Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
September 7
Hormonal contraception and pregnancy
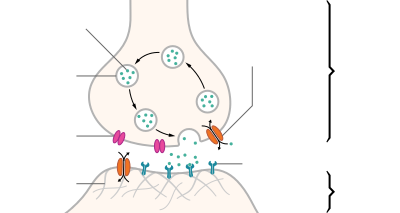
So while hormonal contraceptives are relatively decent at preventing pregnancy, what happens in the event that either the woman becomes pregnant sometime between soon before starting taking them and when they become active or while actively using them? What effects do the contraceptives have on the pregnancy? Ks0stm (T•C•G•E) 01:55, 7 September 2012 (UTC)
- The pill (in various versions) doesn't necessarily work by preventing pregnancy. It can work by causing menstruation whether or not fertilization or implantation have occurred. It is an abortifacient. μηδείς (talk) 04:35, 7 September 2012 (UTC)
- That was news to me, Medeis. I am having difficulty in finding any mention in the articles you reference to this function of the pill. One only menstruates on stopping taking the active-component pill, which is the purpose of the placebos in most monthly packs. And the primary mechanism of the pill is the prevention of ovulation. See:
- Combined oral contraceptive pills were developed to prevent ovulation by suppressing the release of gonadotropins. Combined hormonal contraceptives, including COCPs, inhibit follicular development and prevent ovulation as a primary mechanism of action
- What have I missed? Bielle (talk) 04:50, 7 September 2012 (UTC)
- Indeed. This seems like a complete fabrication. Combined_oral_contraceptive_pill#Mechanism_of_action mentions nothing at all about "causing menstruation whether or not fertilization or implantation have occurred." Indeed, AFAIK, many hormonal-based birth control stops menstruation by stopping ovulation altogether, though many formulations use placebo pills to allow normal menstuation at times when ovulation isn't happening. Extended cycle combined hormonal contraceptives don't have any menstruation at all. It should also be noted that this is often called "withdrawal bleeding", as it isn't true menstruation, as no ovulation occurred. I'm quite interested where Medeis got their information from, as birth control is half a century old, and its mechanisms have been known for quite some time. --Jayron32 04:58, 7 September 2012 (UTC)
- Back to the original question: according to the Mayo Clinic web site, the likelihood of a detrimental effect on the pregnancy or the foetus is small. However, the linked article suggests stopping taking the pill if you believe you are pregnant until you know for sure, and use other birth control methods until you do. Bielle (talk) 04:58, 7 September 2012 (UTC)
- Oh yes, and the article also says: See your doctor. Bielle (talk) 05:01, 7 September 2012 (UTC)
- Haha fortunately that won't be necessary given my gender prevents me from getting pregnant (supposedly). But thanks, that was pretty much the information I was looking for. Ks0stm (T•C•G•E) 05:11, 7 September 2012 (UTC)
From the NIH[1]: "Combinations of estrogen and progestin work by preventing ovulation (the release of eggs from the ovaries). They also change the lining of the uterus (womb) to prevent pregnancy from developing..." I had always thought that was the "main" mechanism, but preventing implantation is obviously a hugely important mechanism. μηδείς (talk) 16:18, 7 September 2012 (UTC)
- Ok, but nowhere in there does it use the term "abortifacient," nor does it indicate that such birth control leads to the abortion or miscarriage of an already formed embryo, fetus, or even fertilized egg. It says that it prevents pregnancy from developing, which is just a tautological definition of birth control. --Jayron32 18:58, 7 September 2012 (UTC)
- Well, I did use the word abortifacient meaning only that it will effectively expel a fertilized egg, rather than letting it implant or remain implanted. The rest, embryo, miscarriage, is what you have apparently read into it. I nowhere said it gets to that stage. That does clarify to me why you accused me of "complete fabrication". Next time please only make that charge on what I have myself actually said, or perhaps be polite enough to ask for a clarification? μηδείς (talk) 20:53, 7 September 2012 (UTC)
- This appears to be rapidly heading into serious error. Medeis, your first response to the question consisted of three sentences:
- The pill (in various versions) doesn't necessarily work by preventing pregnancy. False. Preventing pregnancy is exactly the way it works whether the prevention is due to the principle mechanism of prevention of ovulation, or secondarily, by the prevention of the formation of a lining to the uterus.
- It can work by causing menstruation whether or not fertilization or implantation have occurred. False. It prevents menstruation because it has prevented ovulation.
- It is an abortifacient. False. Even the National Catholic Register is clear on this. From the article What an abortifacient is -- and what it isn't:
- The most important point that emerges from all of this research is that, so far, there is no scientific evidence that any FDA-approved contraception is capable of destroying an embryo. To say that any of these drugs are abortifacient is not only misleading, it does a profound disservice to women who find themselves in a situation where they might have to use one of these drugs or devices. In short, if it isn't at least an embryo, it cannot be aborted. Bielle (talk) 21:39, 7 September 2012 (UTC)
- This appears to be rapidly heading into serious error. Medeis, your first response to the question consisted of three sentences:
- Well, I did use the word abortifacient meaning only that it will effectively expel a fertilized egg, rather than letting it implant or remain implanted. The rest, embryo, miscarriage, is what you have apparently read into it. I nowhere said it gets to that stage. That does clarify to me why you accused me of "complete fabrication". Next time please only make that charge on what I have myself actually said, or perhaps be polite enough to ask for a clarification? μηδείς (talk) 20:53, 7 September 2012 (UTC)
- Equating the contraceptive pill (which includes the morning after pill) to an abortifactant is grossly misleading. Depending on which hormones, contraceptive pills work by suppressing luteinizing hormone release and inhibiting ovulation. Progesterone also decreases the chance of fertilization itself by decreasing the effectiveness of sperm and inhibiting implantation.ISBN 0323033091, page 390 In other words they prevent ovulation itself.Tekoa King, Mary C. Brucker, Pharmacology for Women's Health, page 885.
- As to the original question, there are cases of pregnancy before the pill takes affect. The reasons that they fail when being taken correctly are complicated and I think not well understood. Shadowjams (talk) 22:45, 7 September 2012 (UTC)
- Indeed. I know personally of at least two people who were born while the mother was taking contraception as indicated. Me and my brother. It is rare, but it happens. --Jayron32 23:03, 7 September 2012 (UTC)
- Wow, there seems to be a lot of underlying emotion here. I don't give a fuck whether anyone wants to define something that kills a fertilized egg an abortifacient or not. I am not interested in debate. I think I have clarified my point not only enough, but too much. This is not the place for debates. Let's try to get back to sources please. I am not one. μηδείς (talk) 23:06, 7 September 2012 (UTC)
- Just an aside: you do little to dispel the notion that you are not yourself emotionally attached to the answers by putting in curses in italics and all bold. As far as I can tell, you're the one using emotional language here — everyone else is, fairly calmly, just trying to tell you that you're incorrect. To this disinterested reader it appears you are the one who is reacting to this emotionally, not the others. --Mr.98 (talk) 01:34, 8 September 2012 (UTC)
- Wow, there seems to be a lot of underlying emotion here. I don't give a fuck whether anyone wants to define something that kills a fertilized egg an abortifacient or not. I am not interested in debate. I think I have clarified my point not only enough, but too much. This is not the place for debates. Let's try to get back to sources please. I am not one. μηδείς (talk) 23:06, 7 September 2012 (UTC)
- The pill doesn't kill a fertilized egg. That's the point, and a fact, and supported by the unemotional sources, one of which is not me. Bielle (talk) 23:17, 7 September 2012 (UTC)
- Yes, that's absolutely right. But I am not aware of anything, actually, that directly kills a fertilized egg as such, and I don't think I said it did so. By abortifacient I meant what the word seems to mean at that stage (and as it is used re RU486, a name which will show my age, I guess), it causes the zygote to be aborted rather than implanted. μηδείς (talk) 01:18, 8 September 2012 (UTC)
- RU486 is an abortifactant and there's a large difference between its mechanism of action and the contraceptive pill's. RU 486 works well past implantation, whereas progesterone based pills do not. Shadowjams (talk) 06:04, 8 September 2012 (UTC)
- The fact that it also works as a medical abortifacient for two months past fertilization in no way negates the fact that it prevents implantation if taken early enough. This is logic 101. You might as well say a herbicide doesn't kill acorns because it also kills oaklings. μηδείς (talk) 06:16, 8 September 2012 (UTC)
- RU486 is an abortifactant and there's a large difference between its mechanism of action and the contraceptive pill's. RU 486 works well past implantation, whereas progesterone based pills do not. Shadowjams (talk) 06:04, 8 September 2012 (UTC)
- Yes, that's absolutely right. But I am not aware of anything, actually, that directly kills a fertilized egg as such, and I don't think I said it did so. By abortifacient I meant what the word seems to mean at that stage (and as it is used re RU486, a name which will show my age, I guess), it causes the zygote to be aborted rather than implanted. μηδείς (talk) 01:18, 8 September 2012 (UTC)
- The pill doesn't kill a fertilized egg. That's the point, and a fact, and supported by the unemotional sources, one of which is not me. Bielle (talk) 23:17, 7 September 2012 (UTC)
- From some reliable sources:
- Gabbe: Obstetrics: Normal and Problem Pregnancies, 6th ed., 2012: Oral contraceptives use before or during pregnancy is not associated with fetal loss. The same applies for injectable or implantable contraceptives. (from Chapter 26)
- Martin: Fanaroff and Martin's Neonatal-Perinatal Medicine, 9th ed., 2010: Oral contraceptive agents | No association between first-trimester exposure and malformations. (from Table 38–5, Effects on the Fetus of In Utero Exposure to Physician- or Self-Administered Therapeutic Agents)
- Garfunkel: Pediatric Clinical Advisor, 2nd ed., 2007: Hormonal contraception inhibits ovulation, thickens cervical mucus, inhibits sperm capacitation, slows tubal motility (delaying sperm transport), disrupts transport of the fertilized ovum, and causes endometrial changes that hamper implantation.
- These and other sources I've reviewed lack strong evidence of significant effects on a fetus; in contrast, oral contraceptives can prevent implantation of a fertilized egg, as noted above by others. The original question was broader, i.e. "effect on the pregnancy", and I have found no evidence of adverse effects on the pregnant mother. It is clear that oral contraceptives can increase a woman's risk of blood clots, and pregnancy can have this effect, too. -- Scray (talk) 02:46, 8 September 2012 (UTC)
- Regarding the controversy above, we do seem to have a relevant article: Beginning of pregnancy controversy. This section of that article describes statements from major medical associations defining uterine implantation as the beginning of pregnancy (i.e. that prevention of implantation would not be considered abortifacient). -- Scray (talk) 03:00, 8 September 2012 (UTC)
- I'm not a doctor or expert, but from answering this question and other sources I've looked at, the primary mechanism of progesterone based pills seems to be preventing ovulation in the first place, which means an egg isn't ever even fertilized. There is some suggestion that implantation is impeded by the same too, although at much less frequency. It should be noted too that fertilized and implanted eggs are regularly dispelled by the body. And by no modern definition is a fertilized egg considered an embryo absent implantation. I'm aware certain doctrines believe conception to be the critical moment in terms of life, but even those definitions don't regularly call a fertilized egg an embryo. Shadowjams (talk) 06:00, 8 September 2012 (UTC)
- Implantation has nothing to do with it being called an embryo, Shadowjams, just cell division. See embryo and embryo transfer. μηδείς (talk) 06:08, 8 September 2012 (UTC)
- Does the egg divide before implantation? Shadowjams (talk) 06:11, 8 September 2012 (UTC)
- Yes - implantation occurs during the blastocyst stage, after 6-7 rounds of cell division (cell divisions have actually ceased to be synchronous at that point, so giving an exact number is not possible). Someguy1221 (talk) 06:16, 8 September 2012 (UTC)
- Does the egg divide before implantation? Shadowjams (talk) 06:11, 8 September 2012 (UTC)
- Implantation has nothing to do with it being called an embryo, Shadowjams, just cell division. See embryo and embryo transfer. μηδείς (talk) 06:08, 8 September 2012 (UTC)
- I'm not a doctor or expert, but from answering this question and other sources I've looked at, the primary mechanism of progesterone based pills seems to be preventing ovulation in the first place, which means an egg isn't ever even fertilized. There is some suggestion that implantation is impeded by the same too, although at much less frequency. It should be noted too that fertilized and implanted eggs are regularly dispelled by the body. And by no modern definition is a fertilized egg considered an embryo absent implantation. I'm aware certain doctrines believe conception to be the critical moment in terms of life, but even those definitions don't regularly call a fertilized egg an embryo. Shadowjams (talk) 06:00, 8 September 2012 (UTC)
- Regarding the controversy above, we do seem to have a relevant article: Beginning of pregnancy controversy. This section of that article describes statements from major medical associations defining uterine implantation as the beginning of pregnancy (i.e. that prevention of implantation would not be considered abortifacient). -- Scray (talk) 03:00, 8 September 2012 (UTC)
- I don't want to get too much in to this dispute, but from what I can tell, this hasn't been mentioned yet. The claim that preventing implantation is an important part of the mechanism of COCP seems to be directly disputed by our article, supported by a reliable source:
Insufficient evidence exists on whether changes in the endometrium could actually prevent implantation. The primary mechanisms of action are so effective that the possibility of fertilization during COCP use is very small. Since pregnancy occurs despite endometrial changes when the primary mechanisms of action fail, endometrial changes are unlikely to play a significant role, if any, in the observed effectiveness of COCPs.
- I didn't look at the sources presented above but from what was presented here, it sounds like they are just suggesting the possibility rather then implying it's a significant part of the mode of action.
- Nil Einne (talk) 15:40, 8 September 2012 (UTC)
- Our article Morning after pill#United States legal and ethical controversies suggests that the Catholic church has been maintaining a doctrine that several methods of emergency contraception can cause abortion if the initial contraceptive function fails - citing e.g. [2]. Now that publication arouses a certain degree of skepticism when it uses a 1968 reference for its statement! What this source identifies as problematic is progestagens, Depo-Provera, Norplant, and intrauterine devices. At present I have not evaluated these claims in detail; as they concern the fate of rare eggs which escape the initial contraception to become fertilized, I'm not sure how easy it will be to find information about them. Wnt (talk) 21:06, 11 September 2012 (UTC)
One Severe Pain + Self-Applying Pain elsewhere = Less Pain Overall?
In the subject/headline, I am talking about how, when one is in severe pain, if he or she applies pain elsewhere, let's say, by biting his finger, the first pain seems to become less severe. In theory, kind of mathematically, applying the second pain would make a person's overall pain rating higher, but, in practical terms, it seems to help. So, why is this the case? Any help would be appreciated. Thanks. — Preceding unsigned comment added by 99.124.131.25 (talk) 04:52, 7 September 2012 (UTC)
- What you describe is reminiscent of gate control theory of pain - that thicker, slow nerve fibers carrying pressure, vibration, and/or dull pain might inhibit thinner, fast nerve fibers carrying "sharp" pain. The article I linked, and those linked from there, may interest you. -- Scray (talk) 05:27, 7 September 2012 (UTC)
- I can't say I have a reference for this but I thought it was simply a question of "attention", by biting your finger you are drawing attention away from the other pain. Not the same thing but possibly related I've read that post operative children who are allowed to play video games request far fewer doses of pain medication then kids who do not have access to that kind of distraction. Vespine (talk) 06:17, 7 September 2012 (UTC)
- This one [[3]] looks about right as far as I know. Of course a smarter person would achieve the same thing by clenching their fist on an icecube... pain can be gated by cold. --BozMo talk 10:05, 7 September 2012 (UTC)
- It's kind of like bandwidth but the "newer" pain gets priority, at least momentarily.165.212.189.187 (talk) 12:27, 7 September 2012 (UTC)
- This one [[3]] looks about right as far as I know. Of course a smarter person would achieve the same thing by clenching their fist on an icecube... pain can be gated by cold. --BozMo talk 10:05, 7 September 2012 (UTC)
- I can't say I have a reference for this but I thought it was simply a question of "attention", by biting your finger you are drawing attention away from the other pain. Not the same thing but possibly related I've read that post operative children who are allowed to play video games request far fewer doses of pain medication then kids who do not have access to that kind of distraction. Vespine (talk) 06:17, 7 September 2012 (UTC)
- If you want to do some reading, the phenomenon is known in the literature as pain-induced analgesia. It is widely recognized, but its mechanism is not all that clear. This paper provides some evidence that the effect is mediated by the brain's intrinsic reward circuitry. Looie496 (talk) 00:07, 8 September 2012 (UTC)
- The "gating" Scray referred to may play a part, but the OP was specifically asking about novel pain's effect on existing pain. So Looie's reference may be more specific to the question. Another term, covering this question is Diffuse noxious inhibitory control. -Anthonyhcole (talk) 06:36, 8 September 2012 (UTC)
- Agreed - your response and Looie496's are much more direct answers. I meant for my response to make it clear that what I was describing might interest the original querent, but was not the same thing. -- Scray (talk) 23:10, 8 September 2012 (UTC)
Feces and STDs
I apologize for the disgustingness of this question, but this is a serious and legitimate scientific question:
Can one get STDs (such as AIDS and other STDs) from having one's feces accidentally get inside one's urethra? For the record, this question is purely hypothetical and assume that the person in my hypothetical scenario does not have any STDs at all. Futurist110 (talk) 05:47, 7 September 2012 (UTC)
- You can get infections from getting feces in your urethra; bacteria that thrive in the colon, like E. coli, can cause urinary tract infections. Parents of girls are reminded, when changing diapers, to take care when wiping to avoid exactly this problem. However this is not STDs. AFAIK, if you have a sexually transmitted disease, you can't give it to yourself. If the particular disease you have is transmittable by your feces, you can't then give it to yourself through your urethra if you already have it. Some STDs may be transferable to other people through feces, though it sounds like you are asking if you can give yourself an STD this way. You can't give it to yourself unless you already have it, and thus, if you have it, you aren't giving yourself something you already have. My head hurts trying to figure out exactly what you are asking. I apologize if I misunderstood your question, but it sounds like nonsense as it is written. Could you clarify? --Jayron32 05:55, 7 September 2012 (UTC)
- I'm asking a hypothetical scenario:
- A person who doesn't have any STDs at all accidentally puts some of his own feces inside his own urethra. Both the feces and the urethra came from his own body. Can he get any STDs or any other serious health problems this way? Futurist110 (talk) 05:59, 7 September 2012 (UTC)
- You can most certainly be a host to many diseases in your feces without actually being "infected" by those diseases. So definitely YES to the 2nd question, serious health problems yes, as jayron mentions, feces in urethra is definitely not good, it's a very common (probably most common) and easy way to get a urinary tract infection. However, whether any of those diseases which you can carry around in your feces are actually STDs, that sounds far less likely... Vespine (talk) 06:08, 7 September 2012 (UTC)
- Sorry, that first sentence is very poorly worded by me, you don't have diseases in your feces, you have bacteria in your feces some of which are potentially infectious and disease causing. Vespine (talk) 06:12, 7 September 2012 (UTC)
- Yeah, that's what I thought you meant, Futrurist. In that case, if a person doesn't already have the infectious agent inside their body, then that infectious agent cannot get into their feces. If the infectious agent can't get into their feces, it couldn't get into their urethra from that feces. If they already had the agent that causes the STD, then they already have it. To put it simply: you can't give yourself the clap, because if you don't already have it, you can't pass it on, and if you do already have it, then you already have it. Logically, it makes no sense the scenario you are asking about. --Jayron32 12:46, 7 September 2012 (UTC)
- Sorry, that first sentence is very poorly worded by me, you don't have diseases in your feces, you have bacteria in your feces some of which are potentially infectious and disease causing. Vespine (talk) 06:12, 7 September 2012 (UTC)
- The article on STIs, also known as STDs says: In general, an STI is an infection that has a negligible probability of transmission by means other than sexual contact, but has a realistic means of transmission by sexual contact (more sophisticated means—blood transfusion, sharing of hypodermic needles—are not taken into account). Thus, one may presume that, if a person is infected with an STI, e.g., chlamydia, gonorrhea, genital herpes, it was transmitted to him/her by means of sexual contact. That would seem to be a "no" to the original question with respect to STDs. Bielle (talk) 06:17, 7 September 2012 (UTC)
- You can most certainly be a host to many diseases in your feces without actually being "infected" by those diseases. So definitely YES to the 2nd question, serious health problems yes, as jayron mentions, feces in urethra is definitely not good, it's a very common (probably most common) and easy way to get a urinary tract infection. However, whether any of those diseases which you can carry around in your feces are actually STDs, that sounds far less likely... Vespine (talk) 06:08, 7 September 2012 (UTC)
- A person who doesn't have any STDs at all accidentally puts some of his own feces inside his own urethra. Both the feces and the urethra came from his own body. Can he get any STDs or any other serious health problems this way? Futurist110 (talk) 05:59, 7 September 2012 (UTC)
- There is exactly one disease I know of that you can "catch" from your own feces - pork tapeworm, which is far more harmful if the eggs in the feces are ingested causing cysticercosis than initially when pork meat is eaten causing taeniasis (adult parasites in the intestine which lay those eggs). Of course, I don't know every disease! But I haven't heard of a STD like that. But there are examples of intestinal worms being transmitted sexually, [4][5]; also intestinal protozoa can be STDs [6] and some protozoa have interesting life cycles, so I can't rule out the possibility such a thing could happen - I just haven't heard of it. Also, the scenarios I've described of are for infection by mouth - I don't know if any of these can be caught via the urethra. Wnt (talk) 16:05, 7 September 2012 (UTC)
- To be fair, a point Bielle raised above needs to be re-raised: an STD/STI is generally limited by definition to a disease whose mode of transmission is of a sexual nature only. To quote it again as Bielle has done "an infection that has a negligible probability of transmission by means other than sexual contact, but has a realistic means of transmission by sexual contact". The "negligible probability of transmission by other means" would preclude infections that you can catch otherwise that also one could catch sexually. Intestinal worms can be transmitted by other means, so the fact that you could catch them through some form of sexual activity doesn't make them an STD, at least by the definition we're working with above. which also raises the question of how one could have sexual contact between one's own feces and urethra. I've been told to do that many times in my life, but I never thought it physically possible. --Jayron32 18:53, 7 September 2012 (UTC)
- The point Bielle raised is incorrect (no fault of Bielle's - it's our article that is wrong). If you look at the linked article, that statement is unsupported by any source. I started to make a long list of Pubmed IDs as evidence for my claim, then realized that links to relevant pages at the CDC, WHO, and Mayo Clinic should suffice. Listed STD/STIs there include HIV, HAV, HBV, HPV, Shigella, Cryptosporidium and Giardia lamblia all of which are often transmitted non-sexually. A better definition: "Sexually transmitted diseases (STDs) are infections generally acquired by sexual contact." Perhaps we should improve our article. -- Scray (talk) 01:04, 8 September 2012 (UTC)
- To be fair, a point Bielle raised above needs to be re-raised: an STD/STI is generally limited by definition to a disease whose mode of transmission is of a sexual nature only. To quote it again as Bielle has done "an infection that has a negligible probability of transmission by means other than sexual contact, but has a realistic means of transmission by sexual contact". The "negligible probability of transmission by other means" would preclude infections that you can catch otherwise that also one could catch sexually. Intestinal worms can be transmitted by other means, so the fact that you could catch them through some form of sexual activity doesn't make them an STD, at least by the definition we're working with above. which also raises the question of how one could have sexual contact between one's own feces and urethra. I've been told to do that many times in my life, but I never thought it physically possible. --Jayron32 18:53, 7 September 2012 (UTC)
Discoveries in space (not just developed for the space exploration)
What, if any, are the discoveries made because humankind can travel to space? Excluding discoveries made to travel to space. OsmanRF34 (talk) 13:26, 7 September 2012 (UTC)
- Effect of spaceflight on the human body has some important stuff. None of these developments could have been made had people not actually been in space. Most of the technologies used to get to space already existed on Earth for other purposes: for example, many of the rocket engines used for spaceflight were adapted from weaponry. See Redstone (rocket family), which shows how the first rockets for manned spaceflight developed from ballistic missle rockets. But there have been numerous experiments done in space, such as longterm effects of Weightlessness on various biological and non-biological processes, that could not be done elsewhere. --Jayron32 13:38, 7 September 2012 (UTC)
- Didn't it confirm that all objects fall at the same rate? When a feather and heavy object were dropped on the moon - not sure on the details, although I was shown the clip in class. 86.138.171.71 (talk) 14:58, 7 September 2012 (UTC)
- Well, but that has also been trivially shown to be the case on earth. Dropping two objects in a vacuum can be done on Earth as well as the moon. That's a fairly trivial experiment. There have been a lot of data gathered on physiological changes to living things: plants, animals, and humans, and on the changes in how many chemical reactions happen in weightlessness. But dropping a ball and a feather is hardly a significant scientific discovery from space travel. --Jayron32 15:03, 7 September 2012 (UTC)
- Is the question intentionally limited only to discoveries related to manned spaceflight? Many robotic probes established basic scientific facts - like the characterization of the magnetic field of Jupiter by Voyager - that would not have been possible using Earth-based observation. Nimur (talk) 15:07, 7 September 2012 (UTC)
- Nimur beat me to the gun with his question. Space research is one place to look but are you meaning to restrict to discoveries arising from research by or on humans while they are in space? Does being on the Moon count as "space"? A good deal of our knowledge of the geology of the Moon has come from the Apollo program. The Voyager program and Hubble Space Telescope have made a large number of discoveries in astronomy but perhaps they are not what you mean. Thincat (talk) 15:12, 7 September 2012 (UTC)
- The question is not limited to humans, just excluding things discovered on Earth for leaving the same. OsmanRF34 (talk) 15:50, 7 September 2012 (UTC)
- A good place to start would be our article on Scientific research on the International Space Station. Most of that article except the introduction appears to unfortunately be a list of red links, but each one has a reference to a webpage describing the experiment if you are interested. For a general overview the introduction seems reasonable. Equisetum (talk | contributions) 17:56, 7 September 2012 (UTC)
coffee buzz
I've got a cup of coffee on my desk, made with a drip brewer with a paper cone filter, from dark roasted beans ground maybe a little bit finer than usual for drip brewing, with a few ml of ultra-pasteurized half-and-half stirred in. The damn thing is making a periodic buzzing sound like some kind of insects, definitely audible if I put my ear next to the cup. The buzz starts around 1 khz and descends over about 1-2 seconds to about 500 hz, then jumps back up to 1 khz. (The pitches are rough guesses based on comparing with the 440-hz dial tone from my phone).
What is going on??? I have heard buzzing coming from coffee before, but sort of steady, and I noticed it most when the coffee had powdered creamer, and I figured it was gas bubbles escaping from the creamer. But this has liquid cream and I've never heard this periodic repeating before. I might try making an audio recording and uploading it.
A quick web search about coffee noise finds mostly hits about noisy coffee-making machines.
67.119.15.30 (talk) 17:25, 7 September 2012 (UTC)
- I had a similar experience once (with tea, not coffee). The cause turned out to be a tiny, almost-invisible, crack in the cup that the hot liquid was trying to squeeze through. AndrewWTaylor (talk) 17:59, 7 September 2012 (UTC)
- If the bottom of the cup is wet, and it is resting on a smooth surface, you may be hearing a sound caused by the air trapped under the cup expanding as it gets warmed, and finding a way out as a stream of tiny bubbles. I've noticed a similar effect with a cold drinks can - though presumably the air would have been going the other way. AndyTheGrump (talk) 18:05, 7 September 2012 (UTC)
- Most common cause of this is the cup, not the coffee. One end of the handle has cracked, possibly by a void during manufacture, and there is air trapped inside. Water from its last washing forms a seal. When you add hot liquid inside, the trapped air is heated, expands and is forced out through the crack. This blows bubbles through the water seal, which are just about audible. As they're small bubbles, this is quite a high frequency. Sometimes you can see it happening. Andy Dingley (talk) 18:09, 7 September 2012 (UTC)
- While I think the explanations above are the most likely, another possibility is that the coffee-filled cup is just at the resonant frequency to magnify some random vibration in the office, thus increasing the magnitude to the point where you can hear it. StuRat (talk) 18:36, 7 September 2012 (UTC)
Andy's idea about the cup resting on a surface definitely isn't the explanation. The sound didn't change when I lifted the cup to listen to it. I have doubts about the other explanations but can't rule them out. This was a ceramic cup that might have some invisible cracks. I may try some other time with a plastic cup. The sound did get quieter and lower pitched as the coffee cooled down to drinking temperature. 67.119.15.30 (talk) 21:14, 7 September 2012 (UTC)
- OK, sounds like you have eliminated the possibility I mentioned, as well. StuRat (talk) 21:16, 7 September 2012 (UTC)
1 Photon in all 360 degrees
When something emits one photon in empty space does that only go out from origin in 1 specific directional line or spherically in all directions like an explosion?165.212.189.187 (talk) 17:47, 7 September 2012 (UTC)
- In the particle model, it goes in one direction only. However, the wave-particle duality of light means that it can sometimes be thought of as a wave, which can be focused in one direction, but spreads out from a line. See double-slit experiment for a discussion of this weirdness. StuRat (talk) 18:34, 7 September 2012 (UTC)
- Light is neither a particle nor a wave. It is just light. In some situations, it is helpful to model light like a particle, so we have photons for those situations. In some situations, it is more helpful to model light as a wave. The situation StuRat notes is but one example of where both models break down. All models are inadequate in some way, and neither the photon model nor the wave model are, of themselves, capable of adequately covering all of lights behavior. To the OP's question: a single electron can only emit a single photon in a single direction, so all photons have directionality. An object that contains umteenmillions of electrons will emit in all directions, giving the effect of spericality the OP indicates. So, one needs to be very careful how one defines the terms. If we're talking about the sun giving off light, it is most helpful to think of it as non-directional: the light goes out in all directions. If it is a single electron emiting a single photon, then we have a different situation. --Jayron32 18:47, 7 September 2012 (UTC)
- It also helps us to refine the model: why is something emitting a photon? Often, a photon emission is stimulated by an external event. If so, the symmetry of the problem can be well-defined; the directionality of the source will have some correspondence to the direction of the emitted photon; calculating that correspondence is a scattering problem. In other cases, a photon is emitted spontaneously. If the source emitting the photon is an idealized, spherically-symmetric object, then the photon is equally probable to be observed in any direction. Where it will actually be observed is a different question altogether - and there's where we wrap up all the complexity that we call wave-particle duality. When lots of photons are emitted, we can discern a radiation pattern, which gives us information about the process that is causing the energy to be emitted; this may reveal structure and other physical information about the source. Nimur (talk) 19:07, 7 September 2012 (UTC)
- Well, that gets down to the difference between properties of quantum behavior as observed in the bulk versus observed individually. An event, like the spontaneous emission, that you note will have an equal probability in all directions. Such an event, when it occurs a gajillion times in one place, will result in spherical emission of light equally in all directions, but when observed per particle, each one emission will have directionality. Lots of quantum mechanical behavior has this problem that the behavior on the individual level is not particularly helpful when trying to figure out the behavior of a system of events. The aforementioned double slit experiment is a classic example of the sort of apparent paradoxes this sort of thinking introduces. --Jayron32 19:30, 7 September 2012 (UTC)
- It also helps us to refine the model: why is something emitting a photon? Often, a photon emission is stimulated by an external event. If so, the symmetry of the problem can be well-defined; the directionality of the source will have some correspondence to the direction of the emitted photon; calculating that correspondence is a scattering problem. In other cases, a photon is emitted spontaneously. If the source emitting the photon is an idealized, spherically-symmetric object, then the photon is equally probable to be observed in any direction. Where it will actually be observed is a different question altogether - and there's where we wrap up all the complexity that we call wave-particle duality. When lots of photons are emitted, we can discern a radiation pattern, which gives us information about the process that is causing the energy to be emitted; this may reveal structure and other physical information about the source. Nimur (talk) 19:07, 7 September 2012 (UTC)
- The model I prefer is that a photon, or any other subatomic particle, is a particle, but with a probability function associated with it. This allows you to say it is more likely to be in some spots than others, but you still never know exactly where it is. And note that the probability may not just apply to the three physical directions, but possibly also to temporal dimensions and dimensions beyond those we are familiar with. StuRat (talk) 20:31, 7 September 2012 (UTC)
- Yeah, that works except when it doesn't. Particles don't diffract, for example. Only actual waves do that. That's the problem with any model of light: None of them work all the time. There are some really obvious times when thinking about light as a particle leads you to very wrong places. And other times where it is the only reasonable conclusion. The answer, from my perspective, is to not try to force the answer. Recognize that all human knowledge is a model, even down to the most fundemental level (in a way, that's what knowledge is: the construction of models of the world inside our brains), and that with light, we're stuck with the fact that nothing in our sensory experience prepares us for light, so there is no good single analogy. So we need to use different models for different applications. Yeah, it sucks, but it is what it is. We marry the models with concepts like Debroglie wavelength and wave particle duality, but a lot of that stuff is just the mathematical way of saying "We don't have a good single analogy to make this work for us." In many ways, the exhortation to "shut up and calculate" is valid. In other words, ours is not wonder why, ours is just to do or die. It is what it is. --Jayron32 21:58, 7 September 2012 (UTC)
- It's not necessarily true that particles don't diffract. There are some highly contrived theories of QM that include diffracting particles, and the few physicists who prefer them do so because they hate the idea of waves being a physical thing. Even Isaac Newton's Corpuscular theory of light accurately predicted the brightest diffraction line in something reminiscent of the single-slit experiment, that was popular circa 1700. I don't have links for this because it's something I read in a book when I was in college. The purely-particle QM theory has an article somewhere on Wikipedia. Someguy1221 (talk) 06:29, 8 September 2012 (UTC)
- Well, let me clarify a bit. No particle you have ever had sensory experience with has ever diffracted. That is, part of the normal working definition of "object" for people doesn't include the property "can diffract". The very fact that you call the models "highly contrived" is a clue that it is basically playing fast-and-loose with the definitions. Sure, I can invent a thing that I call a "diffracting particle" and then work with that, but once you redefine common terms, you haven't really changed the models, you've just redefined the language used to describe it. If having a "diffracting particle" makes a model work for whatever purpose, that's fine, but it doesn't make it a more accurate model, per se, nor does it make it any better at describing light. --Jayron32 14:37, 8 September 2012 (UTC)
- It's not necessarily true that particles don't diffract. There are some highly contrived theories of QM that include diffracting particles, and the few physicists who prefer them do so because they hate the idea of waves being a physical thing. Even Isaac Newton's Corpuscular theory of light accurately predicted the brightest diffraction line in something reminiscent of the single-slit experiment, that was popular circa 1700. I don't have links for this because it's something I read in a book when I was in college. The purely-particle QM theory has an article somewhere on Wikipedia. Someguy1221 (talk) 06:29, 8 September 2012 (UTC)
- Yeah, that works except when it doesn't. Particles don't diffract, for example. Only actual waves do that. That's the problem with any model of light: None of them work all the time. There are some really obvious times when thinking about light as a particle leads you to very wrong places. And other times where it is the only reasonable conclusion. The answer, from my perspective, is to not try to force the answer. Recognize that all human knowledge is a model, even down to the most fundemental level (in a way, that's what knowledge is: the construction of models of the world inside our brains), and that with light, we're stuck with the fact that nothing in our sensory experience prepares us for light, so there is no good single analogy. So we need to use different models for different applications. Yeah, it sucks, but it is what it is. We marry the models with concepts like Debroglie wavelength and wave particle duality, but a lot of that stuff is just the mathematical way of saying "We don't have a good single analogy to make this work for us." In many ways, the exhortation to "shut up and calculate" is valid. In other words, ours is not wonder why, ours is just to do or die. It is what it is. --Jayron32 21:58, 7 September 2012 (UTC)
- As far as I recall though for an individual quantum particle the 360 degrees bit is wrong, its 720 for a quantum particles isn't it? something to do with the double cover of SU2 by SO4. Or put it another way a 360 degree rotation is not topologically equivalent to staying still whereas a 720 rotation is. So you can only be sure you are back where you started if you turn around twice and it has to go in all 720 degrees of possibility. --BozMo talk 21:04, 7 September 2012 (UTC)
- I think you may be over-analyzing the Q a bit. StuRat (talk) 21:11, 7 September 2012 (UTC)
- I think, you're thinking of spinors and spin 1/2 particles. That 720 degrees isn't directly relevant to what is being discussed here.Phoenixia1177 (talk) 00:54, 8 September 2012 (UTC)
- Here's a way to focus on the weirdness: if you don't know what happened to the particle(s) that emitted the photon, then you view it as a wave (indeed, one which can diffract with itself, as has been said). But if you know what happened to that particle, then you know by conservation of momentum which way the photon went. And photons that you know where they went ... won't diffract with each other (the double-slit experiment) So not only is the light initially seeming to move in a spherical wave... so is the particle. Wnt (talk) 23:47, 7 September 2012 (UTC)
- There is more than just the 360° too, it is actually 4 pi steradians of solid angle. The wave is not confined to two dimensions. And one interpretation of this situation is that until the photon is absorbed you cannot know which direction it has traveled in. The direction of recoil of the the emitter is entangled with the changed momentum of the absorber of the photon. You can also look at the Uncertainty principle with this. With a spherical wave you have the maximum uncertainty in direction . But if you know exactly which atom absorbs the photon you have a very precisely defined direction for the momentum vector, but at the expense of a very uncertain position. Graeme Bartlett (talk) 23:37, 8 September 2012 (UTC)
- The whole 720 degrees thing isn't really relevant to this question; not to mention that he is asking about a photon which is spin 1, so this wouldn't even apply to it even if it was relevant (fermions are what you are thinking of.) For more information look up spin, spin statistics theorem, the Wigner D-Matrix.Phoenixia1177 (talk) 09:25, 10 September 2012 (UTC)
So could it be that we can't detect in any observable way some objects in space simply because they do not emit any radiation to our discrete loaction in space?165.212.189.187 (talk) 13:42, 10 September 2012 (UTC)
September 8
Gold tunnels
Does anyone know what the long lines located at 64°01′53″N 139°13′56″W / 64.03139°N 139.23222°W are. They are at Bear Creek in the Yukon not far from Dawson City. Bear Creek was a placer mine (http://www.historicplaces.ca/en/rep-reg/place-lieu.aspx?id=6242) and I'm guessing that they might have been formed by hydraulic mining but I would like to know for sure and what they are called. CambridgeBayWeather (talk) 02:56, 8 September 2012 (UTC)
- I see a large number of bluish-white, semicircular plates sticking out of the ground and resting against each other to form long "caterpillars". I was going to suggest that they might be tailings, but I'd expect large, random piles. StuRat (talk) 03:03, 8 September 2012 (UTC)
- If you look at Google street views from the Klondike Highway thereabouts, it's clear that the platelike appearance from the air is just caused by shallow transverse ridges in the "caterpillars". It must have somthing to do with how succesive loads of waste rock were dumped from trucks or otherwise added to the piles. Deor (talk) 14:52, 8 September 2012 (UTC)
- Yes, I agree. From the street view it looks like normal dirt. Odd that the satellite view has the strange blue-white color, although I have seen strange false-color images in the satellite view before. They seem to use some type of algorithm which replaces the color in some circumstances. For example, I've seen them replace grass-less areas in shadow with images of grass. StuRat (talk) 17:36, 8 September 2012 (UTC)
- TopoQuest (one of the links from that maps page) marks it "Waste". —Tamfang (talk) 05:48, 8 September 2012 (UTC)
- They're dredge tailings ([7][8]). Sean.hoyland - talk 15:09, 8 September 2012 (UTC)
- Thanks. I never thought to look through Panoramio or to see if there was a street view. CambridgeBayWeather (talk) 16:02, 8 September 2012 (UTC)
- They're dredge tailings ([7][8]). Sean.hoyland - talk 15:09, 8 September 2012 (UTC)
why does some ceramic heat up in the microwave?
I have two bowls from a set of six, apparently differing only in color. They're a glazed ceramic that chips easily, made in Japan probably 1975–1980. One I can put in the microwave for several minutes, long enough to boil the liquid inside, and then hold by the handle with my bare hand. The other, after half a minute in the oven, will burn my hand through a hot pad, even though the liquid is still cold. Any idea why they should be so different, or why ceramic would heat up in a microwave at all? (I assume it has to be s.t. in the glaze. The one that stays cool is lemon yellow, the one that heats up ocher.) — kwami (talk) 04:39, 8 September 2012 (UTC)
- Well, the fact that some ceramics contain more water than others, may play a role. Plasmic Physics (talk) 05:38, 8 September 2012 (UTC)
- But more than water itself? + should be the same ceramic inside. — kwami (talk) 06:50, 8 September 2012 (UTC)
- My guess is that metal content in the ceramic is more likely. If it is heating faster than the water it seems that water content it unlikely. --BozMo talk 06:53, 8 September 2012 (UTC)
- Maybe a metal-based pigment in the glaze on one. It's remarkably strong effect, though. Also, if metal's in the glaze, I would assume it's oxidized, and you wouldn't get the same heating effect then, right? — kwami (talk) 06:57, 8 September 2012 (UTC)
- "s.t." ? Is that some type of metallic glaze ? I hope it isn't lead. StuRat (talk) 06:59, 8 September 2012 (UTC)
- ("Something".) Yeah, I hope not lead: I've been using it for years. — kwami (talk) 07:37, 8 September 2012 (UTC)
- There is a simple, non-destructive test for lead, some stores here were offering it on their lead-laden toxic Chinese products last xmas. On the plus side, if the heat is all in the glaze, it should cool off quickly, if you just let it sit for a minute. StuRat (talk) 17:11, 8 September 2012 (UTC)
I don't think there's anything particular special about it. Every material is going to have its own opacity to the wavelengths emitted in the microwave. I'm not a material scientist, but I'm willing to bet the initial research into microwave-resistant ceramics amounted to putting them in a microwave and seeing what happened, rather than worrying about what its chemical makeup was. The specific heat of your particular ceramic will also play a role. Someguy1221 (talk) 09:04, 8 September 2012 (UTC)
- "putting them in a microwave and seeing what happened" is what they did for the article, "The Application of Microwave Energy in Mineral Processing – a Review". For interest, it has a table of various minerals together with the temperatures they reached after 1 and 5 minutes in a domestic microwave. Sean.hoyland - talk 09:58, 8 September 2012 (UTC)
- I hope they were large samples, since some small samples might explode after 5 minutes of nuking. StuRat (talk) 17:09, 8 September 2012 (UTC)
- Wouldn't nuking any mineral sample result in the instant vaporization of said sample? And how would they pry loose enough nukes from the federal government to do that? Whoop whoop pull up Bitching Betty | Averted crashes 14:04, 9 September 2012 (UTC)
- Not sure if you are serious or not. "Nuking" is common slang for microwaving, at least here in the US. StuRat (talk) 03:32, 10 September 2012 (UTC)
- 20g samples with a particle size of 0.2-0.5mm. Chalcopyrite reached 780C in 1 minute. The quartz result, 140C, in 1 minute seems surprising. Sean.hoyland - talk 17:23, 8 September 2012 (UTC)
- Geez, 780°C in one minute ? At that rate it probably would explode in 5 minutes. Presumably they stopped nuking it before that ? StuRat (talk) 17:28, 8 September 2012 (UTC)
- Well, chalcopyrite is pretty close to metal in a microwave - it has a resistivity in the 10-3 to 10-5 range, comparable to amorphous carbon. [9] Note it is CuFeS2. Wnt (talk) 19:13, 8 September 2012 (UTC)
"Every material is going to have its own opacity". Sure. But here we have two bowls from the same set, differing apparently only in color, which heat up very differently.
"if the heat is all in the glaze, it should cool off quickly". Actually, no: After 5 minutes it will still burn my fingers. Ceramics tend to have low heat conductivities. — kwami (talk) 21:57, 8 September 2012 (UTC)
- That's surprising, as the heat isn't actually in the ceramic, it's in the glaze. StuRat (talk) 22:00, 8 September 2012 (UTC)
- I am not sure how StuRat gets his answer (I don't contest it but have no reason to accept it), but Someguy's is the correct one, it simply depends on the absorption spectrum of the material (does it get excited by microwaves, reflect them, or let them pass) and is exacerbated by the specific heat. There is no way for us, obviously, to test a ceramic (about as vague a term as metal) for its contents and contaminants over the internet. μηδείς (talk) 03:57, 9 September 2012 (UTC)
- To which answer do you refer ? If it's that the heat must be in the glaze, that follows from the only difference between the ones that heat up and those that don't being the pigments in the glaze. Although I suppose it could somehow work like the greenhouse effect, in that the glaze lets microwaves in, but not out, but I have difficulty imagining how this would work. StuRat (talk) 03:53, 9 September 2012 (UTC)
- That the glaze is the thing. You would seem to be right if you mean in the case of identical ceramics differing only in glaze. Reflection probably matters, not insulation. μηδείς (talk) 03:57, 9 September 2012 (UTC)
- OK, I see what you're saying, that the cool mugs have a glaze which reflects microwaves, while the hot mug's glaze allows the microwaves to pass, where they then heat the ceramic underneath. I was thinking something in the glaze was absorbing the microwaves directly on the hot mug's surface. But, I agree, given the additional info of the mugs staying hot for a long time, it sounds like the microwaves were not absorbed directly by the glaze and converted to heat there. StuRat (talk) 04:04, 9 September 2012 (UTC)
- (edit conflict)Absorption, transmission, reflection. Looking all the way back to the original question, you could have a glaze that reflects the microwave frequencies found in the oven, and another that simply transmits it through to the ceramic underneath. Or one glaze that reflects and another that absorbs. Or one glaze that transmits, one that absorbs, and a ceramic that transmits efficiently in both bowls. Or the ceramic may actually not be the same material even within the set. Or the glaze may be a different thickness on different bowls. And if it's the glaze that's absorbing, how well the heat would transfer to the ceramic is another question entirely. The OP is free to sand the glaze of each bowl and tell us what changes, which may be the only way to find out for sure. Someguy1221 (talk) 04:02, 9 September 2012 (UTC)
- "Huston, we have consensus." μηδείς (talk) 04:14, 9 September 2012 (UTC)
- Do you mean Houston, or are you telling Anjelica Huston ? StuRat (talk) 04:29, 9 September 2012 (UTC)
- I was wondering why that looked wrong when I typed it. μηδείς (talk) 15:32, 9 September 2012 (UTC)
- StuRat and others seem to assume that any heat produced in the glaze will stay there. If the glaze absorbs microwaves, it will tend to heat up the ceramic underneath. Ceramics generally have high heat capacity and will stay hot for a long time when heated. I think it is most likely that one color glaze absorbs microwaves and the other transmits them. This would probably be much more common, except that manufacturers have abandoned glazes that absorb microwaves, so you don't see this on modern products.--Srleffler (talk) 17:57, 10 September 2012 (UTC)
Intelligence and drug use
Why are smart people more likely to do drugs? It doesn't make any intuitive sense, but the data show a definite correlation. --168.7.239.137 (talk) 05:13, 8 September 2012 (UTC)
- [citation needed]. If you're going to ask a question that makes an assumption, you'd need to first establish that your assumption is correct. What data are you using for your assumption here? --Jayron32 05:16, 8 September 2012 (UTC)
- What are you calling an "assumption"? —Tamfang (talk) 05:51, 8 September 2012 (UTC)
- Well, obviously, a question that asks "why?" assumes the statement it asks "why?" about to be itself true. "Why are smart people more likely to do drugs?" assumes the statement "smart people are more likely to do drugs?" do be verified. I have yet seen no evidence presented by the questioner, or anyone speculating below, that that is true. I don't say that it is, and I also don't say that it isn't, but until that is resolved, we can't answer the "why?" part. --Jayron32 14:33, 8 September 2012 (UTC)
- The wording of the question strongly implies that the OP would have assumed the opposite, but was surprised by unspecified "data" to the contrary. The lack of citation does not make the statement an "assumption". —Tamfang (talk) 03:23, 9 September 2012 (UTC)
- Let's make this easier for you to understand. If I ask "Why is the moon made of green cheese", that question is unanswerable because the moon actually isn't made of green cheese. Insofar as we have not yet established that smart people are more likely to do drugs, we can't answer "why" they do more drugs until we can know if they do more drugs. The question as asked has an embedded statement which has not yet been shown to be true yet. --Jayron32 03:30, 9 September 2012 (UTC)
- Perhaps smart people are more curious / more easily bored / less likely to accept "drugs are Bad because I Say So" / ... —Tamfang (talk) 05:52, 8 September 2012 (UTC)
- Or perhaps they are more likely to have money to pay for them. Show us the data... --BozMo talk 06:52, 8 September 2012 (UTC)
- Perhaps they have more brain cells in need of killing ? :-) StuRat (talk) 07:03, 8 September 2012 (UTC)
- That was funny, I hope the mavens don't press you to death under heavy stones because of it. But Tamfang has the right serious answer. μηδείς (talk) 03:36, 9 September 2012 (UTC)
Among the students at the school I'm part of, it's the dopes who smoke dope. HiLo48 (talk) 07:14, 8 September 2012 (UTC)
- Also, "do drugs" is pretty vague and all-encompassing. Different substances show different user demographics, for all kinds of reasons. You'd have to point us in the direction of your data to give us a chance of saying anything useful about it. - Karenjc 09:44, 8 September 2012 (UTC)
- True, especially if you include legal drugs, like caffeine: "Mathematicians are biological machines designed to convert coffee into theorems". StuRat (talk) 17:15, 8 September 2012 (UTC)
- .
- The OP may have skimmed across articles by authors prominent in certain publications, such as Satoshi Kanazawa, who tends to get eye-grabber headlines on his articles such as "Why intelligent people use more drugs" (in which artcle in Pyschology Today - which if you actually read it, does not asert or demonstrate that the title is true, but it does list a number of factors why folk may do drugs in spite of their actual or assumed intelligence.)
- For every journal article that talks about intelligent users doing drugs, there is probably 100 articles demonstrating a negative correlation. Many workers in the field of drug rehabilition consider that drugs ranging from pot to heroin, over time, reduce intelligence. See e.g., http://scienceblog.com/community/older/2002/G/20021645.html.
- However, having significant experience in working in a not-for-profit organistion offerring programmes to assist drug users, I have come to the view that drugs (mainly) do not cause intelligence to fall, instead low intelligence makes drug use more likely. In other words, the lower intellence reported is drug users by many investigators was not due to the drugs reducing intelligence, the the statistically low inteligence reported was due to it being low to start with. After all, drug users generally don't get tested before they take up drugs. When we found a new drug user, or a new (to us) drug user came to us wanting to get off drugs, we had a standard procedure. One of the things we did was review their life with them, for a number of reasons. We pretty often found that they had done lots of dumb things apart from taking drugs, and had lots of indicators - eg poor school performance, trouble with the law, etc, before starting on drugs. In some cases, dumb though it is, their life was so stuffed up, doing drugs was the smartest thing they did!
- Intelligence is multidimensional. A person may score high in applying logic or be able to do math, but may be socially inept. High capability in logical thinking may reduce the chances of doing drugs, but social ineptness may increase it. It is well documented that humans are not good at risk assesment. In balancing the potential risk of addiction against the certainty of getting high by trying a drug your friend is offering is an assesment that many find difficult to properly make.
- Never make the mistake of thinking people who do well at school, or even at university, are thereby or must be intelligent. For example, a kid at school who is socially inept and stupid may end up with no friends - so he can spend more time at home doing his homework. When I was at high school, I did no homework. Naturally I did not score well in tests and exams. That does not make me dumb - it might mean I was lazy. Later on I did however go to uni and graduated with honours - because I found the course interesting, and I wanted the qualification - so I worked hard, had good time management, and the subjects were not actually all that intellectually challenging. That does not make me bright either - maybe just determined.
- So may other factors influence whether or not a person does drugs. These factors are often obscurred or not spelled out in statistical studies, and often overide the otherwise reluctance of an intelleigent person to do drugs. Factors include: whether or not parents or siblings do drugs, peer pressure, availability, in or not in a loving relationship. Loners often don't know where to get drugs - you can't do drugs if you don't know where to get it. But if a loner does get to tray drugs, his/her loneliness may increase the consumption.
- Wickwack121.221.223.160 (talk) 11:00, 8 September 2012 (UTC)
- Given your experience in the field, the conclusions you draw are necessarily based on selection biased data. Ssscienccce (talk) 14:11, 8 September 2012 (UTC)
- Regarding inhalant abuse, studies showed social users to be of average intelligence, isolate users were of above average intelligence. (source NIDA Research Monograph 129 Inhalant Abuse: A Volatile Research Agenda)
- The (second?) most productive mathematician in history was a long-term amphetamine user.
- The Satoshi Kanazawa article can be found here. Ssscienccce (talk) 13:47, 8 September 2012 (UTC)
- Presumably Ssscience's comment about selection biased data is based on it being the folk I came in contact with had, mostly, come to us because they could not succesfully manage their life on drugs. A drug user succesfull in life presumably would not require or ask for help. But how many illegal hard drug users are successful in life? Most users of heroin, e.g., will end up either in jail, dead, or with an assistance programme. That is not nearly so much the case with things like ecstacy, but long term users will have problems.
- Judging from the Paul Edros article linked ("Most productive mathematician"), he was a real weirdo, and perhaps some sort of savant. In any case specific examples do not rule out a trend. Famous examples of illegal drug users include Paul MacCartney and Mick Jagger. These are undoubtably intelligent, capable chaps very succesful in life. But they are certainly exceptions. Wickwack120.145.58.3 (talk) 15:39, 8 September 2012 (UTC)
- The big problem with this question is that "drugs" aren't a clade; they're not even really being defined. What illegal drugs have in common is only that they're illegal. There are some obvious cases where actions such as huffing (which come to think of it more or less isn't an illegal drug...) can damage the brain. In some others the data is dubious or nonexistent. Some people claim that nootropic drugs even increase intelligence, but I would take such claims with a grain of salt - basic evolutionary theory would suggest there is a pretty high bar toward making a drug that is actually good under all circumstances, which means that most positive data of this type should be expected to be a flash in the pan, one circumstance or one specific genetic background that doesn't reliably reproduce, unless perhaps there is some physical or aging-related downside. But I firmly believe there will be many drugs which either have no effect, or whose effects are so pleiotropic and difficult to analyze (even philosophically, if perfect data were available) that no overall conclusion can be made. Wnt (talk) 17:33, 8 September 2012 (UTC)
- The big problem with this question is that "drugs" aren't a clade; they're not even really being defined. What illegal drugs have in common is only that they're illegal. There are some obvious cases where actions such as huffing (which come to think of it more or less isn't an illegal drug...) can damage the brain. In some others the data is dubious or nonexistent. Some people claim that nootropic drugs even increase intelligence, but I would take such claims with a grain of salt - basic evolutionary theory would suggest there is a pretty high bar toward making a drug that is actually good under all circumstances, which means that most positive data of this type should be expected to be a flash in the pan, one circumstance or one specific genetic background that doesn't reliably reproduce, unless perhaps there is some physical or aging-related downside. But I firmly believe there will be many drugs which either have no effect, or whose effects are so pleiotropic and difficult to analyze (even philosophically, if perfect data were available) that no overall conclusion can be made. Wnt (talk) 17:33, 8 September 2012 (UTC)
- Given the indication of the solvent use study, that people with above average intelligence are more likely to use on their own, one could hypothesize that more intelligent drug users are more likely to keep their habit a secret. But I'm not sure there even is a discrepancy to be explained. No one is saying drug users are succesful in life. The claim of Satoshi Kanazawa seems based on reasonable grounds: prospective generational cohort studies are well suited for examining such a correlation. The least one can say is that, barring attrition effects and false answers, the correlation exists in the group of Britains born in one specific week in 1958.
- Been searching for other studies showing a negative correlation between drug use and IQ, but so far nothing. I remember one study about smoking and IQ, that was reported as showing smokers had lower IQs. If I recall correctly the havy smokers scored worst, the group of light smokers scored better than both heavy and non-smokers. I did find this statement in a podcast transcript : "I don’t think we ever found another group of people so defined as a group who had a higher rate of addiction and alcoholism than card-carrying members of Mensa." More support for the OP's assumption... Ssscienccce (talk) 22:42, 8 September 2012 (UTC)
- Can't find any papers showing a negative correlation between drug use and IQ? There's heaps. I gave you one in my ealier post. Here's another: http://www.medicaldaily.com/articles/11758/20120827/teen-marijuna-use-linked-to-lower-iq-in-middle-age.htm. Wickwack120.145.58.3 (talk) 02:59, 9 September 2012 (UTC)
- That study is about the effects of drug use. I was looking for studies that disprove the OP's assumption and Kanazawa's findings, i.e. studies that would show children with higher IQ scores being less likely to use drugs later in life. To quote the Kanazawa article: British children who are more intelligent before the age of 16 are more likely to consume psychoactive drugs at age 42 than less intelligent children. No one claims that drugs make you more intelligent, far from it. That's what makes the findings remarkable, because they show that smart people are more likely to make bad choices. Ssscienccce (talk) 18:30, 9 September 2012 (UTC)
- Yes, I know that. My point that I made earlier is that rather than drug users being more intelligent as the OP claimed, ther is a multitude of papers that show a negative correlation - but researchers have tended to interpret the negative coorrelation as indicating that drugs reduce intelligence, instead of assuming that intelligence was lower to satrt with. (Something that seems obvious from actually working with drug users, instead of just sitting at a desk looking at stats). Wickwack124.178.174.234 (talk) 08:24, 11 September 2012 (UTC)
- That study is about the effects of drug use. I was looking for studies that disprove the OP's assumption and Kanazawa's findings, i.e. studies that would show children with higher IQ scores being less likely to use drugs later in life. To quote the Kanazawa article: British children who are more intelligent before the age of 16 are more likely to consume psychoactive drugs at age 42 than less intelligent children. No one claims that drugs make you more intelligent, far from it. That's what makes the findings remarkable, because they show that smart people are more likely to make bad choices. Ssscienccce (talk) 18:30, 9 September 2012 (UTC)
- Can't find any papers showing a negative correlation between drug use and IQ? There's heaps. I gave you one in my ealier post. Here's another: http://www.medicaldaily.com/articles/11758/20120827/teen-marijuna-use-linked-to-lower-iq-in-middle-age.htm. Wickwack120.145.58.3 (talk) 02:59, 9 September 2012 (UTC)
I suspect drug use is more likely at the top and bottom (but not extreme bottom) of the intelligence curve for different reasons. The top is curious, risk-seeking, self-medicating, and has no respect for authority. The low range also self-medicates and has little respect for authority. The middle range is conventional and less likely to need to or want to self-medicate. That's all OR on my part. μηδείς (talk) 03:42, 9 September 2012 (UTC)
- Really, I can't say that any of my fellow mensans would agree with that generalisation. Plasmic Physics (talk) 08:19, 12 September 2012 (UTC)
elecrostatice
elecrostatice forces between two charges are called central.why? — Preceding unsigned comment added by Parinoor (talk • contribs) 08:24, 8 September 2012 (UTC)
- I'm not sure what you mean by "central". Do you mean it's called a fundamental force? If that's what you're asking, then the answer is in the introduction of that article, "An interaction is fundamental when it cannot be described in terms of other interactions". Someguy1221 (talk) 09:09, 8 September 2012 (UTC)
- A central force in physics is a force that is always directed to, or comming from, an apparent central point. Examples are gravitational force, which for example appears to come from the centre of gravity of any object, and a central electrostatic force, which comes from a point charge/source. Note that electrostatic force may be between two parallel planes each having distributed charge - in this case it is not a central force. Engineers speak of lines of force (like in magnetic lines of force); the lines spread out radially and equidistantly from a central point from an object exerting a central force; for a non central force, the lines of force will be parallel or some other configuration. Wickwack121.221.223.160 (talk) 10:30, 8 September 2012 (UTC)
Diseases crossing the Brain-Blood Barrier
Hello,
I write to you with an inquiry about the Brain-Blood Barrier (BBB). Having read up on its nature on Wikipedia and in my books I have learnt a few things about how through transcytosis matter can flow through the barrier. However, when mentioning diseases such as progressive multifocal leukoencephalopathy and researching into the JC virus, little is uncovered on Wikipedia as to how the virus or other viruses can transfer past the barrier. Could I please ask you which methods are utilized by the virus, and perhaps (since it's so related) how come the ability to cross the barrier seems correlated to the immune system?
Thank you in advance.
88.90.168.15 (talk) 15:35, 8 September 2012 (UTC)
- The lede to our blood-brain barrier article says it stops large objects, like bacteria, and hydrophilic molecules, but allows others through. Viruses certainly are not large. I'm not sure if they are hydrophilic, however. The other approach is to attack the barrier directly, by destroying the cells which compose the barrier. Here the immune system is important in preventing such an attack from succeeding. Once breached (and perhaps repaired), the barrier then can become a hindrance to the immune system, as it also limits the ability of immune cells to pass. StuRat (talk) 17:23, 8 September 2012 (UTC)
- Viruses must have a mechanism of crossing the cell membrane, or they won't be able to reproduce. But only a few types of viruses are able to make it through the BBB if it is intact. One very well known example is rabies, which is taken up by nerves in the periphery and transported along nerve fibers into the CNS. Looie496 (talk) 17:41, 8 September 2012 (UTC)
- The best source I can find is http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2928669/, a freely available paper from 2010 that specifically addresses this question -- it shows evidence that the virus gets into the brain via B cells of the immune system. Looie496 (talk) 17:37, 8 September 2012 (UTC)
- Thank you Looie495, this is an excellent answer to my question. 88.90.168.15 (talk) 23:40, 8 September 2012 (UTC)
How do non-brain cancer cells cross the BBB? Do they just push their way inside? μηδείς (talk) 03:34, 9 September 2012 (UTC)
- This is outside my domain of expertise, but here is what I can make out. The BBB is formed by endothelial cells, which form the lining of blood vessels in the brain. In order for cancer cells to metastasize into the brain, they have to first attach to those endothelial cells and somehow disrupt their ability to form a tight barrier. The way they do that is not clearly understood -- this paper reviews what is known, at least for breast cancer, if you can make sense of it. Looie496 (talk) 03:48, 9 September 2012 (UTC)
- Yes, that's exactly what I suspected and why I said "push their way in". μηδείς (talk) 04:12, 9 September 2012 (UTC)
September 9
Unidentified larva
I am struggling in the identification of this insect found on a wall in the Pantanal. It might be a larva of a bagworm moth or maybe of Phereoeca uterella. However, it seemed that there was no “worm” inside like in these and those pictures, but it still moved. Any ideas? --Leyo 00:42, 9 September 2012 (UTC)
- I think you have just won the prize for most hopeless question of the month. Looie496 (talk) 03:37, 9 September 2012 (UTC)
- Looks like a louse nit or, especially, the unhatched egg of a fly, which might move just before hatching. Makes me want to retch looking at it. μηδείς (talk) 04:10, 9 September 2012 (UTC)
- How long is this egg? Here are images related to fruit fly pupae. Please specify. μηδείς (talk) 21:48, 9 September 2012 (UTC)
- It's not an egg. It moved slowly. It is 1–2 cm long. --Leyo 21:10, 10 September 2012 (UTC)
- Are we sure this isn't a slug of some sort? --Jayron32 21:17, 10 September 2012 (UTC)
- Yes, I am. It has a casing made of sand. --Leyo 22:54, 10 September 2012 (UTC)
- How long is it? μηδείς (talk) 03:11, 11 September 2012 (UTC)
- Yes, I am. It has a casing made of sand. --Leyo 22:54, 10 September 2012 (UTC)
- Are we sure this isn't a slug of some sort? --Jayron32 21:17, 10 September 2012 (UTC)
- It's not an egg. It moved slowly. It is 1–2 cm long. --Leyo 21:10, 10 September 2012 (UTC)
- How long is this egg? Here are images related to fruit fly pupae. Please specify. μηδείς (talk) 21:48, 9 September 2012 (UTC)
- Looks like a louse nit or, especially, the unhatched egg of a fly, which might move just before hatching. Makes me want to retch looking at it. μηδείς (talk) 04:10, 9 September 2012 (UTC)
Mental Capacities of Prenates and Infants Versus Those of Certain Animals
How do the mental capacities of prenates and infants compare with those of certain animals such as pigs, chickens, and fish? BTW, by prenates I meant prenatal human specimens--zygotes, embryos, and fetuses. Futurist110 (talk) 06:18, 9 September 2012 (UTC)
- Unfortunately the only way we have to measure mental capacity is to give various sorts of stimuli and look at the responses. Just about the only response a fetus can make is uncoordinated movement -- and just about the only mental capacity we can recognize is a tendency to move in response to movement by the mother or to loud sounds. Newborn infants can make some basic coordinated movements, but the most sophisticated thing they can do is move their eyes. Their eye movements say that they that they can make some interesting distinctions, for example between faces and other types of objects. Also the sophistication of their responses increases rapidly with age. But basically, the motor control capabilities of adult animals such as pigs and chickens are so much better developed than those of human infants that it's hard to figure out how to do a meaningful comparison. Looie496 (talk) 07:03, 9 September 2012 (UTC)
- Winthrop Kellogg raised Gua (chimpanzee) with his son Donald, beginning when Gua was 7 1/2 months old and Donald was 10 months old. "Although the chimp progressed faster than the boy in the earliest stages, it became evident towards the end of the experiment that she was falling behind, especially ‘in the matter of intellectual adaptation to human demands’."[10] Clarityfiend (talk) 09:36, 9 September 2012 (UTC)
Neurons and viruses
- How large are most (human) cerebral neurons compared to common viruses (say, the influenza A virus)
- How large are synapses between these neurons?
- Is it theoretically possible that viruses, after somehow reaching the brain, could place themselves between (or around) these synapses and cause an interuption between electrical impulses? If so, what problems could arise from this?
Thanks, 64.229.152.217 (talk) 06:19, 9 September 2012 (UTC)
- The synaptic cleft at a chemical synapse is generally 20-40 nm wide, and the smallest viruses are only about 20 nm in length (although most are a good bit larger), so I suppose it is possible in principle, but I have never heard of anything like that happening. The goal in life of a virus is to inject its DNA into a cell so that it can exploit the cell's machinery to reproduce itself, and I can't see how fooling around in the synaptic cleft would do anything to promote that goal. But it's largely a moot point: because of the blood-brain barrier, viruses can't even get access to synapses unless something has gone very badly wrong. Looie496 (talk) 07:19, 9 September 2012 (UTC)
- Neurotropic viruses like rabies infect nerve cells and spread neurally, thereby evading the immune system and the blood-brain barrier. These viruses pass through the synapses so in theory it is possible. That's assuming the infection is otherwise asymptomatic. Infection by some viruses result in degeneration of the neuron as a self-destructive defense mechanism to slow the spread of the virus. a virus blocking the synaptic cleft wouldn't matter much in that case.
- Interestingly, neurotropic viruses could provide researchers an opportunity to trace connections among neurons. Tracers used in neuroanatomy research are limited in their ability because they don't cross synapses and in the past, after identifying neurons of interest, other techniques like electron microscopy were needed to further explore the interconnections between them. Neurotropic viruses could function as synapse crossing tracers, and they would be self-replicating. The paper was published in 1998, so this info is a bit dated. Ssscienccce (talk) 20:17, 9 September 2012 (UTC)
- A protein in a misfolded form called Prion causes mad cow disease, but it is not a virus. -- RexRowan Talk 19:13, 9 September 2012 (UTC)
Efficiency of Vaccinations against Hepatitis B
Comparative strength of fundamental forces
Sometimes we hear things like "gravity is <large number> times weaker than <other fundamental force>", but how is that number calculated, given that different forces depend on different factors (e.g. charge or mass)? What exactly is being compared? 86.167.124.146 (talk) 11:30, 9 September 2012 (UTC)
- Regardless of the what the force operates on, mass or electric charge or whatever, it is always a force, measured in some (tiny) fraction of a Newton, the unit of force (or measured in units of kilogram-force). Gravity causes two bodies having a finite mass to be attracted to each other by a force; two bodies each having an opposite electric charge are attracted to each other by a force. Thus for any two bodies each having a certain mass, each having a certain electric charge, and separated by a certain distance, will be attracted (or repelled) by a net force being the sum of the gravitational force and the electric force. Thus, for any two bodies having mass, charge, and a separation, the components of the net force are calculable and comparable. Ratbone124.178.174.234 (talk) 13:16, 9 September 2012 (UTC)
- Thanks, I understand all that, but that wasn't the question I intended to ask. The statements I am talking about are not comparing forces on some specific bodies with some specific charge or mass in some specific circumstance. They are saying (or purport to be saying) that gravity is <large number> times weaker in some absolute, or general, sense. Therefore the question arises as to what exactly is being compared. 86.167.124.146 (talk) 13:54, 9 September 2012 (UTC)
- That doesn't make much sense. Which of the various forces are the stronger or weaker depends on the circumstances and distances between bodies. Can you cite a reference where you saw this, or, preferably, if you have an online example, can you provide the url? Ratbone124.178.174.234 (talk) 14:30, 9 September 2012 (UTC)
- You can easily give specific values for a specific particle pair - electron and electron, or two sodium ions. But you are right that no general rule exists to get a solid number. Yet... gravity seems weaker than electromagnetism. An exercise I won't actually do right now would be to evaluate the strength of attraction between two Planck masses (thought to be the greatest mass a particle can have before collapsing into a black hole), compared to the strength of repulsion of two +1/3 charges (the smallest possible value I know of, for quarks). I suspect this will indicate that gravity is very much weaker than electromagnetism even for the largest mass and the smallest charge. Wnt (talk) 15:14, 9 September 2012 (UTC)
- No, the gravitational force between two Planck-mass particles with charge 1 is similar to the electromagnetic force between them. -- BenRG (talk) 23:32, 9 September 2012 (UTC)
- Hmmmm... looking further, the Planck particle actually has the square root of pi more mass than the Planck mass. And they are really ridiculously dense - 4.90 x 1094 kg/m3. I'm getting that the mass of the Earth (5.98 x 1024 kg), formed in contiguous Planck particles in fcc packing (0.74) would occupy 1.65 x 10-70 m3 - the area of a sphere with radius 1.58 x 10-23, or about 50 million times smaller than a proton. That's dense! Now anyway, for two identical particles 1 meter apart with this mass and charge 1
- No, the gravitational force between two Planck-mass particles with charge 1 is similar to the electromagnetic force between them. -- BenRG (talk) 23:32, 9 September 2012 (UTC)
- You can easily give specific values for a specific particle pair - electron and electron, or two sodium ions. But you are right that no general rule exists to get a solid number. Yet... gravity seems weaker than electromagnetism. An exercise I won't actually do right now would be to evaluate the strength of attraction between two Planck masses (thought to be the greatest mass a particle can have before collapsing into a black hole), compared to the strength of repulsion of two +1/3 charges (the smallest possible value I know of, for quarks). I suspect this will indicate that gravity is very much weaker than electromagnetism even for the largest mass and the smallest charge. Wnt (talk) 15:14, 9 September 2012 (UTC)
- That doesn't make much sense. Which of the various forces are the stronger or weaker depends on the circumstances and distances between bodies. Can you cite a reference where you saw this, or, preferably, if you have an online example, can you provide the url? Ratbone124.178.174.234 (talk) 14:30, 9 September 2012 (UTC)
- Thanks, I understand all that, but that wasn't the question I intended to ask. The statements I am talking about are not comparing forces on some specific bodies with some specific charge or mass in some specific circumstance. They are saying (or purport to be saying) that gravity is <large number> times weaker in some absolute, or general, sense. Therefore the question arises as to what exactly is being compared. 86.167.124.146 (talk) 13:54, 9 September 2012 (UTC)
- F = (3.86x10-8kg)2*(6.67x10-11N (m/kg)2/1 m2 = 0.99x10-25 N
- F = (1.60x10-19C)2*(8.99x109N (m/C)2/1 m2 = 2.30x10-28 N
- Unless I fouled up... Oddly enough, this ratio is 3.1415 x 137.01 ... so if we multiplied by that fine structure constant, and compare to the Planck mass rather than the mass of the Planck particle, the numbers become equal, within the low precision of this calculation. Not sure what that means... Wnt (talk) 04:26, 10 September 2012 (UTC)
- It's a statement that gets made from time to time in popular science contexts. I could not give exact references. It's interesting though... arguably the greater electromagnetic force between charged particles shows that particles have vastly more charge than they do mass, rather than that gravity is weak. 86.167.124.146 (talk) 17:22, 9 September 2012 (UTC)
- I think they are all measured against the change of velocity between the masses' interactions. -- RexRowan Talk 17:48, 9 September 2012 (UTC)
- It's a statement that gets made from time to time in popular science contexts. I could not give exact references. It's interesting though... arguably the greater electromagnetic force between charged particles shows that particles have vastly more charge than they do mass, rather than that gravity is weak. 86.167.124.146 (talk) 17:22, 9 September 2012 (UTC)
- The "strength" of an interaction is given by the value of its coupling constant. This is a dimensionless number (for electromagnetism, it is 1/137, the fine-structure constant) and is independent of which particles participate in a concrete interaction event. See here for a list of the values of the coupling constants (at low energy). In order to do calculations on particular events, the coupling constants are multiplied by the charges, masses, etc. of the particles involved. For a more intuitive approach, you can e.g. calculate the electric and gravitational forces between two electrons at a certain separation, but note that this changes when you replace the electrons by protons, for instance. --Wrongfilter (talk) 18:05, 9 September 2012 (UTC)
- So, to be clear, is this coupling constant the unique way to describe the "absolute" strength of a fundamental force, independent of its action in any particular situation? It means, therefore, that we can say, for example, "gravity is <exact large number> times weaker than the electromagnetic force"? 86.128.4.46 (talk) 19:23, 9 September 2012 (UTC)
- No, it's dependent, there must be an interaction between the known value of the masses to apply the constant. -- RexRowan Talk 19:34, 9 September 2012 (UTC)
- Are you sure you aren't confusing this with the gravitational constant? 86.128.4.46 (talk) 19:55, 9 September 2012 (UTC)
- I don't know. I think the condition is there must be an interaction between the masses under the effect of a certain type of force to apply the coupling constant. For example, a stone and magnet both with mass but only have gravitational force with each other but no electromagnetic force so the coupling constant of electromagnetic force does not apply. -- RexRowan Talk 19:34, 9 September 2012 (UTC)
- Are you sure you aren't confusing this with the gravitational constant? 86.128.4.46 (talk) 19:55, 9 September 2012 (UTC)
- No, it's dependent, there must be an interaction between the known value of the masses to apply the constant. -- RexRowan Talk 19:34, 9 September 2012 (UTC)
- So, to be clear, is this coupling constant the unique way to describe the "absolute" strength of a fundamental force, independent of its action in any particular situation? It means, therefore, that we can say, for example, "gravity is <exact large number> times weaker than the electromagnetic force"? 86.128.4.46 (talk) 19:23, 9 September 2012 (UTC)
- Hey, try this, you can ask a real physicist here: [11] -- RexRowan Talk 20:02, 9 September 2012 (UTC)
- They are talking about the force between electrons or protons. The difference is around 1037 for protons, 1043 for electrons, or 1040 for one of each. Asking "why is gravity so weak?" is the same as asking "why are electrons and nucleons so light?". -- BenRG (talk) 23:32, 9 September 2012 (UTC)
- Are you disagreeing with Wrongfilter, who says there is a way to measure the strength of these things independently of any specific interaction event? 86.128.4.46 (talk) 00:49, 10 September 2012 (UTC)
- I suppose I am. The electromagnetic fine-structure constant is defined in terms of the electron/proton charge. A corresponding unitless gravitational coupling constant would have to be defined in terms of a particular mass. If you picked the electron mass you'd get a value 1043 times smaller than the electromagnetic constant. But it doesn't make much sense to pick the electron mass since it isn't a fundamental mass the way the electron charge is a fundamental charge. Also, the whole thing is just a needlessly complicated way of saying that the ratio of force strengths for two electrons is ~1043. -- BenRG (talk) 03:34, 10 September 2012 (UTC)
- I concede that the definitions of the coupling constants are to some extent arbitrary and the numbers are therefore indicative more than anything else. I don't know about "needlessly complicated". The force comparison works for electromagnetism and gravity, but how would you calculate the force due to the weak or strong interactions? The "strength" of an interaction manifests itself rather through interaction cross sections, decay rates and stuff like that, and in order to calculate those one uses the coupling constants (attached to the vertices of the Feynman diagrams, if I remember correctly). Also, the coupling constants are used to parameterize the dependence of the interaction strength on the interaction energy. Admittedly, this is probably more abstract than the original question intended. --Wrongfilter (talk) 12:14, 10 September 2012 (UTC)
- Well, according to the calculation I did above, if you use the Planck mass, it appears the coupling constant is something pretty close to 1... Wnt (talk) 03:46, 11 September 2012 (UTC)
- I concede that the definitions of the coupling constants are to some extent arbitrary and the numbers are therefore indicative more than anything else. I don't know about "needlessly complicated". The force comparison works for electromagnetism and gravity, but how would you calculate the force due to the weak or strong interactions? The "strength" of an interaction manifests itself rather through interaction cross sections, decay rates and stuff like that, and in order to calculate those one uses the coupling constants (attached to the vertices of the Feynman diagrams, if I remember correctly). Also, the coupling constants are used to parameterize the dependence of the interaction strength on the interaction energy. Admittedly, this is probably more abstract than the original question intended. --Wrongfilter (talk) 12:14, 10 September 2012 (UTC)
- I suppose I am. The electromagnetic fine-structure constant is defined in terms of the electron/proton charge. A corresponding unitless gravitational coupling constant would have to be defined in terms of a particular mass. If you picked the electron mass you'd get a value 1043 times smaller than the electromagnetic constant. But it doesn't make much sense to pick the electron mass since it isn't a fundamental mass the way the electron charge is a fundamental charge. Also, the whole thing is just a needlessly complicated way of saying that the ratio of force strengths for two electrons is ~1043. -- BenRG (talk) 03:34, 10 September 2012 (UTC)
- Are you disagreeing with Wrongfilter, who says there is a way to measure the strength of these things independently of any specific interaction event? 86.128.4.46 (talk) 00:49, 10 September 2012 (UTC)
speed of light and spin
I know that theoretically, if a particle travels at the speed of light, it shall be mass-less, otherwise it would have infinite energy. Should this kind of particle satisfy any other condition? e.g. should its spin have some certain value?--37.117.25.125 (talk) 14:52, 9 September 2012 (UTC)
- This isn't my field, so caution, but - Photons are spin-1 particles and have an angular momentum of Planck's constant (well, its helicity in the direction of travel is that; there's a square root of 2 that enters into it which is beyond my understanding). The W and Z bosons also have spin 1. But gravitons are believed to be a spin-2 particle, if they exist. Wnt (talk) 15:18, 9 September 2012 (UTC)
- A particle moves at the speed of light if, and only if, it is massless. So you could rephrase your question as aking what special properties are required to be massless. The only phenomena I know of related to spin is that helicity and chirality will be the same; our article on chirality may be of interest to you, the helicity article is not so useful. To date the only observed massless particle is the photon, though the gluon is strongly suspected to be massless as is the graviton (if it exists), so all known massless particles are bosons; though, not all bosons are massless, the Higgs, W boson, and Z boson are massive. The standard model originally assumed that neutrinos (a fermion) were massless, and this wasn't conclusively disproven till '98 when flavour oscillations were observed in Japan; thus, as far as I can tell, there is no reason a fermion couldn't be massless, and hence travel at light speed. [If you don't know already, fermions have half-integer spins, bosons have integer spins.]Phoenixia1177 (talk) 09:47, 10 September 2012 (UTC)
Medical Examination Beds
Hello. What are those rings that doctors can pull and fold out from the sides of medical examination beds called? Thanks in advance. --Mayfare (talk) 15:33, 9 September 2012 (UTC)
- I'm not sure what you're talking about, but this page has many examples, all with different names for different functions. --TammyMoet (talk) 16:32, 9 September 2012 (UTC)
- I think your talking about stirrups, which are for holding the legs up and separated for vaginal examinations and the like. Dominus Vobisdu (talk) 16:42, 9 September 2012 (UTC)
- Do please clarify. Do you mean this? μηδείς (talk) 21:47, 9 September 2012 (UTC)
- I think they mean bed rails, like these: [12]. They are meant to keep the patient from falling out of bed. They fold down for examinations, to exit and enter the bed, etc., where they would otherwise be in the way. They may also contain electronics, such as the nurse call button, bed adjustment controls, and TV/radio controls. StuRat (talk) 03:25, 10 September 2012 (UTC)
Snow in Muslim nations
Which Muslim nations do tend to receive snow during the winter season? — Preceding unsigned comment added by 65.92.155.47 (talk) 16:18, 9 September 2012 (UTC)
- Iran gets snow in its mountains, enough for quite a few skiing areas. -- Finlay McWalterჷTalk 16:26, 9 September 2012 (UTC)
- (ec)It depends how you define "Muslim nation." For example, Kazakhstan is a secular republic, but its population is about 70% Muslim. It is a fairly arid place, but it has cold winters with snow. The climate of the Islamic Republic of Iran is very diverse, but there are high-altitude basins, and numerous very tall mountain ranges; heavy snowfall is common in most of the country. Afghanistan is very snowy, and last time I checked, it is officially an Islamic Republic. And, I'd be remiss if I did not mention Lebanon... the Lebanese people are diverse, but there are many Muslims. As I learned the etymology, "Lebanon" and "lebneh" both come from the same root-word, describing the snowy mountains. Though, this is not in universal agreement. In fact, a senseless war was fought over it. Nimur (talk) 16:42, 9 September 2012 (UTC)
- The mountains of Pakistan, such the Pakistani parts of the Karakoram, get a lot of snow. -- Finlay McWalterჷTalk 18:10, 9 September 2012 (UTC)
- There are the Caucasus mountains. μηδείς (talk) 20:07, 9 September 2012 (UTC)
- This past February Bosnia and Albania were both hit by heavy snowfall, although this was unusual. Some parts of Turkey receive snow, as any reader of Orhan Pamuk will remember. Of course, there is a particular place in Dubai where it snows every night. LANTZYTALK 21:34, 9 September 2012 (UTC)
- The IP seems to be from Canada, (Toronto per geolocate), which may explain the implied bias. μηδείς (talk) 21:44, 9 September 2012 (UTC)
- What helpful information is conveyed by your comment on "implied bias"? Bielle (talk) 22:02, 9 September 2012 (UTC)
- Um, the point of view that southern lands don't get snow? What in the world did you think I meant? μηδείς (talk) 18:40, 10 September 2012 (UTC)
- What helpful information is conveyed by your comment on "implied bias"? Bielle (talk) 22:02, 9 September 2012 (UTC)
- The IP seems to be from Canada, (Toronto per geolocate), which may explain the implied bias. μηδείς (talk) 21:44, 9 September 2012 (UTC)
See Syria: desperate Homs residents collect snow to drink as water is cut off and SNOW IN JORDAN Weird but True. Alansplodge (talk) 22:34, 9 September 2012 (UTC)
- And I found Bethlehem SNOW! - a mainly Christian town in the Muslim dominated West Bank. Alansplodge (talk) 22:42, 9 September 2012 (UTC)
- On a roll now - It Snows in Alexandria (Egypt), Snowfall in Saudi Arabia and UAE's 'once in a lifetime' snow fall although the last sounds rather exceptional - there's a nice video of some chaps in traditional Bedouin robes building a snowman. Alansplodge (talk) 22:47, 9 September 2012 (UTC)
Is it true that point particles like quarks and electrons have no volume?
Topic says it all. ScienceApe (talk) 17:01, 9 September 2012 (UTC)
- I suspect that (a) the definition of "volume" becomes tricky at these scales and/or (b) no one really knows. No doubt someone will be along soon to give you a better answer! 86.167.124.146 (talk) 17:24, 9 September 2012 (UTC)
- Please read Point particles. In standard particle physics, volume does not apply to points because it makes calculations easier when the result doesn't have to go to details. But in quantum mechanics which describes particles on a small scale, point particles are not points anymore, they have volume, mass and electrical charges, including strings. -- RexRowan Talk 17:30, 9 September 2012 (UTC)
- (ec)First off, by definition "point particles" have no volume. They're points - there's no width, length or depth to them, and as such, there's no volume. But then are quarks and electrons true point particles? The Standard Model (the most widely accepted and best validated theory of subatomic particles) treats them as such, although there are other theories (like string theory) which give them non-zero spacial extent (though offhand I'm not aware of ones which give them full 3D volume). But, as 86.167 indicates, you have to be careful about your definition of "volume". For instance, the Heisenberg uncertainty principle means it's difficult to pick out a single point in space where the particle is located - instead it's delocalized over a area - does that count as "volume"? Also, depending on how you set up the calculations, you can come up with a non-zero "size" of things like an electron (see classical electron radius), which means that the electron behaves like it has a non-zero volume (see also cross section (physics)) - does that count? -- 71.35.118.235 (talk) 17:46, 9 September 2012 (UTC)
- String theory posits that the elementary particles (ie. electrons and quarks) within an atom are not 0-dimensional objects, but rather 1-dimensional oscillating lines ("strings"). So, 1 dimensional objects still have no volume. Vespine (talk) 22:42, 9 September 2012 (UTC)
- It should be noted that even atoms don't have a well-defined volume. Well, I suppose they do, but it depends on what definition of atomic radius you are working with. --Jayron32 22:53, 9 September 2012 (UTC)
- String theory posits that the elementary particles (ie. electrons and quarks) within an atom are not 0-dimensional objects, but rather 1-dimensional oscillating lines ("strings"). So, 1 dimensional objects still have no volume. Vespine (talk) 22:42, 9 September 2012 (UTC)
- There are different notions of volume. The technical sense in which electrons are point particles (in the standard model) is that the electron field interacts with other fields (like the electromagnetic field) separately at each point. You can define a proton field too, but the value of that field at a point only tells you whether the proton is centered there. Since the proton has a small size, about 1 fm, that point of the field will interact with the electromagnetic field at points up to ~1 fm away.
- On the other hand, the thing that prevents two objects from occupying the same region of space is the Pauli exclusion principle applied to electrons, so in that sense electrons do occupy space, if anything can be said to. -- BenRG (talk) 23:08, 9 September 2012 (UTC)
- It depends, of course, on what you mean by "electron" and "space". Two electrons can occupy identical orbitals as long as they have orthogonal Spins, but you can still have exactly two electrons within the same "space" (if you take an orbital to be the space an electron occupies). --Jayron32 23:19, 9 September 2012 (UTC)
- Not necessarily. if an "orbital" "contains" electron(s) it must be X-times larger than any 1 electron, right?165.212.189.187 (talk) 13:35, 10 September 2012 (UTC)
- The difference in thinking between an orbital "containing" and electron and an orbital "being" an electron are entirely moot, given that it doesn't really matter to the model what you believe about the "true" nature of the electron. The fact remains that electrons lack a definite location around the atom (see Uncertainty principle and particle in a box), that is it isn't just that we don't know where the electron is, it is that we cannot know where the electron is, so it isn't meaningful to speculate that it has a singular existance at any one point within the orbital. The Pauli exclusion principle doesn't treat electrons as objects in motion around the nucleus, because it doesn't work that way. If it did, you would have a non-zero chance of finding more than two electrons within an orbital. Actually, you have a zero chance of finding them that way. You get at most two, and only two, and only if their spins are orthogonal. That's why the whole discussion about the "volume" of the electron becomes silly when you get to the more advanced models, the notion that an electron can be treated like a little ball just stops making sense. --Jayron32 17:01, 10 September 2012 (UTC)
- Not necessarily. if an "orbital" "contains" electron(s) it must be X-times larger than any 1 electron, right?165.212.189.187 (talk) 13:35, 10 September 2012 (UTC)
- It depends, of course, on what you mean by "electron" and "space". Two electrons can occupy identical orbitals as long as they have orthogonal Spins, but you can still have exactly two electrons within the same "space" (if you take an orbital to be the space an electron occupies). --Jayron32 23:19, 9 September 2012 (UTC)
See Degenerate matter for an example of electrons packed as tightly together as possible. When you add mass to a white dwarf it gets smaller because it is able to squeeze the electrons to higher energy levels and so therefore is able to put in more electrons per unit of volume. Hcobb (talk) 14:14, 10 September 2012 (UTC)
Physical inderpredation
From a Jackson textbook. The time averaged potential of a neutral hydrogen atom is
where q is the magnitude of the electronic charge, , being the Bohr radius.
It first asks to find the distribution of charge, both continuous and discrete. By Posson's equation you get .
What does it mean by asking for discrete charge and is this even possible?
Next it asks for the physical interpretation of the result. What is it. Widener (talk) 19:22, 9 September 2012 (UTC)
Some nonsense
|
|---|
|
- discrete charge of nucleus and probability distribution of electron charge? (i'm guessing here, it's been a very long time)
- This or this may help. Ssscienccce (talk) 21:45, 9 September 2012 (UTC)
Continuation of onsense
|
|---|
|
September 10
Average age of first childbirth
In a "natural" human (as in before the Neolithic Revolution), what was the average age at which a woman had her first child? How about a man? --140.180.254.18 (talk) 05:24, 10 September 2012 (UTC)
- The Wikipedia article titled Advanced maternal age has some information on recent trends, but I don't see anything from Neolithic times. Still, its a lead and there are references. You could start your research there. --Jayron32 05:55, 10 September 2012 (UTC)
- Would that even be possible to determine? I guess women dying during pregnancy leave two skeletons, but apart from that... Ssscienccce (talk) 08:05, 10 September 2012 (UTC)
- Actually, it would not be that difficult at all, provided there were enough skeletal remains from the population in question. The pelvic inlet in the skeleton of a woman who has given birth is significantly wider than in the skeleton of a woman who hasn't. Add to that demographic data about fertility, mortality and age structure, and you could get a very good idea about the age of first birth in a given population. All the more so as childbirth itself was one of the leading causes of death among women of that time, especially in young adolescents, so there would be plenty of early adolescent female skeletons that probably died giving birth or shortly thereafter. Dominus Vobisdu (talk) 08:40, 10 September 2012 (UTC)
- Are you sure about the "leading cause"? Childbirth complications were a leading cause of death for women in early modern times, but the major risk factor there are infectious diseases like childbed fever. These would be a lot less widespread in hunter/gatherer societies than in more densely populated civilisations. Child birth would still be a significant risk, but possibly less than one would think from simple extrapolation. --Stephan Schulz (talk) 09:46, 10 September 2012 (UTC)
- If the average age of first childbirth is hard to determine, how about the average age of childbirth in general? I'd look at the molecular clock to see how many generations had passed between times X and Y, and use radiocarbon dating to determine the absolute time difference. --140.180.247.208 (talk) 17:06, 10 September 2012 (UTC)
- Couldn't one assume that the average age is not long after the earliest possible age for reproduction? What would be the factors tending to delay reproduction? The question as originally posed referred to a "natural" human and one before the Neolithic revolution. I can only imagine cultural factors as held by the group of people to which one belonged inhibiting the natural biological urge to reproduce, but I find myself doubting that such cultural constraints would exist as their efficacy in promoting the wellbeing of the group are not obvious to me. Bus stop (talk) 17:12, 10 September 2012 (UTC)
- One doesn't 'assume' an average, one measures it. AndyTheGrump (talk) 17:33, 10 September 2012 (UTC)
- AndyTheGrump—The original question wonders aloud about the "average age at which a woman had her first child".[13] Whether we "assume" or "measure", we are still addressing the question. I think in some instances assumptions can be worthwhile. The original question was not necessarily concerned with the average age of all births, but rather the average age at which a woman had her first child. Also the original question concerned the average age that a man sired his first child. Yes, measuring for this would be distinctly preferable. But how could one measure for this? Bus stop (talk) 17:49, 10 September 2012 (UTC)
- List of youngest birth mothers may be relevant. Bus stop (talk) 18:25, 10 September 2012 (UTC)
- Oh, don't be silly Bus stop. All the Grump has to do is go back in his time-machine and select a sufficiently large representative cohort and follow them for a few generations, (as our current-day archaeological evidence of the neolithic period is far to sparse top draw any firm affirmation). However, the earliest time that a female can conceive (whether they primate, meerkat or pussycat) is not a good datum (or posit) (or assumption) as females tend to mate when they are ready. I don't wont to get into the nature/nurture on this, because obviously, they can still be wearingly short-white-ankle-cotton-socks, when they meet some rich guy/pop star/etc., and the money pheromone brings them into heat. --Aspro (talk) 18:57, 10 September 2012 (UTC)
- List of youngest birth mothers may be relevant. Bus stop (talk) 18:25, 10 September 2012 (UTC)
- AndyTheGrump—The original question wonders aloud about the "average age at which a woman had her first child".[13] Whether we "assume" or "measure", we are still addressing the question. I think in some instances assumptions can be worthwhile. The original question was not necessarily concerned with the average age of all births, but rather the average age at which a woman had her first child. Also the original question concerned the average age that a man sired his first child. Yes, measuring for this would be distinctly preferable. But how could one measure for this? Bus stop (talk) 17:49, 10 September 2012 (UTC)
- One doesn't 'assume' an average, one measures it. AndyTheGrump (talk) 17:33, 10 September 2012 (UTC)
- Couldn't one assume that the average age is not long after the earliest possible age for reproduction? What would be the factors tending to delay reproduction? The question as originally posed referred to a "natural" human and one before the Neolithic revolution. I can only imagine cultural factors as held by the group of people to which one belonged inhibiting the natural biological urge to reproduce, but I find myself doubting that such cultural constraints would exist as their efficacy in promoting the wellbeing of the group are not obvious to me. Bus stop (talk) 17:12, 10 September 2012 (UTC)
- Actually, it would not be that difficult at all, provided there were enough skeletal remains from the population in question. The pelvic inlet in the skeleton of a woman who has given birth is significantly wider than in the skeleton of a woman who hasn't. Add to that demographic data about fertility, mortality and age structure, and you could get a very good idea about the age of first birth in a given population. All the more so as childbirth itself was one of the leading causes of death among women of that time, especially in young adolescents, so there would be plenty of early adolescent female skeletons that probably died giving birth or shortly thereafter. Dominus Vobisdu (talk) 08:40, 10 September 2012 (UTC)
black sunglasses or brown sunglasses for maximum UV protection
I should use black or brown sunglasses for uv protection? how sunglasses work? — Preceding unsigned comment added by 101.63.161.197 (talk) 07:12, 10 September 2012 (UTC)
- Blocking UV rays would have no connection with glasses looking brown or black in visible light. It would be an entirely separate attribute of the glasses. HiLo48 (talk) 07:46, 10 September 2012 (UTC)
- How do you know that? ←Baseball Bugs What's up, Doc? carrots→ 07:55, 10 September 2012 (UTC)
- As well as the comment below, it's worth pointing out that UV rays are invisible to humans, so the visible colours of glasses are irrelevant to whether they block UV. HiLo48 (talk) 08:37, 10 September 2012 (UTC)
- Because it's in our very article, Sunglasses. The color and protection factor can be varied independently. Now to answer the actual question, the original purpose of having a variety of colors for the lenses stems from the fact that sunglasses distort colors and contrast, and can impact your depth perception. The different colors have subtly different effects, and so you may prefer one over the other, although some colors are used purely for their cosmetic appearance. See Sunglasses#Lens for more. For the actual protection offered by the sunglasses, you'll have to look at the labeling on the sunglasses themselves. In the United States, European, and Australia, there are regulations on Sunglasses that require labeling to indicate the level of protection offered, and you can read about those at Sunglasses#Protection. I don't know if such regulations exist in India, to which your IP address belongs, but hopefully you will find some manner of label. Someguy1221 (talk) 08:23, 10 September 2012 (UTC)
- That was the right answer. :) ←Baseball Bugs What's up, Doc? carrots→ 08:45, 10 September 2012 (UTC)
- Because it's in our very article, Sunglasses. The color and protection factor can be varied independently. Now to answer the actual question, the original purpose of having a variety of colors for the lenses stems from the fact that sunglasses distort colors and contrast, and can impact your depth perception. The different colors have subtly different effects, and so you may prefer one over the other, although some colors are used purely for their cosmetic appearance. See Sunglasses#Lens for more. For the actual protection offered by the sunglasses, you'll have to look at the labeling on the sunglasses themselves. In the United States, European, and Australia, there are regulations on Sunglasses that require labeling to indicate the level of protection offered, and you can read about those at Sunglasses#Protection. I don't know if such regulations exist in India, to which your IP address belongs, but hopefully you will find some manner of label. Someguy1221 (talk) 08:23, 10 September 2012 (UTC)
- The color of the glass doesn't tell you if it blocks UV. Cheap dark plastic sunglasses may be worse than no sunglasses at all. And what Someguy says... Ssscienccce (talk) 08:30, 10 September 2012 (UTC)
Allergies
This is not a request for medical advice. I am just wondering, what can cause allergies to spontaneously develop? When I was younger, I never had any allergies at all, but whilst in Japan, after about seven years I started to develop hay fever - or, in this case, specifically an allergy to the pollen of rice plants (which was hell, because I was living in the countryside surrounded by rice fields). Then, after a while I noticed that my stomach just below my navel developed a criminally insane itchy rash, which I later found out was an allergy to nickel (my belt buckle was causing it). My friend told me that in the past five years he has developed an allergy to something or other, but has no idea what. What causes allergies to develop? KägeTorä - (影虎) (TALK) 08:52, 10 September 2012 (UTC)
- Some allergies can develop after a certain amount of exposure. For example, this paper starts out with "Beekeepers are strongly exposed to honey bee stings and therefore at an increased risk to develop IgE-mediated allergy to bee venom." [14]. Increased exposure may or may not play a role in the allergies that you describe. My understanding is that allergy development is not well understood in general. (start WP:OR): I was "immune" (not allergic) to poison ivy for years, until I had a very large exposure. Now I am normally susceptible (/end OR). This is not medical advice, but reference information, etc. etc. SemanticMantis (talk) 14:36, 10 September 2012 (UTC)
- Very true SemanticMantis. Shortly after leaving school I had a very mild exposer to 'work'. Even now, 50 years on, I'm still trying to recover. The only respite I have found, is to lay down in a darked room and stare at the back of my eyelids. Fortunately, during those episodes (which are frequent) I can often relieve the boredom by turning my monitor's screen brightness level way-down-low and edit Wikipedia. Note: My method of staring at the back of the eyelids is in no way to be taken as medical advice for this very serious condition – consult a specialist.--Aspro (talk) 18:19, 10 September 2012 (UTC)
- More on "not well understood in general". This [15] and similar studies have been in the popular science news over the past few years. The authors suggest that increased exposure during childhood may be linked to lower incidence of allergies in rural-raised children, compared to their urban counterparts. So timing and amount of exposure seems to regulate whether the exposure will increase or decrease the likelihood of (some) allergies. In short, there is no simple answer. SemanticMantis (talk) 19:23, 10 September 2012 (UTC)
- All allergies require a period of sensitization to the allergen -- sometimes it is rapid, sometimes slow. Our allergy article describes the process, in the "Pathophysiology" section, but unfortunately in terms that probably only a biologist can make sense of. Looie496 (talk) 19:24, 10 September 2012 (UTC)
- Also of note is that there is a significant genetic component to allergies, see Allergy#Genetic_basis. This is again one of those nature-nurture unseperatable things: a person may have a genetic predisposition for an allergy, but without preliminary exposure to sensitize them to the allergan, the allergy itself may never fully develop. A person without a genetic predisposition could happily bathe in the same allergan all day and never develop any problems, a person with that predisposition may be fine on a few exposures, but then get "sensitized" and go all anaphalactic without any warning. My father developed a severe bee-venom allergy in his 40s after a being stung by a half-dozen bees; he'd been stung before and never had a bad reaction. Other people can be stung regularly and hundreds of times, and never develop that allergy. It is likely that it was in his genetic makeup to be allergic, but needed a trigger to bring the allergy on. If it wasn't in his genetic make up to have the sensitivity, it may have never developed into a full allergy. --Jayron32 19:31, 10 September 2012 (UTC)
Relativity theory: a body moving while emitting an energetic radiation
According to Einstein's formula, when one calculates the current mass of a resting body that has just emitted an energetic radiation , one should subtract from the original mass the body had had before it emitted the radiation. Note that Einstein did not have to explain whether the original mass he referred to - is the original total mass - or the original rest mass, because he referred to a resting body, i.e. to a body whose rest mass is equal to its total mass. However, how about calculating the current mass of a moving body that has just emitted an energetic radiation ? Should one subtract from the original total mass - or from the original rest mass? Note that this matters very much, because if one subtracts from the original rest mass, then one subtracts from the original total mass. If you think you know the answer (which I don't), then please explain also the full considerations which could have enabled one to get the answer by oneself. 77.127.218.31 (talk) 09:28, 10 September 2012 (UTC)
- I'll give it a try. There are three quantities involved here: energy, momentum and mass. These are related through the four-momentum of a particle. This is a vector with four components (E, px c, pyc, pzc). The length of this vector is computed as , where m is the mass of the particle, this is invariant (what you call "rest mass"). For a particle at rest, the four-momentum is (Ep, 0, 0, 0) = (mc2, 0, 0, 0). Let that particle emit a photon of energy E in the positive x direction; the four-momentum of the
particlephoton is (E, pxc, 0, 0) = (E, E, 0, 0) (the momentum is equal to the energy because the photon has mass zero. Now apply conservation of energy (for the first - actually zeroth -- component) and momentum (for the other components), to determine the four-momentum of the particle after emission (just the difference between the four-momentum of the particle before emission and the four-momentum of the photon), it is (Ep − E, −E, 0, 0). With Ep = m, you find that the mass of the particle after emission is . This shows that the particle after the emission cannot be the same as the one before. Hence, if the particle is an elementary particle it decays to another particle type (examples?). If it is an atom, it changes its internal structure (an electron drops to a lower energy level). If you insist on using "total mass" (but this concept is redundant because it is nothing but the energy), then you can simply say that the photon's energy is subtracted from the total mass, although most physicists today prefer saying that the photon's energy is subtracted from the particle's energy. In terms of rest mass, it is somewhat more complicated, as I've shown. --Wrongfilter (talk) 15:03, 10 September 2012 (UTC)
.
- Thank you for your detailed response. I appreciate it. I have two comments, the second one being more important:
.
- 1. You write: "the four-momentum of the particle is (E, pxc, 0, 0)", but you probably meant: "the four-momentum of the photon is (E, pxc, 0, 0)". Later you write: "the momentum is equal to the energy", but you probably meant "the momentum multiplied by the velocity is equal to the energy". Later you write: "With Ep = m", but you probably meant: "With Ep = mc2". Later you write "it is nothing but the energy", but you probably meant "it is nothing but the energy divided by c2". Later you write: "you can simply say that the photon's energy is subtracted from the total mass", but you probably meant: "you can simply say that the photon's energy divided by c2 is subtracted from the total mass".
.
- 2. Let me take your sentence that contains (in my opinion) the most direct answer to my question: "If you insist on using 'total mass'...then you can simply say that [in order to get the mass of the particle after the emission] the photon's energy [divided by c2] is subtracted from the total mass [the particle had before the emission]". Please notice that the total mass of a particle at rest is simply its mass - i.e. its "rest mass" (as I call it), so your sentence mentioned above lets me conclude that: "[in order to get the mass of the resting particle after the emission], the photon's energy [divided by c2] is subtracted from the [rest] mass [the resting particle had before the emission]". However, this conclusion contradicts your (well-reasoned) conclusion that "the mass of the particle after emission is ", doesn't it?
.
- By the way, what did you mean by :"I'll give it a try"? Did you mean a try to give the real answer - although you're not sure whether it's the real answer, or a try to explain the answer to me - although you're not sure whether you will succeed to explain it to me?
.
- Again, I appreciate very much your detailed response. 77.127.218.31 (talk) 19:15, 10 September 2012 (UTC)
- ad 1): "four-momentum of the photon" is what I meant, I've corrected it above. I usually do these things in units where c=1, hence I usually don't write c in any of of these equations. I tried to pay attention to this, but apparently I failed repeatedly... You're right on all accounts. ad 2): I understood "total mass" as being "relativistic mass", i.e. energy divided by c2, i.e. the first component of the four-momentum. That's a frame-dependent and redundant quantity, and I very much dislike it. Before emission, the particle's "relativistic mass" is equal to its "rest mass", because we chose to work in a frame where the particle is at rest initially. Incidentally, the equation for the (rest) mass still has the frame-dependent E; in order to make it valid in any frame, E should be divided (right?) by . By "I'll give it a try", I mean "I'll try to remember/figure out how to do this" and "I'll try to explain it in a way that people can understand it". --Wrongfilter (talk) 20:44, 10 September 2012 (UTC)
- Unfortunately, I suspect I didn't understand your reply to my second comment: Please note that (in my previous response) I was talking about a particle at rest (in our reference frame), i.e. about a particle whose equals 1. How can this remove the contradiction I've pointed at? Namely: on one hand you claim that "If you insist on using 'total mass'...then you can simply say that [in order to get the mass of the particle after the emission] the photon's energy [divided by c2] is subtracted from the total mass [the particle had before the emission]". So, in our reference frame - in which the particle's equals 1, the mass of the particle after the emission must be , where is the (rest) mass the particle had before the emission; However, on the other hand you claim that "the mass of the particle after emission is ". Can't you see the contradiction (at least in our reference frame in which the particle's equals 1)? 77.127.218.31 (talk) 21:38, 10 September 2012 (UTC)
- ad 1): "four-momentum of the photon" is what I meant, I've corrected it above. I usually do these things in units where c=1, hence I usually don't write c in any of of these equations. I tried to pay attention to this, but apparently I failed repeatedly... You're right on all accounts. ad 2): I understood "total mass" as being "relativistic mass", i.e. energy divided by c2, i.e. the first component of the four-momentum. That's a frame-dependent and redundant quantity, and I very much dislike it. Before emission, the particle's "relativistic mass" is equal to its "rest mass", because we chose to work in a frame where the particle is at rest initially. Incidentally, the equation for the (rest) mass still has the frame-dependent E; in order to make it valid in any frame, E should be divided (right?) by . By "I'll give it a try", I mean "I'll try to remember/figure out how to do this" and "I'll try to explain it in a way that people can understand it". --Wrongfilter (talk) 20:44, 10 September 2012 (UTC)
- Again, I appreciate very much your detailed response. 77.127.218.31 (talk) 19:15, 10 September 2012 (UTC)
- The emitting body isn't at rest after the emission—there's a recoil. So is not its final rest mass, though it is its final relativistic mass in the frame where it's initially at rest. -- BenRG (talk) 22:58, 10 September 2012 (UTC)
Does anyone know what this plant is?
Hi all,
I have a small bush growing in my yard in Cambridge, MA. Its leaves are smooth and almond-shaped, and they have a marking on the top like someone covered them with a smaller spikier leaf and spray painted over them. The branches end in long tendrils with dozens of tiny tiny red buds on them. It grows out of multiple thin stems, and the whole thing is about two feet tall.
http://img.skitch.com/20120910-jk4qjt9rt6sqkg54casg23mh8a.jpg
(Is there a way to embed non-Wikipedia images?)
Thanks! — Sam 24.128.48.26 (talk) 11:32, 10 September 2012 (UTC)
- Looks like this Persicaria. --TammyMoet (talk) 11:47, 10 September 2012 (UTC)

- Thanks! The picture linked looked quite different, but looking it up online finds that you're completely right. Persicaria virginiana — Lance Corporal Knotweed. Thank you so much! — Sam 24.128.48.26 (talk) 12:30, 10 September 2012 (UTC)
- It's often easier to identify plants once they are in flower. Yours appears to have finished flowering so we can't go much on the stalk that's left. But I've seen that plant quite a bit over the years. Some varieties of Persicaria are edible. --TammyMoet (talk) 18:16, 10 September 2012 (UTC)
- Thanks! The picture linked looked quite different, but looking it up online finds that you're completely right. Persicaria virginiana — Lance Corporal Knotweed. Thank you so much! — Sam 24.128.48.26 (talk) 12:30, 10 September 2012 (UTC)
Maldives in the Ice Age
Hi. I'm not sure how coral islands work. But I've read in the last ice age, sea levels were about 120 metres lower than now. So were the Maldives then bigger islands? Would they have had land as much as 100 metres above sea level? How big would they have been and where could I find a map. Thank you. — Preceding unsigned comment added by 142.150.38.84 (talk) 13:30, 10 September 2012 (UTC)
- Probably not. The way that coral reefs form (see Coral_reef#Formation), they generally stay just ahead of sea-level rise. Coral atolls like the Maldives aren't that old, geologically. Most are likely less than 10,000 years old, according to our article, and thus if the Maldives existed during the lower sea levels of the last ice age, they would have also been comparitively lower in elevation, so they may not have been considerably larger. Atoll#Formation also has some information, but also notes the dynamic nature of atolls. --Jayron32 16:55, 10 September 2012 (UTC)
- That makes sense logically, but this study reports finding the remains of a 120-thousand-year old reef only 14 meters below current sea level -- so it seems likely that there would have been about 100 meters of exposure at the LGM. Looie496 (talk) 19:12, 10 September 2012 (UTC)
- When another ice age rolls around and the sea level drops again, the reefs will die, but they won't disintegrate, and the Maldives will grow until the sea level starts rising again. Whoop whoop pull up Bitching Betty | Averted crashes 23:30, 10 September 2012 (UTC)
Cat
Can someone identify the brownish cat in the 21st (or third from bottom) picture in this article for me - [16]? - JuneGloom Talk 19:44, 10 September 2012 (UTC)
- It's a caracal. μηδείς (talk) 20:01, 10 September 2012 (UTC)
- I just ECed with Medeis, but was about to give the same answer. It looks like a caracal, or perhaps a cross including the caracal, such as the Caraval. --Jayron32 20:06, 10 September 2012 (UTC)
- Our article suggests the caraval is spotted. Not to be confused with the also quite handsome Caracalla. μηδείς (talk) 20:43, 10 September 2012 (UTC)
- Thank you guys. I couldn't remember what it was, but knew I'd seen one before. I assume the one in the article is domesticated and lives alongside the Cheetah. - JuneGloom Talk 23:07, 10 September 2012 (UTC)
- Our article suggests the caraval is spotted. Not to be confused with the also quite handsome Caracalla. μηδείς (talk) 20:43, 10 September 2012 (UTC)
- I just ECed with Medeis, but was about to give the same answer. It looks like a caracal, or perhaps a cross including the caracal, such as the Caraval. --Jayron32 20:06, 10 September 2012 (UTC)
Plate tectonics censored?
(Not sure if this is the right section.) From the agnatology article: "For example, knowledge about plate tectonics was censored and delayed for at least a decade because key evidence was classified military information related to underseas warfare." I can't find any reference to this online, nor am I sure what is meant by 'classified military information related to underseas warfare' in this case. Ratzd'mishukribo (talk) 21:08, 10 September 2012 (UTC)
- Well, knowledge of the location of undersea trenches and ridges is important if you want to hide a sub from enemy detection, and those are caused by plate movements. And, when you plot all of those trenches and ridges, it's clear that they are like the seams on a baseball, not just the result of isolated events. StuRat (talk) 21:17, 10 September 2012 (UTC)
- Much of the knowledge of the sea floor was gathered through military surveys, and the military's default state is "classify everything we know". It wasn't until the 1950s that a significant civilian effort to map the sea floor was undertaken. However, I don't know that the suppression was all that significant or that it was as widespread and obfuscatory as that statement makes it out to be. Harry Hammond Hess, a U.S. naval sonar operator and later geologist, was key in developing the modern theory of Plate Tectonics. I don't know that he was deliberately censored. --Jayron32 21:35, 10 September 2012 (UTC)
- So if that is what was meant, is there indeed any source to the allegation that it was ever censored, or perhaps someone needed an example of subject matter for the the topic "agnatology"? Ratzd'mishukribo (talk) 02:25, 11 September 2012 (UTC)
- 'Censored' is the wrong word, to quote from a book by Naomi Oreskes "Another group at Lamont had focused on bathymetric data - measurements of the depth of the sea floor - primarily in the Atlantic. These data were highly classified, but Bruce Heezen (1924-1977) and Marie Tharp had found a creative means around security restrictions: a physiographic map, essentially an artist's rendition of the what the sea floor would look like drained of water, based on quantitative measurements, but without actually revealing them."[17]. So even if the data were classified, researchers still had access to them and found ways around the restrictions. Mikenorton (talk) 04:24, 11 September 2012 (UTC)
- Is the concept of "hiding submarines among ridges" real or happens only in The Hunt for Red October (film)? I would have thought that mid-ocean ridges are way deeper than where submarines can go (a couple of deep sea research vessels excepted, and assuming they want to come back up.) 88.112.47.131 (talk) 05:50, 11 September 2012 (UTC)