Glycerophosphorylcholine
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.044.496 |
| Chemical and physical data | |
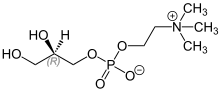
| Formula | C8H20NO6P |
| Molar mass | 257.223 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
L-Alpha glycerylphosphorylcholine (alpha-GPC, choline alfoscerate) is a natural choline compound found in the brain. It is also a parasympathomimetic acetylcholine precursor[1] which has been investigated for its potential for the treatment of Alzheimer's disease[2] and other dementias.[3]
Alpha-GPC rapidly delivers choline to the brain across the blood–brain barrier and is a biosynthetic precursor of acetylcholine.[2] It is a non-prescription drug in most countries. The FDA determined that intake of no more than 196.2 mg/person/day is considered generally recognized as safe (GRAS).[4]
Research
An Italian multicentre clinical trial on 2,044 patients suffering from recent stroke were supplied alpha-GPC in doses of 1,000 mg/day for 28 days and 400 mg three times per day for the five ensuing months. The trial confirmed the therapeutic role of alpha-GPC on the cognitive recovery of patients based on four measurement scales (Mathew Scale (MS), Mini Mental State Test (MMST), Crichton Rating Scale (CRS) and the Global Deterioration Scale (GDS)) three of which reached statistical significance.[5][6][non-primary source needed] In trials utilizing alpha-GPC in vascular dementia, alpha-GPC administration was reported to improve performance on psychometric tests and to be well tolerated.[7]
Small scale studies focusing on the effects of alpha-GPC on physical performance have also reported that alpha-GPC supplementation can increase maximum power and velocity in specified tests (counter-movement jump test) and increase lower body force (isometric mid-thigh pull test).[8][9] In iron deficient women, alpha-GPC supplementation has also been reported to enhance non-heme iron uptake from dietary sources.[10]
Production
Industrially, alpha-GPC is produced by the chemical or enzymatic deacylation of phosphatidylcholine enriched soya phospholipids followed by chromatographic purification. Alpha-GPC may also be derived in small amounts from highly purified soy lecithin as well from purified sunflower lecithin.
External links
References
- ^ De Jesus Moreno Moreno M (January 2003). "Cognitive improvement in mild to moderate Alzheimer's dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomized, placebo-controlled trial". Clinical Therapeutics. 25 (1): 178–93. doi:10.1016/S0149-2918(03)90023-3. PMID 12637119.
- ^ a b Parnetti L, Mignini F, Tomassoni D, Traini E, Amenta F (June 2007). "Cholinergic precursors in the treatment of cognitive impairment of vascular origin: ineffective approaches or need for re-evaluation?". Journal of the Neurological Sciences. 257 (1–2): 264–9. doi:10.1016/j.jns.2007.01.043. PMID 17331541. S2CID 34661218.
- ^ Doggrell SA, Evans S (October 2003). "Treatment of dementia with neurotransmission modulation". Expert Opinion on Investigational Drugs. 12 (10): 1633–54. doi:10.1517/13543784.12.10.1633. PMID 14519085. S2CID 46175609.
- ^ "Generally Recognized as Safe (GRAS) Determination for the Use of AlphaSize® Alpha-Glycerylphosphoryl Choline" (PDF). United States Food and Drug Administration. 25 January 2012. Archived from the original (PDF) on 24 December 2013.
- ^ Barbagallo Sangiorgi G, Barbagallo M, Giordano M, Meli M, Panzarasa R (June 1994). "alpha-Glycerophosphocholine in the mental recovery of cerebral ischemic attacks. An Italian multicenter clinical trial". Annals of the New York Academy of Sciences. 717: 253–69. doi:10.1111/j.1749-6632.1994.tb12095.x. PMID 8030842. S2CID 86029937.
- ^ Traini, Enea; Bramanti, Vincenzo; Amenta, Francesco (December 2013). "Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline- containing phospholipid with a still interesting profile as cognition enhancing agent". Current Alzheimer Research. 10 (10): 1070–1079. doi:10.2174/15672050113106660173. ISSN 1875-5828. PMID 24156263.
- ^ Di Perri, R.; Coppola, G.; Ambrosio, L. A.; Grasso, A.; Puca, F. M.; Rizzo, M. (July 1991). "A multicentre trial to evaluate the efficacy and tolerability of alpha-glycerylphosphorylcholine versus cytosine diphosphocholine in patients with vascular dementia". The Journal of International Medical Research. 19 (4): 330–341. doi:10.1177/030006059101900406. ISSN 0300-0605. PMID 1916007. S2CID 33715399.
- ^ Bellar, David; LeBlanc, Nina R.; Campbell, Brian (2015). "The effect of 6 days of alpha glycerylphosphorylcholine on isometric strength". Journal of the International Society of Sports Nutrition. 12: 42. doi:10.1186/s12970-015-0103-x. ISSN 1550-2783. PMC 4650143. PMID 26582972.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Marcus, Lena; Soileau, Jason; Judge, Lawrence W.; Bellar, David (2017). "Evaluation of the effects of two doses of alpha glycerylphosphorylcholine on physical and psychomotor performance". Journal of the International Society of Sports Nutrition. 14: 39. doi:10.1186/s12970-017-0196-5. ISSN 1550-2783. PMC 5629791. PMID 29042830.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Armah, Charlotte N.; Sharp, Paul; Mellon, Fred A.; Pariagh, Sandra; Lund, Elizabeth K.; Dainty, Jack R.; Teucher, Birgit; Fairweather-Tait, Susan J. (May 2008). "L-alpha-glycerophosphocholine contributes to meat's enhancement of nonheme iron absorption". The Journal of Nutrition. 138 (5): 873–877. doi:10.1093/jn/138.5.873. ISSN 1541-6100. PMID 18424594.
