Chloromethyl chloroformate
Appearance
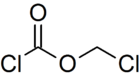
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Chloromethyl carbonochloridate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.040.707 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H2Cl2O2 | |
| Molar mass | 128.94 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.45 g/ml[1] |
| Boiling point | 107–108 °C (225–226 °F; 380–381 K)[1] |
| Related compounds | |
Related chloroformates
|
Chloroethyl chloroformate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chloromethyl chloroformate (CClO2CH2Cl), also known as palite gas,[2] is a chemical compound developed into gas form and used for chemical warfare during World War I. It is a tearing agent designed to cause temporary blindness. It is a colorless liquid with a penetrating, irritating odor.
Industrially, chloromethyl chloroformate is used to manufacture other chemicals.
References
- ^ a b "Chloromethyl chloroformate". Sigma-Aldrich.
- ^ Auld, S.J.M. (February 4, 1918). "Methods of gas warfare". Journal of the Washington Academy of Sciences. 8 (3): 58. Bibcode:1918Natur.101R.215.. doi:10.1038/101215b0. JSTOR 24521564. S2CID 32522395.
