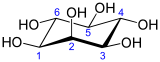
Inositol

| |
| |
| Names | |
|---|---|
| IUPAC name
(1R,2S,3r,4R,5S,6s)-cyclohexane-1,2,3,4,5,6-hexol
| |
| Other names
cis-1,2,3,5-trans-4,6-cyclohexanehexol
cyclohexanehexol mouse antialopecia factor nucite phaseomannite phaseomannitol rat antispectacled eye factor scyllite (for the structural isomer scyllo-inositol) vitamin B8 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.295 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.16 g/mol |
| Density | 1.752 g/cm3 |
| Melting point | 225 to 227 °C (437 to 441 °F; 498 to 500 K) |
| Pharmacology | |
| A11HA07 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 143 °C (289 °F; 416 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
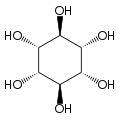
Inositol, or more precisely myo-inositol, is a carbocyclic sugar that is abundant in brain and other mammalian tissues, mediates cell signal transduction in response to a variety of hormones, neurotransmitters and growth factors and participates in osmoregulation.[2] It is a sugar alcohol with half the sweetness of sucrose (table sugar). It is made naturally in humans from glucose. A human kidney makes about two grams per day. Other tissues synthesize it too, and the highest concentration is in the brain where it plays an important role making other neurotransmitters and some steroid hormones bind to their receptors.[3] Since about 2008 myo-inositol gained importance in the management of polycystic ovary syndrome (PCOS) due to its efficacy, safety profile and worldwide availability.[4]
Overview
myo-Inositol plays an important role as the structural basis for a number of secondary messengers in eukaryotic cells, the various inositol phosphates. In addition, inositol serves as an important component of the structural lipids phosphatidylinositol (PI) and its various phosphates, the phosphatidylinositol phosphate (PIP) lipids.
Inositol or its phosphates and associated lipids are found in many foods, in particular fruit, especially cantaloupe and oranges.[5] In plants, the hexaphosphate of inositol, phytic acid or its salts, the phytates, serve as phosphate stores in seed, for example in nuts and beans.[6] Phytic acid also occurs in cereals with high bran content. Phytate is, however, not directly bioavailable to humans in the diet, since it is not digestible. Some food preparation techniques partly break down phytates to change this. However, inositol in the form of glycerophospholipids, as found in certain plant-derived substances such as lecithins is well-absorbed and relatively bioavailable.
myo-Inositol (free of phosphate) was once considered a member of the vitamin B complex, called Vitamin B8 in this context. However, because it is produced by the human body from glucose, it is not an essential nutrient.[7]
Isomers and structure
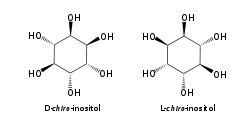
The isomer myo-inositol is a meso compound, and hence optically inactive, because it has a plane of symmetry. For this reason, meso-inositol is an obsolete name for this compound. Besides myo-inositol, the other naturally occurring stereoisomers are scyllo-, muco-, D-chiro-, and neo-inositol, although they occur in minimal quantities in nature. The other possible isomers are L-chiro-, allo-, epi-, and cis-inositol. As their names denote, L- and D-chiro inositol are the only pair of inositol enantiomers, but they are enantiomers of each other, not of myo-inositol.[8]
In its most stable conformation, the myo-inositol isomer assumes the chair conformation, which moves the maximum number of hydroxyls to the equatorial position, where they are farthest apart from each other. In this conformation, the natural myo isomer has a structure in which five of the six hydroxyls (the first, third, fourth, fifth, and sixth) are equatorial, whereas the second hydroxyl group is axial.[9]
Biosynthesis
myo-Inositol is synthesized from glucose 6-phosphate (G6P) in two steps. First, G6P is isomerised by an inositol-3-phosphate synthase enzyme (for example, ISYNA1) to myo-inositol 1-phosphate, which is then dephosphorylated by an inositol monophosphatase enzyme (for example, IMPA1) to give free myo-inositol. In humans, most inositol is synthesized in the kidneys, followed by testicles, typically in amounts of a few grams per day.[2] at the peripheral level, myo-inositol is converted to D-chiro-inositol by a specific epimerase. The activity of this epimerase is insulin dependent. Worthy of note, only a small quantity of myo-inositol is converted into D-chiro-inositol and the conversion is irreversible.[10]
Inositol, phosphatidylinositol and some of their mono- and polyphosphates function as secondary messengers in a number of intracellular signal transduction pathways. They are involved in a number of biological processes, including:
- Insulin signal transduction[11]
- Cytoskeleton assembly
- Nerve guidance (epsin)
- Intracellular calcium (Ca2+) concentration control[12]
- Cell membrane potential maintenance[13]
- Breakdown of fats[14]
- Gene expression[15][16]
In one important family of pathways, phosphatidylinositol 4,5-bisphosphate (PIP2) is stored in cellular membranes until it is released by any of a number of signalling proteins and transformed into various secondary messengers, for example diacylglycerol and inositol triphosphate.[17]
Phytic acid in plants
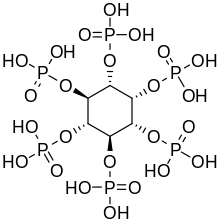
Inositol hexaphosphate, also called phytic acid or IP6, is the principal storage form of phosphorus in many plant tissues, especially bran and seed.[18] Phosphorus and inositol in phytate form are not generally bioavailable to non-ruminant animals because these animals lack the digestive enzyme phytase required to remove the phosphate groups. Ruminants are readily able to digest phytate because of the phytase produced by rumen microorganisms.[19] Moreover, phytic acid also chelates important minerals such as calcium, magnesium, iron, and zinc, making them unabsorbable, and contributing to mineral deficiencies in people whose diets rely highly on bran and seeds for their mineral intake, such as occurs in developing countries.[20][21]
Inositol penta- (IP5), tetra- (IP4), and triphosphate (IP3) are also called "phytates".
Use in explosives manufacture
At the 1936 meeting of the American Chemical Society, professor Edward Bartow of the University of Iowa presented a commercially viable means of extracting large amounts of inositol from the phytic acid naturally present in waste corn. As a possible use for the chemical, he suggested 'inositol nitrate' as a more stable alternative to nitroglycerin.[22] Today, inositol nitrate is used to gelatinize nitrocellulose, thus can be found in many modern explosives and solid rocket propellants.[23]
Counter to road salt
When plants are exposed to increasing concentrations of road salt, the plant cells become dysfunctional and undergo apoptosis, leading to inhibited growth. Inositol pretreatment could reverse these effects.[24]
Research and clinical applications
Large doses of inositol have been studied for treatment of depression, but further study is needed to determine whether this is an effective treatment.[25]
Inositol has been found to have modest effects in patients with panic disorder or obsessive-compulsive disorder.[26][27]
Inositol should not be routinely implemented for the management of preterm babies who have or are at a risk of infant respiratory distress syndrome (RDS).[28] Noteworthily, myo-inositol helps prevent neural tube defects with particular efficacy in combination with folic acid.[29]
Inositol is considered a safe and effective treatment for polycystic ovary syndrome (PCOS). It works by increasing insulin sensitivity, which helps to improve ovarian function and reduce hyperandrogenism.[30] It is also shown to reduce the risk of metabolic disease in people with PCOS.[31] In addition, thanks to its role as FSH second messenger, myo-inositol is effective in restoring FSH/LH ratio and menstrual cycle regularization.[32] myo-Inositol's role as FSH second messenger leads to a correct ovarian follicle maturation and consequently to a higher oocyte quality. Improving the oocyte quality in both women with or without PCOS, myo-inositol can be considered as a possible approach for increasing the chance of success in assisted reproductive technologies.[33][34] In contrast, D-chiro-inositol can impair oocyte quality in a dose-dependent manner.[35] The high level of DCI seems to be related to elevated insulin levels retrieved in about 70% of PCOS women.[36] In this regard, insulin stimulates the irreversible conversion of myo-inositol to D-chiro-inositol causing a drastic reduction of myo-inositol. myo-Inositol depletion is particularly damaging to ovarian follicles because it is involved in FSH signaling, which is impaired due to myo-inositol depletion.[10] Recent evidence reports a faster improvement of the metabolic and hormonal parameters when these two isomers are administered in their physiological ratio. The plasmatic ratio of myo-inositol and D-chiro-inositol in healthy subjects is 40:1 of myo- and D-chiro-inositol respectively.[37] The use of the 40:1 ratio shows the same efficacy of myo-inositol alone but in a shorter time. In addition, the physiological ratio does not impair oocyte quality.[38]
The use of inositols in PCOS is gaining more importance, and an efficacy higher than 70% with a strong safety profile is reported. On the other hand, about 30% of patients could show as inositol-resistant.[39] New evidence regarding PCOS aetiopathogenesis describes an alteration in the species and the quantity of each strain characterizing the normal gastrointestinal flora. This alteration could lead to a chronic low grade of inflammation and malabsorption.[40] A possible solution could be represented by the combination of myo-inositol and α-lactalbumin. This combination shows a synergic effect in increasing myo-inositol absorption.[41] A recent study reported that the myo-inositol and α-lactalbumin combination is able to increase myo-inositol plasmatic content in inositol-resistant patients with a relative improvement of hormonal and metabolic parameters.[42]
Use as a cutting agent
Inositol has been used as an adulterant or cutting agent for many illegal drugs, such as cocaine, methamphetamine, and sometimes heroin,[43] probably because of its solubility, powdery texture, or reduced sweetness (50%) compared to more common sugars.
Inositol is also used as a stand-in film prop for cocaine in filmmaking.[44]
Nutritional sources
myo-Inositol is naturally present in a variety of foods, although tables of food composition do not always distinguish between lecithin, the relatively bioavailable lipid form and the biounavailable phytate/phosphate form.[5] Foods containing the highest concentrations of myo-inositol and its compounds include fruits, beans, grains, and nuts.[5] Fruits in particular, especially oranges and cantaloupe, contain the highest amounts of myo-inositol.[45] It is also present in beans, nuts, and grains, however, these contain large amounts of myo-inositol in the phytate form, which is not bioavailable.
In humans, myo-inositol is naturally made from glucose through enzymatic dephosphorylation.[45]
References
- ^ Merck Index (11th ed.). p. 4883.
- ^ a b Parthasarathy, L. K.; Seelan, R. S.; Tobias, C.; Casanova, M. F.; Parthasarathy, R. N. (2006). "Mammalian inositol 3-phosphate synthase: its role in the biosynthesis of brain inositol and its clinical use as a psychoactive agent". Sub-Cellular Biochemistry. Subcellular Biochemistry. 39: 293–314. doi:10.1007/0-387-27600-9_12. ISBN 978-0-387-27599-4. PMID 17121280.
- ^ Croze, M. L.; Soulage, C. O. (October 2013). "Potential role and therapeutic interests of myo-inositol in metabolic diseases". Biochimie. 95 (10): 1811–1827. doi:10.1016/j.biochi.2013.05.011. PMID 23764390.
- ^ Gateva, A.; Unfer, V.; Kamenov, Z. (2018). "The use of inositol (S) isomers in the management of polycystic ovary syndrome: a comprehensive review". Gynecological Endocrinology. 34 (7): 545–550. doi:10.1080/09513590.2017.1421632. PMID 29309199.
- ^ a b c Clements, R. S.; Darnell, B. (September 1980). "myo-Inositol content of common foods: development of a high-myo-inositol diet". The American Journal of Clinical Nutrition. 33 (9): 1954–1967. doi:10.1093/ajcn/33.9.1954. PMID 7416064.
- ^ "Phytic acid". www.phytochemicals.info. Archived from the original on 2017-08-06. Retrieved 2017-10-02.
- ^ Reynolds, J. E. F. (1993). Martindale: The Extra Pharmacopoeia. Vol. 30. Pennsylvania: Rittenhouse Book Distributors. p. 1379. ISBN 978-0-85369-300-0.
An isomer of glucose that has traditionally been considered to be a B vitamin although it has an uncertain status as a vitamin and a deficiency syndrome has not been identified in man
- ^ Majumder, A. L.; Biswas, B. B. (2006-10-03). Biology of Inositols and Phosphoinositides. Springer Science & Business Media. ISBN 9780387276007.
- ^ Brady, S.; Siegel, G.; Albers, R. W.; Price, D. (2005). Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Academic Press. p. 348. ISBN 9780080472072.
- ^ a b Carlomagno, G.; Unfer, V.; Roseff, S. (2011). "The D-chiro-inositol paradox in the ovary". Fertility and Sterility. 95 (8): 2515–6. doi:10.1016/j.fertnstert.2011.05.027. PMID 21641593.
- ^ Larner, J. (2002). "D-chiro-Inositol—its functional role in insulin action and its deficit in insulin resistance". International Journal of Experimental Diabetes Research. 3 (1): 47–60. doi:10.1080/15604280212528. PMC 2478565. PMID 11900279.
- ^ Gerasimenko, J. V.; Flowerdew, S. E.; Voronina, S. G.; Sukhomlin, T. K.; Tepikin, A. V.; Petersen, O. H.; Gerasimenko, O. V. (December 2006). "Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors". The Journal of Biological Chemistry. 281 (52): 40154–40163. doi:10.1074/jbc.M606402200. PMID 17074764.
- ^ Kukuljan, M.; Vergara, L.; Stojilković, S. S. (February 1997). "Modulation of the kinetics of inositol 1,4,5-trisphosphate-induced [Ca2+]i oscillations by calcium entry in pituitary gonadotrophs". Biophysical Journal. 72 (2 Pt 1): 698–707. Bibcode:1997BpJ....72..698K. doi:10.1016/S0006-3495(97)78706-X. PMC 1185595. PMID 9017197.
- ^ Rapiejko, P. J.; Northup, J. K.; Evans, T.; Brown, J. E.; Malbon, C. C. (November 1986). "G-proteins of fat-cells. Role in hormonal regulation of intracellular inositol 1,4,5-trisphosphate". The Biochemical Journal. 240 (1): 35–40. doi:10.1042/bj2400035. PMC 1147372. PMID 3103610.
- ^ Shen, X.; Xiao, H.; Ranallo, R.; Wu, W.-H.; Wu, C. (January 2003). "Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates". Science. 299 (5603): 112–114. Bibcode:2003Sci...299..112S. doi:10.1126/science.1078068. PMID 12434013.
- ^ Steger, D. J.; Haswell, E. S.; Miller, A. L.; Wente, S. R.; O'Shea, E. K. (January 2003). "Regulation of chromatin remodeling by inositol polyphosphates". Science. 299 (5603): 114–116. Bibcode:2003Sci...299..114S. doi:10.1126/science.1078062. PMC 1458531. PMID 12434012.
- ^ Mathews, C. K.; Van Holde, K. E.; Ahern, K. G. (2000). Biochemistry (3rd ed.). San Francisco, CA: Benjamin Cummings. p. 855. ISBN 978-0805330663. OCLC 42290721.
- ^ "Phytic acid". www.phytochemicals.info. Archived from the original on 7 March 2018. Retrieved 2018-05-02.
- ^ Klopfenstein, T. J.; Angel, R.; Cromwell, G.; Erickson, G. E.; Fox, D. G.; Parsons, C.; Satter, L. D.; Sutton, A. L.; Baker, D. H. (July 2002). "Animal diet modification to decrease the potential for nitrogen and phosphorus pollution". Council for Agricultural Science and Technology. 21. Archived from the original on 2011-06-11.
- ^ Hurrell, R. F. (September 2003). "Influence of vegetable protein sources on trace element and mineral bioavailability". The Journal of Nutrition. 133 (9): 2973S–2977S. doi:10.1093/jn/133.9.2973S. PMID 12949395.
- ^ Committee on Food Protection; Food and Nutrition Board; National Research Council (1973). "Phytates". Toxicants Occurring Naturally in Foods. National Academy of Sciences. pp. 363–371. ISBN 978-0-309-02117-3.
- ^ Laurence, W. L. (April 17, 1936). "Corn by-product yields explosive". The New York Times. p. 7. Archived from the original on 2013-05-12.
- ^ Ledgard, J. (2007). The Preparatory Manual of Explosives. p. 366. ISBN 9780615142906.
- ^ Chatterjee, J.; Majumder, A. L. (September 2010). "Salt-induced abnormalities on root tip mitotic cells of Allium cepa: prevention by inositol pretreatment". Protoplasma. 245 (1–4): 165–172. doi:10.1007/s00709-010-0170-4. PMID 20559853.
- ^ Taylor, M. J.; Wilder, H.; Bhagwagar, Z.; Geddes, J. (2004). "Inositol for depressive disorders". The Cochrane Database of Systematic Reviews (2): CD004049. doi:10.1002/14651858.CD004049.pub2. PMC 6984679. PMID 15106232.
- ^ Benjamin, J. (July 1995). "Double-blind, placebo-controlled, crossover trial of inositol treatment for panic disorder". Am J Psychiatry. 152 (7): 1084–6. doi:10.1176/ajp.152.7.1084. PMID 7793450.
- ^ Fux, J. (1996). "Inositol treatment of obsessive-compulsive disorder". Am J Psychiatry. 153 (9): 1219–21. doi:10.1176/ajp.153.9.1219. PMID 8780431.
- ^ Howlett, Alexandra; Ohlsson, Arne; Plakkal, Nishad (8 July 2019). "Inositol in preterm infants at risk for or having respiratory distress syndrome". The Cochrane Database of Systematic Reviews. 7: CD000366. doi:10.1002/14651858.CD000366.pub4. ISSN 1469-493X. PMC 6613728. PMID 31283839.
- ^ Cavalli, P.; Ronda, E. (2017). "Myoinositol: the bridge (PONTI) to reach a healthy pregnancy". International Journal of Endocrinology. 2017: 5846286. doi:10.1155/2017/5846286. PMC 5274721. PMID 28243254.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Monastra, G.; Unfer, V.; Harrath, A. H.; Bizzarri, M. (January 2017). "Combining treatment with myo-inositol and D-chiro-inositol (40:1) is effective in restoring ovary function and metabolic balance in PCOS patients". Gynecological Endocrinology. 33 (1): 1–9. doi:10.1080/09513590.2016.1247797. PMID 27898267.
- ^ Nordio, M.; Proietti, E. (May 2012). "The combined therapy with myo-inositol and D-chiro-inositol reduces the risk of metabolic disease in PCOS overweight patients compared to myo-inositol supplementation alone". European Review for Medical and Pharmacological Sciences. 16 (5): 575–581. PMID 22774396.
- ^ Unfer, V. (2012). "Effects of myo-inositol in women with PCOS: a systematic review of randomized controlled trials". Gynecological Endocrinology. 28 (7): 509–15. doi:10.3109/09513590.2011.650660. PMID 22296306.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Ciotta, L. (2011). "Effects of myo-inositol supplementation on oocyte's quality in PCOS patients: a double blind trial". European Review for Medical and Pharmacological Sciences. 15 (5): 509–14. PMID 21744744.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Papaleo, E. (2009). "Contribution of myo-inositol to reproduction". European Journal of Obstetrics & Gynecology and Reproductive Biology. 147 (2): 120–3. doi:10.1016/j.ejogrb.2009.09.008. PMID 19800728.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Isabella, R.; Raffone, E. (2012). "Does ovary need D-chiro-inositol?". Journal of Ovarian Research. 5 (1): 14. doi:10.1186/1757-2215-5-14. PMC 3447676. PMID 22587479.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Moghetti, P. (2016). "Insulin resistance and polycystic ovary syndrome". Current Pharmaceutical Design. 22 (36): 5526–5534. doi:10.2174/1381612822666160720155855. PMID 27510482.
- ^ Facchinetti, F. (2015). "Results from the International Consensus Conference on myo-Inositol and D-chiro-Inositol in Obstetrics and Gynecology: the link between metabolic syndrome and PCOS". European Journal of Obstetrics & Gynecology and Reproductive Biology. 195: 72–6. doi:10.1016/j.ejogrb.2015.09.024. PMID 26479434.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Colazingari, S. (2013). "The combined therapy myo-inositol plus D-chiro-inositol, rather than D-chiro-inositol, is able to improve IVF outcomes: results from a randomized controlled trial". Archives of Gynecology and Obstetrics. 288 (6): 1405–11. doi:10.1007/s00404-013-2855-3. PMID 23708322.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Kamenov, Z.; et al. (2015). "Ovulation induction with myo-inositol alone and in combination with clomiphene citrate in polycystic ovarian syndrome patients with insulin resistance". Gynecological Endocrinology. 31 (2): 131–5. doi:10.3109/09513590.2014.964640. PMID 25259724.
- ^ González, F. (2012). "Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction". Steroids. 77 (4): 300–5. doi:10.1016/j.steroids.2011.12.003. PMC 3309040. PMID 22178787.
- ^ Monastra, G. (2018). "alpha-Lactalbumin effect on myo-inositol intestinal absorption: in vivo and in vitro". Current Drug Delivery. 15 (9): 1305–1311. doi:10.2174/1567201815666180509102641. PMID 29745333.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Oliva, M. M. (2018). "Effects of myo-inositol plus alpha-lactalbumin in myo-inositol-resistant PCOS women". Journal of Ovarian Research. 11 (1): 38. doi:10.1186/s13048-018-0411-2. PMC 5944130. PMID 29747700.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ "Inositol, Nerve guidance, Cutting agent manufacturer". Tianyu Feed Additive. Archived from the original on 2014-09-08. Retrieved 2013-07-21.
- ^ Golianopoulos, T. (2012-05-12). "Drug doubles: What actors actually toke, smoke and snort on camera". Wired. Wired Magazine. Archived from the original on 2012-05-14. Retrieved 2012-05-14.
- ^ a b Awuchi, Chinaza (2017). "Sugar Alcohols: Chemistry, Production, Health Concerns and Nutritional Importance of Mannitol, Sorbitol, Xylitol, and Erythritol". International Journal of Advanced Academic Research. 5 (11): 1954–1967.