List of miscellaneous 5HT2A agonists: Difference between revisions
Content deleted Content added
←Created page with 'This is a list of agonists of the serotonin receptor subtype 5-HT<sub>2A</sub> which fall outside the common structural classes. Most agonists at this receptor are either substituted phenethylamine derivatives from the 2C, DOx and 25-NB groups, or substituted tryptamines along with more complex derivatives such as lysergamides and iboga-type alkaloids. Ther...' |
No edit summary |
||
| Line 1: | Line 1: | ||
This is a list of [[agonist]]s of the [[serotonin]] [[Receptor (biochemistry)|receptor]] subtype [[5-HT2A receptor|5-HT<sub>2A</sub>]] which fall outside the common structural classes. Most agonists at this receptor are either [[substituted phenethylamine]] derivatives from the [[2C (psychedelics)|2C]], [[DOx]] and [[25-NB]] groups, or [[substituted tryptamine]]s along with more complex derivatives such as [[lysergamides]] and [[iboga-type alkaloid]]s. There are however numerous examples of 5-HT<sub>2A</sub> receptor agonists which do not fall within any of these groups, some representative examples of which are listed below. |
This is a list of [[agonist]]s of the [[serotonin]] [[Receptor (biochemistry)|receptor]] subtype [[5-HT2A receptor|5-HT<sub>2A</sub>]] which fall outside the common structural classes. Most agonists at this receptor are either [[substituted phenethylamine]] derivatives from the [[2C (psychedelics)|2C]], [[DOx]] and [[25-NB]] groups, or [[substituted tryptamine]]s along with more complex derivatives such as [[lysergamides]] and [[iboga-type alkaloid]]s.<ref>Nichols DE. Chemistry and Structure-Activity Relationships of Psychedelics. ''Curr Top Behav Neurosci''. 2018;36:1-43. {{doi|10.1007/7854_2017_475}} {{pmid|28401524}}</ref> There are however numerous examples of 5-HT<sub>2A</sub> receptor agonists which do not fall within any of these groups, some representative examples of which are listed below. |
||
{| class="wikitable sortable" |
{| class="wikitable sortable" |
||
Revision as of 09:38, 20 January 2024
This is a list of agonists of the serotonin receptor subtype 5-HT2A which fall outside the common structural classes. Most agonists at this receptor are either substituted phenethylamine derivatives from the 2C, DOx and 25-NB groups, or substituted tryptamines along with more complex derivatives such as lysergamides and iboga-type alkaloids.[1] There are however numerous examples of 5-HT2A receptor agonists which do not fall within any of these groups, some representative examples of which are listed below.
| Structure | Name | Chemical name | h5-HT2A Ki (EC50) (nM) | PubChem | CAS number | Reference |
|---|---|---|---|---|---|---|

|
Compound 23 | 9-Chloro-7-(2-ethoxy-phenyl)-2,3,4,5-tetrahydro-1H-[1,4]diazepino[1,7-a]indole | 32 | 44315398 | 599173-25-8 | [2] |
|
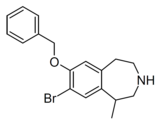
Compound 10d | 7-Benzyloxy-8-bromo-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine | 22 | 10472780 | 616201-60-6 | [3] |
|
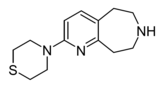
Example 22.67 | 4-(6,7,8,9-tetrahydro-5H-pyrido[2,3-d]azepin-2-yl)thiomorpholine | 21 | 44124494 | [4] | |
|
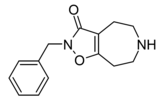
Compound 3d (N-Bn-THAZ) | 2-benzyl-5,6,7,8-tetrahydro-4H-[1,2]oxazolo[4,5-d]azepin-3-one | (549) | 14515725 | 125115-66-4 | [5] |
|
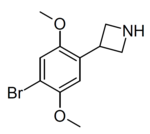
Example 1 (ZC-B) | 3-(4-bromo-2,5-dimethoxyphenyl)azetidine | (1.6) | 156337249 | 2641630-65-9 | [6] |

|
Compound 11 | (3R)-N,N-diethyl-5-(1H-indol-4-yl)-1-methyl-3,6-dihydro-2H-pyridine-3-carboxamide | (<10) | 156278040 | [7] | |
|
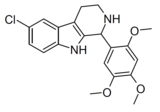
Compound 106 | 6-chloro-1-(2,4,5-trimethoxyphenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole | 4376990 | 528525-37-3 | [8] | |
|
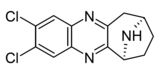
Compound 6c | (6S)-2,3-dichloro-7,8,9,10-tetrahydro-6H-6,9-epiminocyclohepta[b]quinoxaline | (400) | [9] | ||
|
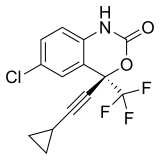
Efavirenz | (4S)-6-Chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-2,4-dihydro-1H-3,1-benzoxazin-2-one | 64139 | 154598-52-4 | [10] | |

|
IHCH-7113 | (6bR,10aS)-3-methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline | 21302499 | 313368-85-3 | [11] | |

|
Mefloquine | 2,8-bis(trifluoromethyl)quinolin-4-yl-(2-piperidyl)methanol | 40692 | 53230-10-7 | [12] | |

|
ORG-37684 | (3S)-3-[(2,3-dihydro-5-methoxy-1H-inden-4-yl)oxy]pyrrolidine | 9794656 | 213007-95-5 | [13] | |

|
P-54 | 2-(5-methoxypyrazolo[1,5-a]pyridin-3-yl)-N,N-dimethylethanamine | 168946740 | [14] | ||

|
(R)-69 | 3-[(5R)-5-methyl-1,2,5,6-tetrahydropyridin-3-yl]-1H-pyrrolo[2,3-b]pyridine | 164513426 | [15] | ||

|
RH-34 | 3-[2-(2-methoxybenzylamino)ethyl]-1H-quinazoline-2,4-dione | 10041987 | 1028307-48-3 | [16] | |
|
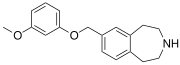
SCHEMBL5334361 | 7-[(3-methoxyphenoxy)methyl]-2,3,4,5-tetrahydro-1H-3-benzazepine | (0.4) | 59027940 | 959867-47-1 | [17] |
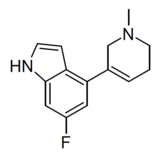
|
WXVL_BT0793LQ2118 | 6-fluoro-4-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl)-1H-indole | [18] | |||

|
Z2825713589 | (4-amino-3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrol-2-yl)-(6-methoxypyrazin-2-yl)methanone | 167788805 | [18] | ||

|
Z2876442907 | ethyl 2-[[2-(4-methyl-1H-indol-3-yl)ethylamino]methyl]-1,3-thiazole-5-carboxylate | 167850865 | [18] | ||
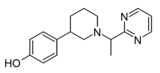
|
Z3517967757 | 4-[1-(1-pyrimidin-2-ylethyl)piperidin-3-yl]phenol | 167949972 | [18] | ||

|
Z3881312504 | 2-bromo-4-[2-[methyl-[2-(1,3-thiazol-2-yl)ethyl]amino]ethyl]phenol | 167904469 | [18] | ||

|
Z4154032166 | 2,2,2-trifluoro-1-[6-(1,2,3,6-tetrahydropyridin-5-yl)pyridin-2-yl]ethanol | 167878716 | [18] | ||

|
Z5247692566 | 4-[(3,3-dimethyloxolan-2-yl)methyl]-3-[(1H-indol-3-yl)methyl]morpholine | [18] | |||

|
Z5247692629 | 1-(1-bicyclo[1.1.1]pentanyl)-4-[[5-(4-chlorophenyl)-1H-pyrazol-4-yl]methyl]piperazine | 166358273 | [18] |
References
- ^ Nichols DE. Chemistry and Structure-Activity Relationships of Psychedelics. Curr Top Behav Neurosci. 2018;36:1-43. doi:10.1007/7854_2017_475 PMID 28401524
- ^ Ennis MD, Hoffman RL, Ghazal NB, Olson RM, Knauer CS, Chio CL, Hyslop DK, Campbell JE, Fitzgerald LW, Nichols NF, Svensson KA, McCall RB, Haber CL, Kagey ML, Dinh DM. 2,3,4,5-tetrahydro- and 2,3,4,5,11,11a-hexahydro-1H-[1,4]diazepino[1,7-a]indoles: new templates for 5-HT(2C) agonists. Bioorg Med Chem Lett. 2003 Jul 21;13(14):2369-72. doi:10.1016/s0960-894x(03)00403-7 pmid|12824036}}
- ^ Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, Sengupta D, Thomsen WJ, Saldana HR, Whelan KT, Menzaghi F, Webb RR, Beeley NR. Discovery and SAR of new benzazepines as potent and selective 5-HT(2C) receptor agonists for the treatment of obesity. Bioorg Med Chem Lett. 2005 Mar 1;15(5):1467-70. doi:10.1016/j.bmcl.2004.12.080 PMID 15713408
- ^ Slassi A, et al. Compounds with activity at the 5-ht2c receptor. WO 2009/079765
- ^ Jensen AA, Plath N, Pedersen MH, Isberg V, Krall J, Wellendorph P, Stensbøl TB, Gloriam DE, Krogsgaard-Larsen P, Frølund B. Design, synthesis, and pharmacological characterization of N- and O-substituted 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol analogues: novel 5-HT(2A)/5-HT(2C) receptor agonists with pro-cognitive properties. J Med Chem. 2013 Feb 14;56(3):1211-27. doi:10.1021/jm301656h PMID 23301527
- ^ US 2021/0137908, Kristensen JL, Jensen AA, Märcher-Rørsted E, "5-HT2A Agonists for Use in Treatment of Depression.", published 13 May 2021, assigned to Lophora ApS.
- ^ WO 2021/076572, Olson DE, et al., "Ergoline-like compounds for promoting neural plasticity"
- ^ Orr MJ, et al. Discovery of Highly Potent Serotonin 5-HT2 Receptor Agonists Inspired by Heteroyohimbine Natural Products. ACS Med. Chem. Lett. 2022; 13(4):648–657. doi:10.1021/acsmedchemlett.1c00694
- ^ Yao R, Jensen AA, Bryce-Rogers HP, Schultz-Knudsen K, Zhou L, Hovendal NP, et al. (August 2023). "Identification of 5-HT2 Serotonin Receptor Modulators through the Synthesis of a Diverse, Tropane- and Quinuclidine-alkaloid-Inspired Compound Library". Journal of Medicinal Chemistry. 66 (16): 11536–11554. doi:10.1021/acs.jmedchem.3c01059. PMID 37566000. S2CID 260806387.
- ^ Gatch MB, Kozlenkov A, Huang RQ, Yang W, Nguyen JD, González-Maeso J, Rice KC, France CP, Dillon GH, Forster MJ, Schetz JA. The HIV antiretroviral drug efavirenz has LSD-like properties. Neuropsychopharmacology. 2013 Nov;38(12):2373-84. doi:10.1038/npp.2013.135 PMID 23702798
- ^ Cao D, Yu J, Wang H, Luo Z, Liu X, He L, et al. (January 2022). "Structure-based discovery of nonhallucinogenic psychedelic analogs". Science. 375 (6579): 403–411. doi:10.1126/science.abl8615. PMID 35084960. S2CID 246360313.
- ^ Janowsky A, Eshleman AJ, Johnson RA, Wolfrum KM, Hinrichs DJ, Yang J, Zabriskie TM, Smilkstein MJ, Riscoe MK. Mefloquine and psychotomimetics share neurotransmitter receptor and transporter interactions in vitro. Psychopharmacology (Berl). 2014 Jul;231(14):2771-83. doi:10.1007/s00213-014-3446-0 PMID 24488404
- ^ Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M (August 2004). "Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 370 (2): 114–23. doi:10.1007/s00210-004-0951-4. PMID 15322733. S2CID 8938111.
- ^ WO 2023115165, Banister S, Jorgensen W, Jinlong T, "Compounds", published 29 June 2023, assigned to Psylo Pty Ltd.
- ^ Kaplan AL, Confair DN, Kim K, Barros-Álvarez X, Rodriguiz RM, Yang Y, et al. (September 2022). "Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity". Nature. 610 (7932): 582–591. Bibcode:2022Natur.610..582K. doi:10.1038/s41586-022-05258-z. PMC 9996387. PMID 36171289. S2CID 252598838.
- ^ Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT(2A) receptor". Journal of Computer-aided Molecular Design. 25 (1): 51–66. Bibcode:2011JCAMD..25...51S. CiteSeerX 10.1.1.688.2670. doi:10.1007/s10822-010-9400-2. PMID 21088982. S2CID 3103050.
- ^ WO 2007149728, Mohapatra S, Hellberg MR, Feng Z, "Aryl and heteroaryl tetrahydrobenzazepine derivatives and their use for treating glaucoma", assigned to Alcon Manufacturing, Ltd.
- ^ a b c d e f g h Lyu J, Kapolka N, Gumpper R, Alon A, Wang L, Jain MK, et al. (December 2023). "AlphaFold2 structures template ligand discovery". bioRxiv. doi:10.1101/2023.12.20.572662. PMC 10769324. PMID 38187536.
