and pewter does not convert. --Chemicalinterest (talk) 20:31, 10 July 2010 (UTC)
- Possibilities:
- a. Electroplate from a tin(II) solution
- b. Electrorefine a tin solder.
- If you decide to try either of these further advice could be given depending on what you're working from.87.102.85.197 (talk) 20:46, 10 July 2010 (UTC)
- Out of curiosity, isn't a tin(II) solution going to have cation impurities anyway, some of which will inevitably deposit on electroplating? I suppose on could set up two cathode-anode structures, with one tin terminal on each attracting all things with higher electronegativity and all things with lower, respectively, right? Or how would one purify, say, from a tin can? SamuelRiv (talk) 16:35, 11 July 2010 (UTC)
- (from a solution of the metal ions) the deposition of more electropositive metals can be avoided by using a deposition voltage lower than their deposition voltage see Standard electrode potential (data page) - so a solution of tin(II) can be deposited to tin in the precense of Fe(ii) etc.
- Conversely less electropositive metals (eg Ag+) can be deposited out first, using the method above.
- Electrorefining (ie starting from metal) is more complex since the net reaction is metal > metal (accross electrodes) and technically EMF=0 (excluding effects due to impurities) .. in practice it also works, there's a good introduction here [26] 178.78.64.206 (talk) 18:49, 11 July 2010 (UTC)
- (Separating tin from iron using electrorefining I think will be tricky though).178.78.64.206 (talk) 19:22, 11 July 2010 (UTC)
- Out of curiosity, isn't a tin(II) solution going to have cation impurities anyway, some of which will inevitably deposit on electroplating? I suppose on could set up two cathode-anode structures, with one tin terminal on each attracting all things with higher electronegativity and all things with lower, respectively, right? Or how would one purify, say, from a tin can? SamuelRiv (talk) 16:35, 11 July 2010 (UTC)
Stoichiometry
How many mL of 0.3M KMnO4 is required to titrate 0.02 moles of sodium oxalate.
Isn't the answer 100 mL?--478jjjz (talk) 20:47, 10 July 2010 (UTC)
- Have you got the reaction? It's given at [27] 1 mole permanganate reacts with 2.5 moles of oxalate.
- From Sodium oxalate:
5H2C2O4 + 2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 10CO2 + 8H2O
I have
Na2C2O4 + 2KMnO4 → 2 NaMnO4 + K2C2O4
--478jjjz (talk) 20:56, 10 July 2010 (UTC)
- Reduction to manganate(VI) is right for alkaline conditions. But your equation is wrong - the oxalate needs to be oxidised, and the permanganate reduced.87.102.85.197 (talk) 20:59, 10 July 2010 (UTC)
My instructor has given the following answer
[0.02 x (2/5) /0.3] x 1000 = 26.67 mL
He doesn't explain a diddly-squat, so I am baffled.
I am supposed to use 16 H+ + 2 MnO4− + 5 C2O42-→ 2 Mn2+ + 8 H2O + 10 CO2
, but I don't understand why.--478jjjz (talk) 21:18, 10 July 2010 (UTC)
- That's the reaction: Permanganate is reduced to manganese 2+ ions, and oxalate is oxidised to carbon dioxide
- These are called redox reactions. Didn't you get a class about them? There's an introduction here http://www.chemtutor.com/redox.htm I think you need to understand anout redox reactions first - then you'll understand the equation you were given.87.102.85.197 (talk) 21:26, 10 July 2010 (UTC)
- This was on a lab quiz in the 1st semester of my general chemistry course. Redox reactions will be studied in detail in the next semester. The lab manual was written by my college professors who cover the topics in a different order than my acutal textbook. The lab manual keeps jumping back and forth in terms of the topics covered in the textbook.--478jjjz (talk) 21:31, 10 July 2010 (UTC)
- It appears that 87's reaction works for this example. If there is 0.02 moles of oxalate, and the ratio of oxalate to permanganate is 5·2, then there needs to be 0.02 · 2 ÷ 5 moles of permanganate, or 0.008M. The concentration is 0.3M per liter and there is 0.008M; divide 0.3 ÷ 0.008 = 37.5. 1000 mL in a liter ÷ 37.5 = 26.66(repeating). The last problem could also be a proportion; 0.3M over 0.008M = 1000 ml over ??? ml. Hope this helps. --Chemicalinterest (talk) 22:20, 10 July 2010 (UTC)
- This was on a lab quiz in the 1st semester of my general chemistry course. Redox reactions will be studied in detail in the next semester. The lab manual was written by my college professors who cover the topics in a different order than my acutal textbook. The lab manual keeps jumping back and forth in terms of the topics covered in the textbook.--478jjjz (talk) 21:31, 10 July 2010 (UTC)
Molniya orbit
Does this table make sense? Within a range of 500 to 39,900 km above the Earth, 1.5 to 10.0 km/s?--Email4mobile (talk) 15:20, 11 July 2010 (UTC)
- It doesn't make sense, actually. Some of these orbital speeds are calculated with respect to the tangential speed at the surface of the Earth (8km/s), and some are calculated with respect to a stationary frame. I'll have to fix this. SamuelRiv (talk) 17:09, 11 July 2010 (UTC)
Aircraft tails
If the vertical stabilizers on an aircraft are tilted outward (e.g. Lockheed Martin F-22 Raptor) or inward (e.g. Lockheed Have Blue), does this make any difference in maneuverability or drag? Also, is there a name for this tilt? --The High Fin Sperm Whale 20:50, 10 July 2010 (UTC)
- That would be the dihedral of the vertical stabilizer(s). This certainly impacts drag, lift, and maneuverability, especially because the rudder or rudders serve as elevators) as well. The exact parameters are complex, of course, because the airframe and the rest of the aircraft dynamics are all interconnected. Nimur (talk) 16:25, 11 July 2010 (UTC)
- Following on that, as one gets to very steeply angled vertical stabilizers it becomes possible to discard the elevators entirely, leading to ruddervators which combine the function of rudder and elevator in the same control surfaces. TenOfAllTrades(talk) 16:32, 11 July 2010 (UTC)
- So I'm assuming that tails that point out from the centre are more maneuverable since they are used on many fighter aircraft (e.g. McDonnell Douglas F/A-18 Hornet, Lockheed Martin F-22 Raptor, Lockheed Martin F-35 Lightning II)? --The High Fin Sperm Whale 16:35, 11 July 2010 (UTC)
- They also reduce the radar signature of the aircraft by avoiding the right angle between the horizontal and vertical fins that acts like a corner reflector for the radar. But I'm not sure whether that is the dominant reason for using them. When you have a computer flying the plane, it's easy to have the software decode simple left/right and up/down commands from the pilot into the complicated mix of flapperons (aileron/flaps) and ruddervators needed to make the plane do that. In the past, when those controls were essentially connected directly to the joystick with cables and push-rods, the various control surfaces had to map onto the pilot's inputs directly and simply. SteveBaker (talk) 16:58, 11 July 2010 (UTC)
- This page discusses several examples, and the reasoning behind them. Canted vertical stabilizers were first added to military aircraft in the 1960s to reduce radar cross-section (the SR-71 spy plane may be the first example, with its unique inward-canted tails), more recent aircraft also definitely take advantage of the canted stabilizers to increase manoeuverability and performance. (The link I provided discusses some of the specific benefits in the F-18, as one example.) As SteveBaker notes, the advent of fully fly-by-wire craft made it much easier for aircraft designers to take advantage of 'non-traditional' control surface orientation and placement. TenOfAllTrades(talk) 17:19, 11 July 2010 (UTC)
Diatomaceous earth

I have just produced a very large image of diatomaceous earth, a powdery rock made up of the skeletons of dead diatoms. Does anyone know much about diatoms and can help identify some of the classes of diatoms present in this sample? - Zephyris Talk 21:59, 10 July 2010 (UTC)
- Probably they are deformed and compacted so much that they are unrecognizable. --Chemicalinterest (talk) 12:36, 11 July 2010 (UTC)
- They probably aren't too compressed/distorted (the rock has not been greatly compacted as you can tell its the low density and absorbency), and there are definately fairly complete shells (look, for example, at the centre bottom). I'm sure some general level of classification would be possible... - Zephyris Talk 14:40, 11 July 2010 (UTC)
July 11
Ground connection
Why do some electrical devices have plugs with three "sticks" (don't know their names) instead of two? I heard that the third one is a connection to the ground. If so, 1) why include this, and 2) how would the current go to the ground? Thanks. 74.15.137.192 (talk) 00:53, 11 July 2010 (UTC)
- The Ground pin or prong, also known as the Earth, is indeed a connection to the ground/Earth. 1) Appliances designed for such systems have internal connections between their external cases or other touchable components (if conducting) and the Earth pin on their power plug so that if a fault develops in the appliance making such parts live, there will be a lowest-resistance path for the electrical current to pass harmlessly to Earth rather than taking the only somewhat higher-resistance path via the appliance's user, electrocuting them in the process. 2) Power supply circuits using this system include connections between the Earth socket (which takes the Earth pin) and the ground, often but not necessarily utilising existing good Earth connections such as metal water-supply pipes that pass through the ground.
- Not all appliances used with 3-pin plugs necessarily have an Earth connection: table lamps, for example, often use cables or flexes with only two internal wires, one for the Live and one for the Neutral; in this case the Earth pin has no electrical function, but on most modern 3-pin sockets, it is still necessary for pushing aside the internal shrouds that otherwise block off the other two socket holes to prevent such things as small children poking sharp objects into them, and to discourage irresponsible adults attempting to insert flex wires without using a plug. 87.81.230.195 (talk) 01:32, 11 July 2010 (UTC)
- I'm guessing '87 is from the UK. Here in the US, there aren't shrouds in the socket to be pushed aside by the third pin. There are far too many two-pin devices still being made to allow that possibility. Fortunately, 110v is a lot less dangerous than 240v. But you're right otherwise. Devices with plastic cases can't really become dangerous even if an internal wire breaks loose - so two pins is enough. Devices with metal cases or exposed metal parts can potentially become live in the event of some kind of internal fault - and internally connecting all of the metal parts to the earth wire provides both an efficient way to route the current away - and a way to rapidly trip an earth leakage circuit breaker to make everything safe. SteveBaker (talk) 04:04, 11 July 2010 (UTC)
But what prevents the current from always flowing to the earth? 74.15.137.192 (talk) 09:39, 11 July 2010 (UTC)
- Most of the components are isolated from contact with the ground to prevent this from happening. They are either physically kept away, or are insulated by wrapping them in plastic or some other nonconductive material. This is why (among other reasons) wires are usually coated in plastic. Staecker (talk) 10:21, 11 July 2010 (UTC)
- Yes: to clarify a little further, the Earth pin is connected (via a third wire inside the cable) only to parts of the appliance that should not be live, but may become live if a fault inside the appliance occurs. 87.81.230.195 (talk) 16:42, 11 July 2010 (UTC)
- Indeed, if you pull apart a device with a 3 pin plug and carefully trace the earth wire, you'll find that it doesn't connect to anything at all besides the metal parts of the case of the machine. When the gizmo is functioning normally, the earth wire simply isn't connected and no electricity flows along it. Where it comes into play is if someone yanks hard on the power cord or drops the device or something - at that point, either the live or neutral wire could come loose and touch one of the metal parts of the case. If you're holding it at the time, you're going to get a powerful - and possibly lethal - electric shock. This is so dangerous that we have that earth wire there as a "just in case" measure. So if the live wire should come loose, the current won't flow through your body because the earth wire is a "shorter" path (less resistance) to ground than your flesh. But when the machine is working perfectly - the earth wire doesn't do anything at all! You could disconnect it and the machine would continue to work perfectly well (but please don't because that's dangerous!)...of course none of this is an absolute guarantee - it could be that you dropped an electrical device and that it broke such that the earth wire became disconnected AND the live wire ended up touching the case. But it is generally assumed that such 'double fault' conditions are sufficiently rare as to be of little concern. Personally, if I drop an electrical device, I'll first unplug it at the wall before picking it up and checking it for obvious damage. SteveBaker (talk) 16:51, 11 July 2010 (UTC)
- Yes: to clarify a little further, the Earth pin is connected (via a third wire inside the cable) only to parts of the appliance that should not be live, but may become live if a fault inside the appliance occurs. 87.81.230.195 (talk) 16:42, 11 July 2010 (UTC)
Quantum mechanics, psychology, and free will
Up until now, I thought that neuroscientists were generally in favor of hard determinism (I know philosophy falls outside their field) because of Libet's experiment on conscious decision-making, but I came across this: Excitatory postsynaptic potential#Miniature EPSPs
See what it says about the quantal nature of synaptic activity.
Wouldn't that hold relevance to the article on Free Will? I don't see anything there mentioned about it. That is, couldn't miniature EPSPs provide a basis for free-will?
And one more thing: Are there any other interesting experiments or research which involve quantum mechanics and psychology? Aside from EPSPs, apparently IPSPs are probabilistic also? So, are there miniature IPSPs too? ☯ Zenwhat (talk) 05:01, 11 July 2010 (UTC)
- (1) Speaking as a neuroscientist, I can tell you that neuroscientists are generally materialists, but don't care very much whether the world is deterministic at the subatomic level. Only a small fraction of neuroscientists know about the Libet experiments in any detail, and of the ones that do, attitudes are mixed.
- (2) The "quanta" that are involved in synaptic release have absolutely nothing to do with quantum mechanics. They are subcellular structures that are large enough to be visible in an electron microscope -- they are called quanta because they come in discrete packets, called synaptic vesicles. They are orders of magnitude too large for quantum mechanical phenomena to come into play.
- (3) I don't believe there have been any experiments that involve quantum mechanics and neuroscience or psychology. A few people in the "physics of consciousness" camp have suggested ideas for experiments, but I'm not aware of any having been conducted. You might look at our article on quantum mind for further pointers. (I think it's ridiculous myself.) Looie496 (talk) 06:12, 11 July 2010 (UTC)
- I'll add (from a different perspective) that random phenomena is a bad direction in which to start searching for free will. free will is not random (in fact, there is no decent analytical definition of what free will means, and so there is no possibility of testing for or measuring it). when a scientist uses a random model to study human/cognitive behavior it's not because he necessarily thinks there is or isn't anything random about human/cognitive behavior, it's only because human/cognitive behavior is too subtle and complex to be handled accurately using a deterministic model under current empirical limitations. --Ludwigs2 07:10, 11 July 2010 (UTC)
- Modern mainstream physics does not describe a "free will" in any shape or form. A system may exhibit a pronounced quantum-mechanical behavior if it is small enough. This usually has no effect on a large scale (indeed, you do not need to know the rotational and vibrational state of every molecule to estimate the thermodynamic properties of the air in the room; knowing pressure and temperature is enough to get a very accurate prediction). On a large (macroscopic) scale a system may exhibit a "deterministically chaotic" behavior, which simply means that to predict the system behavior on a long enough time scale the initial conditions must be known with impossible accuracy (imagine a nitrogen molecule released in a vessel of oxygen gas; no amount of accuracy will help you predict the nitrogen molecule location a minute later). Alternatively, a large system may have a "deterministic" behavior proper (for example, a deep enough attractor state for the probability of escape to be negligible over the longest relevant time scale; put your lab notebook in the desk drawer in the evening and you will find it there the next morning). Finally, a macroscopic system may (by chance or by feat of engineering) reflect in its macroscopic state a state of a much smaller, quantum-mechanical system it is brought into interaction with. When this happens deliberately it is called "a measurement"; when this happens by chance it may be referred to as "a butterfly effect". Note that nothing I described above has anything even remotely to do with a free will. Neuroscience, on the other hand, has something to say about the free will. It has been shown repeatedly that the brain prepares for an action (or for a particular decision) before the decision is consciously taken or the action is is consciously initiated. Some human data are summarized in the Neuroscience of free will article, with due caveats. There are also some monkey electrophysiology + behavior data reported by several groups on a few different tasks, all pointing to the same thing. However, again, this does not imply that there is no free will; rather, this implies that the brain is indeed involved in the early stages of decision-making :) . Indeed, when we decide to turn our head to attend to a particular stimulus, the brain must have already compared its relative importance to the other stimuli for us. And when we speak with other people, we do not consciously prepare every word we say, yet we say what we want and do not spew random nonsense. The unconscious decision precedes the conscious one, but it is still our decision and not Erwin Schrödinger's. It is still a free will, or at least it feels like a free will, so at least some people assume it is; and our entire moral system (and the entire criminal justice system, too) are based on this assumption. On the other hand, as Ludwigs2 said above, any sufficiently complicated but ultimately chaotic system is indistinguishable from a system with a free will; at least not at the Turing test level. Yet no-one seriously blames a computer's free will for coming down with a Blue Screen of Death or mucking up its own file-system at the worst possible moment. A very compelling scientific evidence against the existence of the human free will would necessarily lead to abolition of the notion of crime or responsibility for one's actions; and the human civilization will need to adjust accordingly (as it has adjusted to the fact that the Earth is round, or that the sky does not separate the waters above from the waters below). I personally do not see this forthcoming anytime soon. --Dr Dima (talk) 08:30, 11 July 2010 (UTC)
- I would just like to point out, as a philosophical thing, that there is no recourse in the probabilistic for free will. Showing something is not classically deterministic does not give the individual agent any freedom. Doing what you do because of a cosmic dice rolling is not free will in any sense. It is still deterministic, just with the dice as the determiner. Free will is a complicated and quite fuzzy concept, one rooted more in moral questions than physical ones, and looking for a physical analogy for it has been, so far as I have been able to see, quite fruitless. The world is, as far as anyone can tell, ultimately mechanistic, even if some of that mechanism is, at a very low level, inherently probabilistic. (I would contrast this with things that are "apparently probabilistic," like shuffling a deck of cards, which appears randomly determined only because we aren't tracking all of the initial conditions and variables involved. The quantum level is truly probabilistic so far as we know—the information required to understand it in a deterministic way just simply does not exist, according to reigning thought on these things.) At the levels of complexity one is talking about, speaking of the world, or the human brain, in strictly deterministic terms is not always the most sensible way for coming to terms with moral problems, that much I agree. But searching relentlessly for some tiny part that might be somehow outside the rest of the physical system seems to me quite pointless on the face of it, and probabilistic functions are no less determined, even if their outcome cannot be predicted ahead of time. --Mr.98 (talk) 12:30, 11 July 2010 (UTC)
- I concur with the point of view that free will is not random. Therefore deterministic models of human mind are likely better models to explain our perceived free will. Dauto (talk) 13:21, 11 July 2010 (UTC)
- I somewhat disagree (but not with your conclusion). Randomness does not equate to what most people think of as "free will". When you roll a dice - does the dice have "free will" to decide what number will come up? No! It's just obeying the laws of physics. That doesn't stop it from coming up with different numbers each time you roll it - and it doesn't mean that there aren't 'weighted' dice that come up with a '6' more often than the other numbers.
- There are undoubtedly random effects going on here - between quantum effects, external 'noise' sources impinging on the neural network and chaos theory effects, there are plenty of reasons to believe in randomness in the brain. The brain is a massively parallel, asynchronous machine, without timing interlocks of any kind. Which means that (in computing terms) it's going to be super-sensitively dependent on internal timing and 'race-conditions'. To try to put that into concrete terms: Suppose someone asks you a question that doesn't have an automatically "right" answer. One part of your brain starts assembling data that would result in a "Yes" answer and another part is coming up with other information that would produce a "No" - then differences in speed between those two parts (which could depend on anything from what you ate for breakfast to the phase of the moon!) might cause you to answer the question differently each time you are asked. That might make it 'seem' like you were making a 'free choice' - when in fact, it was essentially just a random fluctuation.
- However, I don't see how any of those things results in an assumption of "free will". After all, we can plug random number sources and timing weirdnesses just like this into any 100% deterministic computer and have the result of a question be somewhat randomly determined - and we certainly wouldn't say that was "free will"! I have had plenty of programs that I've written that have "race-conditions" that I hadn't noticed that would cause the program to behave differently each time I ran it. In software, we'd generally call those "bugs". Some programmers use the specific term "heisenbug" to describe them because any attempt to pin the bug down causes it to behave differently!
- So I definitely don't hold with the idea of "free will" - that smacks of something highly non-scientific, verging into matters of "the soul" and all manner of other metaphysical bullshit. However, that's not because of a lack of randomness - it's because the brain is just another computing machine (albeit a somewhat flaky one) - and it has to obey the exact same laws of physics as the laptop on your desk or that rock over there.
- I fully agree that free will has nothing to do with randomness -- the idea that it does is an artifact of most people's intuitive dualism. And I also agree with Steve that there is a lot of randomness in the human mind at the macroscopic level -- and not just the human mind. I've studied the behavior of rats running on a T-maze, where on every trial they have a choice of going right or left, with no motivation to choose one direction over the other. The rats have a pretty strong natural tendency to alternate, choosing the opposite direction from the previous trial, but there are lots of exceptions, and it is extremely difficult to find any deterministic pattern in them. It even goes beyond mammals: Paul Grobstein has studied decision-making in frogs, and found that their choices seem to contain a large component of unpredictable randomness. Why does this happen? Nobody really knows -- my guess is that it happens because operant conditioning requires a basic mechanism for randomizing behavior as a starting point for learning. In other words, you can't find novel solutions to problems if you can't start out by experimenting randomly. Looie496 (talk) 17:08, 11 July 2010 (UTC)
- I would just point out, to both Steve and Looie, that there are two different (among many) definitions of "randomness" here. One is like dice—they are only random in the sense that our model of them and their conditions is insufficiently complicated enough to calculate the outcome, but with a sufficiently omniscient model of the initial conditions and the rolling mechanism, you could hypothetically calculate out what the next roll would be at "classical" (non-quantum) scales. It is only "random" in the sense that it is complex. The other is a quantum definition, where the information to predict the end-state simply is not there. The probabilistic nature of quantum effects is, as far as our theory tells us, utterly and irreconcilably random. It is not random because it is complex—in fact, it is quite simple, by comparison. But knowing all of the initial conditions will not tell you whether a given atom of a given radioactive element will decay at a particular point in time. From a philosophical point of view I would consider those to be quite different. A decision-making process that is "random" because it is a complex mixture of variables and conditions is not quite the same thing as one that is "random" in the sense that it is non-deterministic. Now I do agree that in both cases, as I said before, there is no way that I can see to turn either of those forms of randomness into something like "free will," which I do agree with you is not a scientific concept anyway. --Mr.98 (talk) 18:36, 11 July 2010 (UTC)
Condition with monologuing, stream-of-consciousness speech
Is there a psychiatric or neurological condition characterized by extreme talkativeness (e.g., a monologue that may go on for 15 minutes or more), stream-of-consciousness-like speech in which a particular theme is carried for maybe 15 seconds, followed by another, tangentially-related theme, and so forth, and attention to irrelevant detail (such as giving the home addresses of persons whose names come up)? Thanks in advance for your attention. 70.50.64.16 (talk) 06:00, 11 July 2010 (UTC)
- Williams Syndrome can look a lot like that. So can some forms of mental retardation or autism. Looie496 (talk) 06:20, 11 July 2010 (UTC)
- We can give direction if you're looking for an answer to a random question. If you are asking for a real person please consult a medical doctor. --mboverload@ 06:45, 11 July 2010 (UTC)
- talkativeness is a symptom of many different psychological conditions (as well as being part of the spectrum of normal behavior). In and of itself it is not indicative of anything without a proper differential diagnosis. if it's behavior that in some way interferes with the person's life, they should consult a therapist. --Ludwigs2 07:00, 11 July 2010 (UTC)
- The symptoms you describe, I believe, are called Pressure of speech. This is not a diagnosis, this is the answer to your direct question. We can not and do not provide diagnoses. If you suspect that someone you know exhibits these symptoms, please consult a specialist. --Dr Dima (talk) 08:45, 11 July 2010 (UTC)
- Stumbled upon Tachylalia. Consult a specialist. --Ouro (blah blah) 10:23, 11 July 2010 (UTC)
- These conditions may also be seen in Asperger's Syndrome, monomania, and a "verbal" form of hypergraphia. ~AH1(TCU) 14:39, 11 July 2010 (UTC)
- I have Asperger's and I have a definite tendency to "monologue" which I try (but fail) to notice and curtail. But in my experience, 'aspies' don't randomly change the subject every 15 seconds - instead tending to drill down in more and more detail in the same obsessive subject for as long as you let them...and if you happen to catch one of their 'pet' subjects, 15 minutes would barely be enough to get started! In my case (and, in most others I've seen), simply interrupting the person and telling them to please stop talking is a good thing...and it shouldn't offend them. I'd prefer the momentary annoyance of not being able to finish that extended thought - to the realization that I've monopolized the conversation and upset everyone within earshot. Of course if you're interested in every possible detail of how a 1963 Mini Cooper works, how it was designed and which Monte Carlo rallies it won - please feel free to ask and I'll be more than happy to give you a two hour dissertation! It's also not just speech...people who frequent these Reference Desk pages will surely have noticed how my posts are much longer and (arguably) overly-detailed than those of normal people. SteveBaker (talk) 15:50, 11 July 2010 (UTC)
- These conditions may also be seen in Asperger's Syndrome, monomania, and a "verbal" form of hypergraphia. ~AH1(TCU) 14:39, 11 July 2010 (UTC)
- Stumbled upon Tachylalia. Consult a specialist. --Ouro (blah blah) 10:23, 11 July 2010 (UTC)
Resonating chimney creates infrasound in breeze?
When its quiet at night I can often hear or feel an unpleasant very low-pitched noise in one particular room. That room has a chimney that has been closed up, nearly. The total length of the chimney must be about 25 feet. I cannot detect where it is coming from, only guess. Perhaps the chimney is resonating like an organ-pipe or like blowing across the top of a bottle.
What frequency of sound would a 25ft long organ pipe make? Would the frequency of sound of it in bottle mode be similar? Thanks 92.15.3.130 (talk) 11:35, 11 July 2010 (UTC)
- The first harmonic frequency would be 11hz. Slightly lower actually, once you include the diameter (and harmonics at the odd multiples of this). See Acoustic resonance. That said I've never heard of it happening with a chimney, although I have with caves. I'm pretty sure you'd need some fast wind, a regular breeze probably wouldn't work. (But I'm not really sure.) Ariel. (talk) 12:38, 11 July 2010 (UTC)
The formula given in the article suggests about 11Hz, with harmonics at 33hz, 55hz, etc. Assuming the loudness of the harmonics decrease as the Hz rises, and that the unhearable 10Hz modulates the higher harmonics, it may well explain what I've been hearing. 92.15.3.130 (talk) 16:44, 11 July 2010 (UTC)
My next question is - how would the subjective loudness or energy (not the same thing) of the various tones compare? Would the noise I could barely hear be accompanied by more powerful infrasound at 11Hz? 92.15.3.130 (talk) 16:49, 11 July 2010 (UTC)
Parkinson's disease differences from chronic alcoholism
How are the symptoms and diagnosis of these two differentiated? Thanks 92.15.3.130 (talk) 11:43, 11 July 2010 (UTC)
- You may be interested in Parkinson's disease#Diagnosis and Long-term effects of alcohol. The tremor experienced by people with Parkinson's disease is different and usually more severe than that in alcoholism. The Parkinson's disease article describes it very well; the alcoholism article not quite so well. Regards, --—Cyclonenim | Chat 12:40, 11 July 2010 (UTC)
- As for the temors experienced in alcohol withdrawl, see delirium tremens. ~AH1(TCU) 14:37, 11 July 2010 (UTC)
Where is the 2nd dimension?
I was watching this video, http://www.youtube.com/watch?v=UnURElCzGc0 with Carl Sagan explaining the fourth dimension to laymans using the second dimension as an analogy.
I showed it to my friend and he was a bit skeptical. He says that we strictly live in a 3D universe, and he gave a rather convincing argument for it that had me stumped. Rather than paraphrase what he said, I'll just quote him directly so I don't lose any of his arguments.
"Yea, the 1D-2D bullshit. The guy in this video tells you: IMAGINE, that there is a flatland. Okay? Why do you want me to imagine? Isn't there a 2D realm already? He asks you to ignore the height of the square and just try to pretend it doesn't exist.
So, what is he doing now? He is asking you to ignore reality (which is the only thing that exists)and recall a non existing "idea". You see, in our universe, squares don't really exist. Lines don't exist either. Lines, squares circles, and all 2D shapes are all just perfect ideas. They don't exist in our universe. You can draw a line and it can seem to be perfect, but, after you zoom in you will see imperfect and rough edges. You can use laser sharp cutting and it will be perfect this time. But, you zoom in more, still, it's not perfect. You will never ever reach a perfect line.
When you talk about a square, you KNOW you're talking about something that's so perfect that it doens't exist. And when you build a square building, no matter how accurate, you're just immitating the absolute idea of a square. Something your brain had imagined one day and you're trying to achieve it. So, it's WRONG to think that there is 1D, and then comes 2D, then our 3D. Because, all that exists in the universe is 3D. The other "D's" are philosophical ideas. And so is the 4th dimension. The 4th dimension is a "clever" approach to apply perfect ideas to try to imagine how the 4th dimension "would" look like. But, it's not more real than you imagining what would have happened if we lived in a cube planet. You can derive euqations to try to understand how gravity would be like? how the earth orbit would be like? But, that's all just on paper.
People who use the (2D-3D) (3D-4D) analogy try to convince you that there IS a fourth dimension and that you are to it as a 2D realm to 3D one!!!
FUCK! Did we approve 2D realm as a reality now? Didn't we just agree it's just our imagination?
I believe other dimensions are just "other scenarios" of the same 3D universe. This is all I can approve. Because it's a general rule that can be applied on lines and squares, and on our universe too.
2D is a realm of infinite scenarios of 1D. 3D is a realm of infinite scenarios of 2D. and 4D is a realm for infinite scenarios of 3D, and there it stops. And, since there is no 1D or 2D, then it's only 3D with different scenarios and that's ALL."
I thought about what he was saying, and it made sense. There are no true 2D objects except those we think of in our heads or exist in abstraction. Or are there? Could it be that all objects have a projection in 2D but we can't see it because we only see and experience the universe in 3D? ScienceApe (talk) 14:33, 11 July 2010 (UTC)
- Your friend obviously lacks imagination. What Sagan is asking you to do is to IMAGINE a 2D world. Not that there is a 2D world - just use your imagination. The problem he is trying to solve is to explain how a fourth spatial dimension might work. That's difficult because our brains are not well-equipped to imagine a 4D world (and here we're talking strictly about four SPATIAL dimensions - we're not talking about 4D as in three spatial dimensions plus time. To try to help our imaginations along - to understand (at least at some level) what an 'extra' dimension would be like, it's easier to imagine that we're 2D beings in a 2D universe - then to imagine how the 'extra' third dimension would appear to such beings. Most people have no trouble whatever imagining 2D - there have been at least a couple of fictional stories written about 2D universes..."Flatland" (which I, personally, think is a pretty terrible book) and "Planiverse" which makes it onto my personal "top 20" bookshelf.
- So, we have to imagine creatures whose whole existance lies on the surface of (let us say) a sheet of paper. Now we know this 2D universe doesn't really exist (and according to some of the appendices of Planiverse, cannot exist as a meaningful thing because chemical reactions would be impossible in 2D)...but this is just a thought experiment. So these 2D creatures would look to us like drawings on a page.
- This is easy to imagine. Think about the PacMan game. It is set in a 2D world. The 'walls' of the maze have no height. PacMan is a circle with a mouth, not a sphere. The ghosts are just 2D shapes. When PacMan is caught between two walls with a ghost coming towards him from both directions, he can't put on a James Bond jetpack and move out of the screen to avoid them...he's trapped in a 2D world. Now - that's not hard to understand is it? So the question is, if we go to PacMan and tell him that there really is a third dimension - and that he could escape the ghosts easily by moving "up" and out of the screen...or tunneling "down" under them. This would be a very tough concept for his tiny 2D brain to imagine.
- Just as it's hard to imagine (for us) escaping out of a doorless, windowless jail cell with solid walls, roof and floor by moving in the 4th dimension...stepping "up", going past the walls and then back "down" again. Unfortunately, we don't even have good words for "up", and "down" in the 4th dimension...which makes even discussing this problem tricky! Suppose we make some words: Norf and Souf (like North and South) to describe directions in the 4th dimension. We have cell walls to the north, south, east, west, and floors above and below...but the stupid 3D prison cell designers forgot to build walls to the Norf and Souf! So as 4D beings, we walk out of the cell by moving Norf - then a little to the East - then back Souf again! It's just as easy for them as if the jailer forgot to build North and South side walls for our cell!
- This is hard to imagine - but let's do the analogous thing in PacMan-land: PacMan is stuck in a square jail cell - encompassed by four typical pacman game walls to the North, South, East and West. But nobody built a floor or ceiling to his jail. But if a 3D being like you or me were imprisoned in pacman-land, we could simply step over those pathetic 2D walls by moving "up" - then east then back "down" again.
- The analogy here makes it much easier to imagine those "Norf" and "Souf" directions - which our poor little brains simply can't grasp - by imagining how pacman has problems understanding "up" and "down".
- What's worse about your friend's rhetoric is that he claims that 4D is the most you could have...but one of the leading scientific theories that we have to explain how our 'for-real' universe works (string theory) requires there to be somewhere between 15 and 26 actual 'for-real' spatial dimensions! It is quite possible (and some would say, almost certain) that we really do live in a 26D universe!
- But it does require an effort of imagination and a degree of patience to follow Sagan's explanation - and I guess some people are simply incapable of doing that - which is sad. Anyway, if this interests you (and I surely hope YOUR imagination is up to it) - then grab a copy of Planiverse and prepare to have your brain stretched!
for a person who laughed at the idea of sound waves being represented in a physical form for the lack of proof ( Steve Baker) possesses quite a vivid imagination to assume we live in a 26D universe!!! where did the proof come for this 26D universe steve? or do you ask for proof only when it suits you? Most of us could argue that the argument of the OP's friend is perfectly sane... and would suit most scientific minds.... My suggestion is to continuously think out of the box and not to ridicule or laugh at what appears to be fantastic today for lack of proof like how Steve Baker dramatically did using quite a few profanities in the thread about representing physical matter in the form of sound waves.--Fragrantforever 16:27, 11 July 2010 (UTC) —Preceding unsigned comment added by Fragrantforever (talk • contribs)
- Fragrantforever: see Bosonic string theory. – ClockworkSoul 16:56, 11 July 2010 (UTC)
- The "proof" of dimensions is a mathematical construct, thus all theorems associated with them are perfectly correct. The application of those dimensions to the physical world is something that simply works, and works well, thus we see three dimensions around us, referred to as 3D, and it corresponds pretty much exactly with the mathematical form (and for reference, we get another hint on Earth where the pull of gravity marks the z-axis and the surface of the earth is x-y). As physics expands, we find that we can make time into a fourth dimension, with a major difference in metric, and again still fit the dimension model just as easily as we did with space. Note that all proofs still hold, once we fit reality so well to the math. When Steve refers to the possibility of a 10D or 26D universe, that refers to a specific framework of particle physics that is still debatable, but if the clues fall into place, then through the same mathematical theorems we will understand that there are 6 or 22 more "hidden" dimensions that only the borderline-possible fundamental particles see, but we here on Earth still only see four. SamuelRiv (talk) 16:59, 11 July 2010 (UTC)
Really this is a generic know-nothing argument that can be used against any scientific discovery since Copernicus. Of course the Earth isn't moving—isn't it obvious? You know what it's like to be in a moving vehicle. Of course matter isn't made of atoms; have you ever seen an atom? Have you ever seen a new species arise? And so on. The evidence supporting modern science is very subtle. If it weren't, it wouldn't be modern science, because the ancients would have discovered it already. Nevertheless, the evidence is there, and in such overwhelming quantities that you can't help being persuaded by it once you've found it. Incidentally, the three-dimensionality of space isn't obvious either. The experience of space is so immediate and unconscious that it took a long time for anyone to realize that one could associate the number 3 with it. A possible response to this argument would be to say "I don't believe that space is three-dimensional. Can you convince me?" and then see if he can do it without asking you to imagine two-dimensional space. -- BenRG (talk) 18:38, 11 July 2010 (UTC)



