Androgen insensitivity syndrome: Difference between revisions
m →References: removed stray characters |
→References: ref reformatting |
||
| Line 642: | Line 642: | ||
<ref name="2009 nichols 91">Nichols JL, Bieber EJ, Gell JS. Case of sisters with complete androgen insensitivity syndrome and discordant Müllerian remnants. ''Fertil Steril.'' 2009;'''91''':932e15-e18.</ref> |
<ref name="2009 nichols 91">Nichols JL, Bieber EJ, Gell JS. Case of sisters with complete androgen insensitivity syndrome and discordant Müllerian remnants. ''Fertil Steril.'' 2009;'''91''':932e15-e18.</ref> |
||
<ref name="2006 nieschlag 65">Nieschlag E. Testosterone treatment comes of age: new options for hypogonadal men. ''Clin Endocrinol.'' 2006;'''65''':275-281.</ref> |
|||
<ref name="2006 nieschlag 65">{{cite journal | author = Nieschlag E | title = Testosterone treatment comes of age: new options for hypogonadal men | journal = Clin. Endocrinol. (Oxf) | volume = 65 | issue = 3 | pages = 275–81 | year = 2006 | month = September | pmid = 16918944 | doi = 10.1111/j.1365-2265.2006.02618.x | url = | issn = }}</ref> |
|||
<ref name="2006 nihoul-fekete 175">Nihoul-Fékété C, Thibaud E, Lortat-Jacob S, Josso N. Long-term surgical results and patient satisfaction with male pseudohermaphroditism or true hermaphroditism: a cohort of 63 patients. ''J Urol.'' 2006;'''175''':1878-1884.</ref> |
|||
<ref name="1998 nordenskjold 11">Nordenskjöld A, Söderhäll S. An androgen receptor gene mutation (A645D) in a boy with a normal phenotype. ''Hum Mutat.'' 1998;'''11''':339.</ref> |
|||
<ref name="2006 nihoul-fekete 175">{{cite journal | author = Nihoul-Fékété C, Thibaud E, Lortat-Jacob S, Josso N | title = Long-term surgical results and patient satisfaction with male pseudohermaphroditism or true hermaphroditism: a cohort of 63 patients | journal = J. Urol. | volume = 175 | issue = 5 | pages = 1878–84 | year = 2006 | month = May | pmid = 16600787 | doi = 10.1016/S0022-5347(05)00934-1 | url = | issn = }}</ref> |
|||
<ref name="2008 oakes 21">Oakes MB, Eyvazzadeh AD, Quint E, Smith YR. Complete androgen insensitivity syndrome-a review. ''J Pediatr Adolesc Gynecol.'' 2008;'''21''':305-10.</ref> |
|||
<ref name="1971 ohno 1">Ohno S, Lyon MF. X-linked testicular feminization in the mouse as a non-inducible regulatory mutation of the Jacob-Monod type. ''Clin Genet.'' 1970;'''1''':121-127.</ref> |
|||
<ref name="1998 nordenskjold 11">{{cite journal | author = Nordenskjöld A, Söderhäll S | title = An androgen receptor gene mutation (A645D) in a boy with a normal phenotype | journal = Hum. Mutat. | volume = 11 | issue = 4 | pages = 339 | year = 1998 | pmid = 9554755 | doi = | url = | issn = }}</ref> |
|||
<ref name="2010 ozulker 24">Özülker T, Özpaçaci T, Özülker F, Özekici Ü, Bilgiç R, Mert M. Incidental detection of Sertoli–Leydig cell tumor by FDG PET/CT imaging in a patient with androgen insensitivity syndrome. ''Ann Nucl Med.'' 2010;'''24''':35-39.</ref> |
|||
<ref name="2008 oakes 21">{{cite journal | author = Oakes MB, Eyvazzadeh AD, Quint E, Smith YR | title = Complete androgen insensitivity syndrome--a review | journal = J Pediatr Adolesc Gynecol | volume = 21 | issue = 6 | pages = 305–10 | year = 2008 | month = December | pmid = 19064222 | doi = 10.1016/j.jpag.2007.09.006 | url = | issn = }}</ref> |
|||
<ref name="1971 ohno 1">{{cite journal | author = Ohno S, Lyon MF | title = X-Linked testicular feminization in the mouse as a non-inducible regulatory mutation of the Jacob-Monod type | journal = Clinical Genetics | year = 1970 | month = July | volume = 1 | issue = 3-4 | pages = 121–127 | doi = 10.1111/j.1399-0004.1970.tb01627.x}}</ref> |
|||
<ref name="2010 ozulker 24">{{cite journal | author = Ozülker T, Ozpaçaci T, Ozülker F, Ozekici U, Bilgiç R, Mert M | title = Incidental detection of Sertoli-Leydig cell tumor by FDG PET/CT imaging in a patient with androgen insensitivity syndrome | journal = Ann Nucl Med | volume = 24 | issue = 1 | pages = 35–9 | year = 2010 | month = January | pmid = 19957213 | doi = 10.1007/s12149-009-0321-x | url = | issn = }}</ref> |
|||
<ref name="1820 panckoucke 1">Panckoucke CLF, ed. ''Dictionnaire des sciences médicales - biographie médicale, 1st ed.'' Paris: Panckoucke 1820;'''1''':59.</ref> |
<ref name="1820 panckoucke 1">Panckoucke CLF, ed. ''Dictionnaire des sciences médicales - biographie médicale, 1st ed.'' Paris: Panckoucke 1820;'''1''':59.</ref> |
||
<ref name="2006 papadimitriou 65">Papadimitriou DT, Linglart A, Morel Y, Chaussain J. Puberty in subjects with complete androgen insensitivity syndrome. ''Horm Res.'' 2006;'''65''':126-131.</ref> |
|||
<ref name="2006 papadimitriou 65">{{cite journal | author = Papadimitriou DT, Linglart A, Morel Y, Chaussain JL | title = Puberty in subjects with complete androgen insensitivity syndrome | journal = Horm. Res. | volume = 65 | issue = 3 | pages = 126–31 | year = 2006 | pmid = 16491011 | doi = 10.1159/000091592 | url = | issn = }}</ref> |
|||
<ref name="1573 pare">Paré, A. ''Des monstres et prodiges.'' Paris: Dupuys 1573.</ref> |
<ref name="1573 pare">Paré, A. ''Des monstres et prodiges.'' Paris: Dupuys 1573.</ref> |
||
<ref name="1994 patterson 22">Patterson MN, Hughes IA, Gottlieb B, Pinsky L. The androgen reeptor gene mutations database. ''Nucl acids res.'' 1994;'''22''':3560-3562.</ref> |
|||
<ref name="1994 patterson 22">{{cite journal | author = Patterson MN, Hughes IA, Gottlieb B, Pinsky L | title = The androgen receptor gene mutations database | journal = Nucleic Acids Res. | volume = 22 | issue = 17 | pages = 3560–2 | year = 1994 | month = September | pmid = 7937057 | pmc = 308319 | doi = | url = | issn = }}</ref> |
|||
<ref name="1987 perez-palacios 27">Pérez-Palacios G, Chávez B, Méndez JP, McGinley JI, Ulloa-Aguirre A. The syndromes of androgen resistance revisited. ''J Steroid Biochem.'' 1987;'''27''':1101-1108.</ref> |
|||
<ref name="1987 perez-palacios 27">{{cite journal | author = Pérez-Palacios G, Chávez B, Méndez JP, McGinley JI, Ulloa-Aguirre A | title = The syndromes of androgen resistance revisited | journal = J. Steroid Biochem. | volume = 27 | issue = 4-6 | pages = 1101–8 | year = 1987 | pmid = 3320547 | doi = | url = | issn = }}</ref> |
|||
<ref name="1859 peschier">Peschier A, Mozin DJ, eds. ''Supplément au dictionnaire complet des langues française et allemande de l'abbe Mozin.'' Paris: Stuttgart et Augsbourg 1859, p. 333.</ref> |
<ref name="1859 peschier">Peschier A, Mozin DJ, eds. ''Supplément au dictionnaire complet des langues française et allemande de l'abbe Mozin.'' Paris: Stuttgart et Augsbourg 1859, p. 333.</ref> |
||
<ref name="1997 pietila 19">Pietilä K, Grön M, Alvesalo L. The craniofacial complex in karyotype 46,XY females. ''Eur J Orthod.'' 1997;'''19''':383-389.</ref> |
|||
<ref name="1997 pietila 19">{{cite journal | author = Pietilä K, Grön M, Alvesalo L | title = The craniofacial complex in karyotype 46,XY females | journal = Eur J Orthod | volume = 19 | issue = 4 | pages = 383–9 | year = 1997 | month = August | pmid = 9308259 | doi = | url = | issn = }}</ref> |
|||
<ref name="1984 pinsky 36">Pinsky L, Kaufman M, Killinger DW, Burko B, Shatz D, Volpé R. Human minimal androgen insensitivity with normal dihydrotestosterone-binding capacity in cultured genital skin fibroblasts: evidence for an androgen-selective qualitative abnormality of the receptor. ''Am J Hum Genet.'' 1984;'''36''':965-978.</ref> |
|||
<ref name="1989 pinsky 32">Pinsky L, Kaufman M, Killinger DW, Opitz JM, Reynolds JF. Impaired spermatogenesis is not an obligate expression of receptor-defective androgen resistance. ''Am J Med Genet.'' 1989;'''32''':100-104.</ref> |
|||
<ref name="1984 pinsky 36">{{cite journal | author = Pinsky L, Kaufman M, Killinger DW, Burko B, Shatz D, Volpé R | title = Human minimal androgen insensitivity with normal dihydrotestosterone-binding capacity in cultured genital skin fibroblasts: evidence for an androgen-selective qualitative abnormality of the receptor | journal = Am. J. Hum. Genet. | volume = 36 | issue = 5 | pages = 965–78 | year = 1984 | month = September | pmid = 6333813 | pmc = 1684524 | doi = | url = | issn = }}</ref> |
|||
<ref name="1989 pinsky 32">{{cite journal | author = Pinsky L, Kaufman M, Killinger DW | title = Impaired spermatogenesis is not an obligate expression of receptor-defective androgen resistance | journal = Am. J. Med. Genet. | volume = 32 | issue = 1 | pages = 100–4 | year = 1989 | month = January | pmid = 2705470 | doi = 10.1002/ajmg.1320320121 | url = | issn = }}</ref> |
|||
<ref name="1995 quigley 16">{{cite journal | author = Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS | title = Androgen receptor defects: historical, clinical, and molecular perspectives | journal = Endocr. Rev. | volume = 16 | issue = 3 | pages = 271–321 | year = 1995 | month = June | pmid = 7671849 | doi = | url = | issn = }}</ref> |
<ref name="1995 quigley 16">{{cite journal | author = Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS | title = Androgen receptor defects: historical, clinical, and molecular perspectives | journal = Endocr. Rev. | volume = 16 | issue = 3 | pages = 271–321 | year = 1995 | month = June | pmid = 7671849 | doi = | url = | issn = }}</ref> |
||
<ref name="2010 quint 53">Quint EH, McCarthy JD, Smith YR. Vaginal surgery for congenital anomalies. ''Clin Obstet Gynecol.'' 2010;'''53''':115-124.</ref> |
|||
<ref name="2010 quint 53">{{cite journal | author = Quint EH, McCarthy JD, Smith YR | title = Vaginal surgery for congenital anomalies | journal = Clin Obstet Gynecol | volume = 53 | issue = 1 | pages = 115–24 | year = 2010 | month = March | pmid = 20142648 | doi = 10.1097/GRF.0b013e3181cd4128 | url = | issn = }}</ref> |
|||
<ref name="1997 radmayr 158">Radmayr C, Culig Z, Glatzl J, Neuschmid-Kaspar F, Bartsch G, Klocker H. Androgen receptor point mutations as the underlying molecular defect in 2 patients with androgen insensitivity syndrome. ''J Urol.'' 1997;'''158''':1553-1556.</ref> |
|||
<ref name="2007 radpour 28">Radpour R, Rezaee M, Tavasoly A, Solati S, Saleki A. Association of long polyglycine tracts (GGN repeats) in exon 1 of the androgen receptor gene with cryptorchidism and penile hypospadias in Iranian patients. ''J Androl.'' 2007;'''28''':164-169.</ref> |
|||
<ref name="1997 radmayr 158">{{cite journal | author = Radmayr C, Culig Z, Glatzl J, Neuschmid-Kaspar F, Bartsch G, Klocker H | title = Androgen receptor point mutations as the underlying molecular defect in 2 patients with androgen insensitivity syndrome | journal = J. Urol. | volume = 158 | issue = 4 | pages = 1553–6 | year = 1997 | month = October | pmid = 9302173 | doi = | url = | issn = }}</ref> |
|||
<ref name="2006 rajender 27">Rajender S, Rajani V, Gupta NJ, Chakravarty B, Singh L, Thangaraj K. No association of androgen receptor GGN repeat length polymorphism with infertility in Indian men. ''J Androl.'' 2006;'''27''':785-789.</ref> |
|||
<ref name="2002 rajpert-de meyts 359">Rajpert-De Meyts E, Leffers H, Petersen JH, Andersen AG, Carlsen E, Jørgensen N, Skakkebaek NE. CAG repeat length in androgen-receptor gene and reproductive variables in fertile and infertile men. ''Lancet'' 2002;'''359''':44-46.</ref> |
|||
<ref name="2007 radpour 28">{{cite journal | author = Radpour R, Rezaee M, Tavasoly A, Solati S, Saleki A | title = Association of long polyglycine tracts (GGN repeats) in exon 1 of the androgen receptor gene with cryptorchidism and penile hypospadias in Iranian patients | journal = J. Androl. | volume = 28 | issue = 1 | pages = 164–9 | year = 2007 | pmid = 16957138 | doi = 10.2164/jandrol.106.000927 | url = | issn = }}</ref> |
|||
<ref name="2009 rajender 91">Rajender S, Gupta NJ, Chakrabarty B, Singh L, Thangaraj K. Ala 586 asp mutation in androgen receptor disrupts transactivation function without affecting androgen binding. ''Fertil Steril.'' 2009;'''91''':933.e23-e28.</ref> |
|||
<ref name="1947 reifenstein 3">Reifenstein EC Jr. Hereditary familial hypogonadism. ''Clin Res.'' 1947;'''3''':3.</ref> |
|||
<ref name="2006 rajender 27">{{cite journal | author = Rajender S, Rajani V, Gupta NJ, Chakravarty B, Singh L, Thangaraj K | title = No association of androgen receptor GGN repeat length polymorphism with infertility in Indian men | journal = J. Androl. | volume = 27 | issue = 6 | pages = 785–9 | year = 2006 | pmid = 16809273 | doi = 10.2164/jandrol.106.000166 | url = | issn = }}</ref> |
|||
<ref name="2007 reis 50">Reis, E. Divergence or Disorder? The Politics of Naming Intersex. ''Perspect Biol Med.'' 2007;'''50''':535-543.</ref> |
|||
<ref name="2002 rajpert-de meyts 359">{{cite journal | author = Rajpert-De Meyts E, Leffers H, Petersen JH, Andersen AG, Carlsen E, Jørgensen N, Skakkebaek NE | title = CAG repeat length in androgen-receptor gene and reproductive variables in fertile and infertile men | journal = Lancet | volume = 359 | issue = 9300 | pages = 44–6 | year = 2002 | month = January | pmid = 11809188 | doi = | url = | issn = }}</ref> |
|||
<ref name="2009 rajender 91">{{cite journal | author = Rajender S, Gupta NJ, Chakrabarty B, Singh L, Thangaraj K | title = Ala 586 Asp mutation in androgen receptor disrupts transactivation function without affecting androgen binding | journal = Fertil. Steril. | volume = 91 | issue = 3 | pages = 933.e23–8 | year = 2009 | month = March | pmid = 19062009 | doi = 10.1016/j.fertnstert.2008.10.041 | url = | issn = }}</ref> |
|||
<ref name="1947 reifenstein 3">{{cite journal | author = Reifenstein EC Jr. | title = Hereditary familial hypogonadism | journal = Proc Am Fed Clin Res | volume = 3 | issue = | pages = 86 | year = 1947 | pmid = 18909356 | doi = | url = | issn = }}</ref> |
|||
<ref name="2007 reis 50">{{cite journal | author = Reis E | title = Divergence or disorder?: the politics of naming intersex | journal = Perspect. Biol. Med. | volume = 50 | issue = 4 | pages = 535–43 | year = 2007 | pmid = 17951887 | doi = 10.1353/pbm.2007.0054 | url = | issn = }}</ref> |
|||
<ref name="1840 ritter von raimann 22">Ritter von Raiman JN, Edlen von Rosas A, Fischer SC, Wisgrill J, eds. ''Medicinische Jahrbücher des kaiserlich-königlichen österreichischen Staates (volume 22).'' Vienna: Carl Gerold 1840;'''22''':380-384.</ref> |
<ref name="1840 ritter von raimann 22">Ritter von Raiman JN, Edlen von Rosas A, Fischer SC, Wisgrill J, eds. ''Medicinische Jahrbücher des kaiserlich-königlichen österreichischen Staates (volume 22).'' Vienna: Carl Gerold 1840;'''22''':380-384.</ref> |
||
<ref name="1996 rodien 81">Rodien P, Mebarki F, Mowszowicz I, Chaussain JL, Young J, Morel Y, Schaison G. Different phenotypes in a family with androgen insensitivity caused by the same M780I point mutation in the androgen receptor gene. ''J Clin Endocrinol Metab.'' 1996;'''81''':2994-2998.</ref> |
|||
<ref name="1996 rodien 81">{{cite journal | author = Rodien P, Mebarki F, Mowszowicz I, Chaussain JL, Young J, Morel Y, Schaison G | title = Different phenotypes in a family with androgen insensitivity caused by the same M780I point mutation in the androgen receptor gene | journal = J. Clin. Endocrinol. Metab. | volume = 81 | issue = 8 | pages = 2994–8 | year = 1996 | month = August | pmid = 8768864 | doi = | url = | issn = }}</ref> |
|||
<ref name="1965 rosewater 63">Rosewater S, Gwinup G, Hamwi JG. Familial gynecomastia. ''Ann Int Med.'' 1965;'''63''':377-385.</ref> |
|||
<ref name="1999 roy 55">Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B. Regulation of androgen action. ''Vitam Horm.'' 1999;'''55''':309-352.</ref> |
|||
<ref name="1965 rosewater 63">{{cite journal | author = Rosewater S, Gwinup G, Hamwi JG | title = Familial gynecomastia | journal = Ann. Intern. Med. | volume = 63 | issue = | pages = 377–85 | year = 1965 | month = September | pmid = 14327504 | doi = | url = | issn = }}</ref> |
|||
<ref name="1991 rutgers 10">Rutgers JL, Scully RE. The androgen insensitivity syndrome (testicular feminization): a clinicopathologic study of 43 cases. ''Int J Gynecol Pathol.'' 1991;'''10''':126-144.</ref> |
|||
<ref name="1999 roy 55">{{cite journal | author = Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B | title = Regulation of androgen action | journal = Vitam. Horm. | volume = 55 | issue = | pages = 309–52 | year = 1999 | pmid = 9949684 | doi = | url = | issn = }}</ref> |
|||
<ref name="1991 rutgers 10">{{cite journal | author = Rutgers JL, Scully RE | title = The androgen insensitivity syndrome (testicular feminization): a clinicopathologic study of 43 cases | journal = Int. J. Gynecol. Pathol. | volume = 10 | issue = 2 | pages = 126–44 | year = 1991 | pmid = 2032766 | doi = | url = | issn = }}</ref> |
|||
<ref name="1709 ruysch 8">Ruysch F. ''Thesaurus anatomicus octavus.'' Amsterdam: Joannem Wolters 1709. p. 33, Plate II.</ref> |
<ref name="1709 ruysch 8">Ruysch F. ''Thesaurus anatomicus octavus.'' Amsterdam: Joannem Wolters 1709. p. 33, Plate II.</ref> |
||
<ref name="1832 st. hilaire">Saint Hilaire IG. ''Histoire générale et particulière des anomolies de l'organisation.'' Paris: J.-B. Baillière 1832-1836.</ref> |
<ref name="1832 st. hilaire">Saint Hilaire IG. ''Histoire générale et particulière des anomolies de l'organisation.'' Paris: J.-B. Baillière 1832-1836.</ref> |
||
<ref name="1999 shkolny 84">Shkolny DL, Beitel LK, Ginsberg J, Pekeles G, Arbour L, Pinsky L, Trifiro MA. Discordant measures of androgen-binding kinetics in two mutant androgen receptors causing mild or partial androgen insensitivity, respectively. ''J Clin Endocrinol Metab.'' 1999;'''84''':805-810.</ref> |
|||
<ref name="1999 shkolny 84">{{cite journal | author = Shkolny DL, Beitel LK, Ginsberg J, Pekeles G, Arbour L, Pinsky L, Trifiro MA | title = Discordant measures of androgen-binding kinetics in two mutant androgen receptors causing mild or partial androgen insensitivity, respectively | journal = J. Clin. Endocrinol. Metab. | volume = 84 | issue = 2 | pages = 805–10 | year = 1999 | month = February | pmid = 10022458 | doi = | url = | issn = }}</ref> |
|||
<ref name="1991 simental 266">Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. ''J Biol Chem.'' 1991;'''266''':510-518.</ref> |
|||
<ref name="2007 simmonds 92">Simmonds M. Was "variations of reproductive development" considered? ''Arch Dis Child.'' 2007;'''92''':89.</ref> |
|||
<ref name="1991 simental 266">{{cite journal | author = Simental JA, Sar M, Lane MV, French FS, Wilson EM | title = Transcriptional activation and nuclear targeting signals of the human androgen receptor | journal = J. Biol. Chem. | volume = 266 | issue = 1 | pages = 510–8 | year = 1991 | month = January | pmid = 1985913 | doi = | url = | issn = }}</ref> |
|||
<ref name="1976 simpson">Simpson JL. ''Disorders of Sexual Differentiation: Etiology and Clinical Delineation.'' New York: Academic Press, 1976.</ref> |
|||
<ref name="1995 simpson">Simpson JL, Rebar RW. Normal and abnormal sexual differentiation and development. In: Becker KL, ed. ''Principles and practice of endocrinology and metabolism, 3rd ed.'' Philadelphia: Lippincott 2001, pp. 852-885.</ref> |
|||
<ref name="2007 simmonds 92">{{cite journal | author = Simmonds M | title = Was "variations of reproductive development" considered? | journal = Arch. Dis. Child. | volume = 92 | issue = 1 | pages = 89 | year = 2007 | month = January | pmid = 17185456 | pmc = 2083124 | doi = 10.1136/adc.2006.107797 | url = | issn = }}</ref> |
|||
<ref name="2008 simpson">Simpson JL. Male Pseudohermaphroditism Due to Androgen Insensitivity or 5a-Reductase Deficiency. ''Glob libr women's med.'' (ISSN: 1756-2228) 2008; DOI 10.3843/GLOWM.10349</ref> |
|||
<ref name="1976 simpson"><ref name="isbn0-12-644450-1">{{cite book | author = Jirásek JE, Simpson JL | authorlink = | editor = | others = | title = Disorders of sexual differentiation: etiology and clinical delineation | edition = | language = | publisher = Academic Press | location = Boston | year = 1976 | origyear = | pages = | quote = | isbn = 0-12-644450-1 | oclc = | doi = | url = | accessdate = }}</ref> |
|||
<ref name="1995 simpson">{{cite book | author = Simpson JL, Rebar RW | authorlink = | editor = Hung, Wellington; Becker, Kenneth L.; Bilezikian, John P.; William J Bremner | others = | title = Principles and Practice of Endocrinology and Metabolism | edition = | language = | publisher = Lippincott Williams & Wilkins | location = Hagerstwon, MD | year = 2002 | origyear = | pages = 852-885 | quote = | isbn = 0-7817-4245-5 | oclc = | doi = | url = | accessdate = }}</ref> |
|||
<ref name="2008 simpson">{{cite book | author = Simpson JL | title = Glob. Libr. Women's Med. | chapter = Male Pseudohermaphroditism Due to Androgen Insensitivity or 5α-Reductase Deficiency | year = 2008 | doi = 10.3843/GLOWM.10349 }}</ref> |
|||
<ref name="1839 simpson">Simpson JY. Hermaphroditism. In: Todd RB, ed. ''Cyclopaedia of Anatomy and Physiology, Vol II.'' London: Longman, Brown, Green, Longmans, & Roberts 1839;'''2''':684-738.</ref> |
<ref name="1839 simpson">Simpson JY. Hermaphroditism. In: Todd RB, ed. ''Cyclopaedia of Anatomy and Physiology, Vol II.'' London: Longman, Brown, Green, Longmans, & Roberts 1839;'''2''':684-738.</ref> |
||
<ref name="1989 sinnecker 68">Sinnecker G, Köhler S. Sex hormone-binding globulin response to the anabolic steroid stanozolol: evidence for its suitability as a biological androgen sensitivity test. ''J Clin Endocrinol Metab.'' 1989;'''68''':1195-1200.</ref> |
|||
<ref name="1989 sinnecker 68">{{cite journal | author = Sinnecker G, Köhler S | title = Sex hormone-binding globulin response to the anabolic steroid stanozolol: evidence for its suitability as a biological androgen sensitivity test | journal = J. Clin. Endocrinol. Metab. | volume = 68 | issue = 6 | pages = 1195–200 | year = 1989 | month = June | pmid = 2723028 | doi = | url = | issn = }}</ref> |
|||
<ref name="1997 sinnecker 156">Sinnecker GHG, Hiort O, Nitsche EM, Holterhus P, Kruse K, German Collaborative Intersex Study Group. Functional assessment and clinical classification of androgen sensitivity in patients with mutations of the androgen receptor gene. ''Eur J Pediatr.'' 1997;'''156''':7-14.</ref> |
|||
<ref name="2006 sobel 91">Sobel V, Schwartz B, Zhu YS, Cordero JJ, Imperato-McGinley J. Bone mineral density in the complete androgen insensitivity and 5alpha-reductase-2 deficiency syndromes. ''J Clin Endocrinol Metab.'' 2006;'''91''':3017-3023.</ref> |
|||
<ref name="1997 sinnecker 156">{{cite journal | author = Sinnecker GH, Hiort O, Nitsche EM, Holterhus PM, Kruse K | title = Functional assessment and clinical classification of androgen sensitivity in patients with mutations of the androgen receptor gene. German Collaborative Intersex Study Group | journal = Eur. J. Pediatr. | volume = 156 | issue = 1 | pages = 7–14 | year = 1997 | month = January | pmid = 9007482 | doi = | url = | issn = }}</ref> |
|||
<ref name="1995 soule 43">Soule SG, Conway G, Prelevic GM, Prentice M, Ginsburg J, Jacobs HS. Osteopenia as a feature of the androgen insensitivity syndrome. ''Clin Endocrinol.'' 1995;'''43''':671–675.</ref> |
|||
<ref name="2002 steiner 84">Steiner E, Woernle F, Kuhn W, Beckmann K, Schmidt M, Pilch H, Knapstein PG. Carcinoma of the neovagina: case report and review of the literature. ''Gynecol Oncol.'' 2002;'''84''':171-175.</ref> |
|||
<ref name="2006 sobel 91">{{cite journal | author = Sobel V, Schwartz B, Zhu YS, Cordero JJ, Imperato-McGinley J | title = Bone mineral density in the complete androgen insensitivity and 5alpha-reductase-2 deficiency syndromes | journal = J. Clin. Endocrinol. Metab. | volume = 91 | issue = 8 | pages = 3017–23 | year = 2006 | month = August | pmid = 16735493 | doi = 10.1210/jc.2005-2809 | url = | issn = }}</ref> |
|||
<ref name="1999 stenoien 8">Stenoien DL, Cummings CJ, Adams HP, Mancini MG, Patel K, DeMartino GN, Marcelli M, Weigel NL, Mancini MA. Polyglutamine-expanded androgen receptors form aggregates that sequester heat shock proteins, proteasome components and SRC-1, and are suppressed by the HDJ-2 chaperone. ''Hum Mol Genet.'' 1999;'''8''':731-741.</ref> |
|||
<ref name="2009 stouffs 15">Stouffs K, Tournaye H, Liebaers I, Lissens W. Male infertility and the involvement of the X chromosome. ''Hum Reprod Update.'' 2009;'''15''':623–637.</ref> |
|||
<ref name="1995 soule 43">{{cite journal | author = Soule SG, Conway G, Prelevic GM, Prentice M, Ginsburg J, Jacobs HS | title = Osteopenia as a feature of the androgen insensitivity syndrome | journal = Clin. Endocrinol. (Oxf) | volume = 43 | issue = 6 | pages = 671–5 | year = 1995 | month = December | pmid = 8736267 | doi = | url = | issn = }}</ref> |
|||
<ref name="2001 sultan 7">Sultan C, Paris F, Terouanne B, Balaguer P, Georget V, Poujol N, Jeandel C, Lumbroso S, Nicolas J. Disorders linked to insufficient androgen action in male children. ''Hum Reprod Update.'' 2001;'''7''':314-322.</ref> |
|||
<ref name="2002 sultan 20">Sultan C, Lumbroso S, Paris F, Jeandel C, Terouanne B, Belon C, Audran F, Poujol N, Georget V, Gobinet J, Jalaguier S, Auzou G, Nicolas JC. Disorders of androgen action. ''Semin Reprod Med.'' 2002;'''20''':217-228.</ref> |
|||
<ref name="2002 steiner 84">{{cite journal | author = Steiner E, Woernle F, Kuhn W, Beckmann K, Schmidt M, Pilch H, Knapstein PG | title = Carcinoma of the neovagina: case report and review of the literature | journal = Gynecol. Oncol. | volume = 84 | issue = 1 | pages = 171–5 | year = 2002 | month = January | pmid = 11748997 | doi = 10.1006/gyno.2001.6417 | url = | issn = }}</ref> |
|||
<ref name="1999 stenoien 8">{{cite journal | author = Stenoien DL, Cummings CJ, Adams HP, Mancini MG, Patel K, DeMartino GN, Marcelli M, Weigel NL, Mancini MA | title = Polyglutamine-expanded androgen receptors form aggregates that sequester heat shock proteins, proteasome components and SRC-1, and are suppressed by the HDJ-2 chaperone | journal = Hum. Mol. Genet. | volume = 8 | issue = 5 | pages = 731–41 | year = 1999 | month = May | pmid = 10196362 | doi = | url = | issn = }}</ref> |
|||
<ref name="2009 stouffs 15">{{cite journal | author = Stouffs K, Tournaye H, Liebaers I, Lissens W | title = Male infertility and the involvement of the X chromosome | journal = Hum. Reprod. Update | volume = 15 | issue = 6 | pages = 623–37 | year = 2009 | pmid = 19515807 | doi = 10.1093/humupd/dmp023 | url = | issn = }}</ref> |
|||
<ref name="2001 sultan 7">{{cite journal | author = Sultan C, Paris F, Terouanne B, Balaguer P, Georget V, Poujol N, Jeandel C, Lumbroso S, Nicolas JC | title = Disorders linked to insufficient androgen action in male children | journal = Hum. Reprod. Update | volume = 7 | issue = 3 | pages = 314–22 | year = 2001 | pmid = 11392378 | doi = | url = | issn = }}</ref> |
|||
<ref name="2002 sultan 20">{{cite journal | author = Sultan C, Lumbroso S, Paris F, Jeandel C, Terouanne B, Belon C, Audran F, Poujol N, Georget V, Gobinet J, Jalaguier S, Auzou G, Nicolas JC | title = Disorders of androgen action | journal = Semin. Reprod. Med. | volume = 20 | issue = 3 | pages = 217–28 | year = 2002 | month = August | pmid = 12428202 | doi = 10.1055/s-2002-35386 | url = | issn = }}</ref> |
|||
<ref name="1995 tanaka 43">Tanaka Y, Matsuo N, Aya M, et al. Persistent Müllerian duct remnants in three siblings with partial androgen insensitivity. ''Horumon To Rinsho'' 1995;'''43''':3-8.</ref> |
<ref name="1995 tanaka 43">Tanaka Y, Matsuo N, Aya M, et al. Persistent Müllerian duct remnants in three siblings with partial androgen insensitivity. ''Horumon To Rinsho'' 1995;'''43''':3-8.</ref> |
||
<ref name="2005 taneja 280">Taneja SS, Ha S, Swenson NK, Huang HY, Lee P, Melamed J, Shapiro E, Garabedian MJ, Logan SK. Cell-specific regulation of androgen receptor phosphorylation in vivo. ''J Biol Chem.'' 2005;'''280''':40916-40924.</ref> |
<ref name="2005 taneja 280">Taneja SS, Ha S, Swenson NK, Huang HY, Lee P, Melamed J, Shapiro E, Garabedian MJ, Logan SK. Cell-specific regulation of androgen receptor phosphorylation in vivo. ''J Biol Chem.'' 2005;'''280''':40916-40924.</ref> |
||
Revision as of 03:26, 25 September 2010
| Androgen insensitivity syndrome | |
|---|---|
| Specialty | Endocrinology |
Androgen insensitivity syndrome (AIS) is a condition in which people who are genetically males are partially or completely unaffected by androgens at the cellular level[1][2][3]. The inability of the cell to respond to the presence of androgenic hormones produces a wide spectrum of clinical effects, ranging from a normal male habitus with mild spermatogenic defect or reduced secondary terminal hair, to a full female habitus, despite the presence of a Y chromosome[1][4][5][6][7][8].
AIS is X-linked recessive, causes only genetic males (i.e. a 46,XY karyotype) to be significantly affected[1]. These phenotypes are grouped into three classes that are differentiated by the degree of genital masculinization: complete androgen insensitivity syndrome (CAIS) is indicated when the external genitalia is that of a normal female, mild androgen insensitivity syndrome (MAIS) is indicated when the external genitalia that of a normal male, and partial androgen insensitivity syndrome (PAIS) is indicated when the external genitalia is partially, but not fully masculinized [1][2][4][5][6][9][10][11][12]. Androgen insensitivity syndrome is the largest single entity that leads to 46,XY undermasculinization [13].
Signs and symptoms
AIS is broken down into three classes based on phenotype: complete androgen insensitivity syndrome (CAIS), partial androgen insensitivity syndrome (PAIS), and mild androgen insensitivity syndrome (MAIS) [1][2][4][5][6][9][10][11][12]. A supplemental system of phenotypic grading that uses seven classes instead of the traditional three was proposed by pediatric endocrinologist Charmian A. Quigley et al. in 1995 [3]. The first six grades of the scale, grades 1 through 6, are differentiated by the degree of genital masculinization; grade 1 is indicated when the external genitalia is fully masculinized, grade 6 is indicated when the external genitalia is fully feminized, and grades 2 through 5 quantify four degrees of increasingly feminized genitalia that lie in the interim [3]. Grade 7 is indistinguishable from grade 6 until puberty, and is thereafter differentiated by the presence of secondary terminal hair; grade 6 is indicated when secondary terminal hair is present, whereas grade 7 is indicated when it is absent [3]. The Quigley scale can be used in conjunction with the traditional three classes of AIS to provide additional information regarding the degree of genital masculinization, and is particularly useful when the diagnosis is PAIS [2][14].
Complete AIS

Individuals with complete androgen insensitivity syndrome (grades 6 and 7 on the Quigley scale) are born phenotypically female, without any signs of genital masculinization, despite having a 46,XY karyotype [17]. Symptoms of CAIS do not appear until puberty [2], which may be slightly delayed [18], but is otherwise normal except for absent menses and diminished or absent secondary terminal hair [1]. Axillary hair (i.e. armpit hair) fails to develop in one third of all cases [19]. External genitalia is normal, although the labia and clitoris are sometimes underdeveloped [20][21]. The vaginal depth is widely variable, but is typically shorter than unaffected women [1]; one study of eight women with CAIS measured the average vaginal depth to be 5.9 cm [22] (vs. 11.1 ± 1.0 cm for unaffected women [23]). In some extreme cases, the vagina has been reported to be aplastic (resembling a "dimple"), though the exact incidence of this is unknown [24].
The gonads in these women are not ovaries, but instead, are testes; during the embryonic stage of development, testes form in an androgen-independent process that occurs due to the influence of the SRY gene on the Y chromosome [25][26]. They may be located intra-abdominally, at the internal inguinal ring, or may herniate into the labia majora [1][27][28][29]. Germ cells in the testes are arrested at an early stage and do not mature into sperm, since sensitivity to androgens is required in order for spermatogenesis to complete [30][31]. Germ cell malignancy risk, once thought to be relatively high, is now thought to be approximately 2% [32]. Wolffian structures (the epididymides, vasa deferentia, and seminal vesicles) are typically absent, but will develop at least partially in approximately 30% of cases, depending on which mutation is causing the CAIS [33]. The prostate, like the external male genitalia, cannot masculinize in the absence of androgen receptor function, and thus remains in the female form [17][34][35][36].
The Müllerian system (the fallopian tubes, uterus, and upper portion of the vagina) typically regresses due to the presence of anti-Müllerian hormone originating from the Sertoli cells of the testes [18]. These women are thus born without fallopian tubes, a cervix, or a uterus [18], and the vagina ends "blindly" in a pouch [1]. Müllerian regression does not fully complete in approximately one third of all cases, resulting in Müllerian "remnants" [18]. Although rare, a few cases of women with CAIS and fully developed Müllerian structures have been reported. In one exceptional case, a 22 year old with CAIS was found to have a normal cervix, uterus, and fallopian tubes [37]. In an unrelated case, a fully developed uterus was found in a 22 year old adult with CAIS [36].
Other subtle differences that have been reported include slightly longer limbs and larger hands and feet due to a proportionally greater stature than unaffected women [38][39][40], larger teeth [41][42], minimal or no acne [43], well developed breasts [44], and a greater incidence of meibomian gland dysfunction (i.e. dry eye syndromes and light sensitivity) [45].

Partial AIS
Partial androgen insensitivity syndrome is diagnosed when the degree of androgen insensitivity in an individual with a 46,XY karyotype is great enough to partially prevent the masculinization of the genitalia, but is not great enough to completely prevent genital masculinization [1][2][17][27]. This includes any phenotype resulting from androgen insensitivity where the genitalia is partially, but not completely masculinized. Genital ambiguities are frequently detected during clinical examination at birth, and consequently, a PAIS diagnosis can be made during infancy as part of a differential diagnostic workup [47][48].
Pubertal undervirilization is common, including gynecomastia, decreased secondary terminal hair, and / or a high pitched voice [49]. The phallic structure ranges from a penis with varying degrees of diminished size and hypospadias to a slightly enlarged clitoris [1][2][3]. Wolffian structures (the epididymides, vasa deferentia, and seminal vesicles) are typically partially or fully developed [2]. The prostate is typically small or impalpable [50][51]. Müllerian remnants are rare, but have been reported [52][53].
The gonads in individuals with PAIS are testes, regardless of phenotype [2]; during the embryonic stage of development, testes form in an androgen-independent process that occurs due to the influence of the SRY gene on the Y chromosome [25][26]. Cryptorchidism is common [1][2], and carries with it a 50% risk of germ cell malignancy [32]. If the testes are located intrascrotally, there may still be significant risk of germ cell malignancy; studies have not yet been published to assess this risk [32].
Predominantly male phenotypes vary in the degree of genital undermasculinization to include micropenis, chordee, bifid scrotum, and / or pseudovaginal perineoscrotal hypospadias [1][17][54]. Impotence may be fairly common, depending on phenotypic features; in one study of 15 males with PAIS, 80% of those interviewed indicated that they had some degree of impotence [55]. Anejaculation appears to occur somewhat independently of impotence; some men are still able to ejaculate despite impotence, and others without erectile difficulties cannot [50][19][56][57]. Predominantly female phenotypes include a variable degree of labial fusion and clitoromegaly [3]. Ambiguous phenotypic states include a phallic structure that is intermediate between a clitoris and a penis, and a single perineal orifice that connects to both the urethra and the vagina (i.e. urogenital sinus) [3]. At birth, it may not be possible to immediately differentiate the external genitalia of individuals with PAIS as being either male or female [1][58], although the majority of individuals with PAIS are raised male [1].
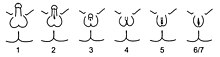
Given the wide diversity of phenotypes associated with PAIS, the diagnosis is often further specified by assessing genital masculinization [2][3]. Grades 2 through 5 of the Quigley scale quantify four degrees of increasingly feminized genitalia that correspond to PAIS [3].
Grade 2, the mildest form of PAIS, presents with a predominantly male phenotype that presents with minor signs of undermasculinized genitalia, such as isolated hypospadias [3], which can be severe [1]. Hypospadias may manifest with a partially formed channel from the urethral opening to the glans [3][59]. Until recently, it was thought that isolated micropenis was not a manifestation of PAIS [1]. However, in 2010, two cases of PAIS manifesting with isolated micropenis were documented [60].

Grade 3, the most common phenotypic form of PAIS [1][55], features a predominantly male phenotype that is more severely undermasculinized, and typically presents with micropenis and pseudovaginal perineoscrotal hypospadias with bifid scrotum [3].
Grade 4 presents with a gender ambiguous phenotype, including a phallic structure that is intermediate between a clitoris and a penis [3]. The urethra typically opens into a common channel with the vagina (i.e. urogenital sinus) [3].
Grade 5, the form of PAIS with the greatest degree of androgen insensitivity, presents with a mostly female phenotype, including separate urethral and vaginal orifices, but also shows signs of slight masculinization including mild clitoromegaly and / or partial labial fusion [1][3].
Previously, it was erroneously thought that individuals with PAIS were always infertile; at least one case report has been published that describes fertile men that fit the criteria for grade 2 PAIS (micropenis, penile hypospadias, and gynecomastia) [62].
Mild AIS

Individuals with mild (or minimal) androgen insensitivity syndrome (grade 1 on the Quigley scale) are born phenotypically male, with fully masculinized genitalia; this category of androgen insensitivity is diagnosed when the degree of androgen insensitivity in an individual with a 46,XY karyotype is great enough to impair virilization or spermatogenesis, but is not great enough to impair normal male genital development [1][4][5][8]. MAIS is the mildest and least known form of androgen insensitivity syndrome [4][64].
The existence of a variant of androgen insensitivity that solely affected spermatogenesis was theoretical at first [65]. Cases of phenotypically normal males with isolated spermatogenic defect due to AR mutation were first detected as the result of male infertility evaluations [1][12][17][31]. Until then, early evidence in support of the existence of MAIS was limited to cases involving a mild defect in virilization [63][66], although some of these early cases made allowances for some degree of impairment of genital masculinization, such as hypospadias or micropenis [49][67][68]. It is estimated that 2-3% of infertile men have AR gene mutations [5].
Examples of MAIS phenotypes include isolated infertility (oligospermia or azoospermia) [4][6], mild gynecomastia in young adulthood, decreased secondary terminal hair, high pitched voice, or minor hypospadias repair in childhood [1][69]. The external male genitalia (penis, scrotum, and urethra) are otherwise normal in individuals with MAIS [1][4][5][8]. Internal genitalia, including Wolffian structures (the epididymides, vasa deferentia, and seminal vesicles) and the prostate, is also normal, although the bitesticular volume of infertile men (both with and without MAIS) is diminished [5]; male infertility is associated with reduced bitesticular volume, varicocele, retractile testes, low ejaculate volume, male accessory gland infections (MAGI), and mumps orchitis [5]. The incidence of these features in infertile men with MAIS is similar to that of infertile men without MAIS [5]. MAIS is not associated with Müllerian remnants.
Kennedy Disease
Kennedy disease, also known as spinal and bulbar muscular atrophy (SBMA), is a severe neurodegenerative syndrome that is associated with a particular mutation of the androgen receptor's polyglutamine tract called a trinucleotide repeat expansion [70][71]. SBMA results when the length of the polyglutamine tract exceeds 40 repetitions [72].
Although technically a variant of MAIS, SBMA's presentation is not typical of androgen insensitivity; symptoms do not occur until adulthood and include neuromuscular defects as well as signs of androgen inaction [70]. Neuromuscular symptoms include progressive proximal muscle weakness, atrophy, and fasciculations. Symptoms of androgen insensitivity experienced by men with SBMA are also progressive [70] and include testicular atrophy, severe oligospermia or azoospermia, gynecomastia, and feminized skin changes [73] despite elevated androgen levels [1]. Disease onset, which usually affects the proximal musculature first, occurs in the third to fifth decades of life, and is often preceded by muscular cramps on exertion, tremor of the hands, and elevated muscle creatine kinase [74]. SBMA is often misdiagnosed as amyotrophic lateral sclerosis (ALS) (aka. Lou Gehrig's disease) [71].
The symptoms of SBMA are thought to be brought about by two simultaneous pathways involving the toxic misfolding of proteins and loss of AR functionality [1]. The polyglutamine tract in affected pedigrees tends to increase in length over generations, a phenomenon known as "anticipation" [75], leading to an increase in the severity of the disease as well as a decrease in the age of onset for each subsequent generation of a family affected by SBMA [70].
Comorbidity

All forms of androgen insensitivity are associated with infertility, though exceptions have been reported for both the mild and partial forms [4][6][62][76][77][78].
CAIS is associated with a decreased bone mineral density [79][80][81][82][83][84]. Some have hypothesized that the decreased bone mineral density observed in women with CAIS is related to the timing of gonadectomy and inadequate estrogen supplementation [83]. However, recent studies show that bone mineral density is similar whether gonadectomy occurs before or after puberty, and is decreased despite estrogen supplementation, leading some to hypothesize that the deficiency is directly attributable to the role of androgens in bone mineralization [79][80][81][82].
CAIS is also associated with an increased risk for gonadal tumors (e.g. germ cell malignancy) in adulthood if gonadectomy is not performed [32][85][86][87]. The risk of malignant germ cell tumors in women with CAIS increases with age and has been estimated to be 3.6% at 25 years and 33% at 50 years [87]. The incidence of gonadal tumors in childhood is thought to be relatively low; a recent review of the medical literature [85] found that only three cases of malignant germ cell tumors in prepubescent girls have been reported in association with CAIS in the last 100 years. Some have estimated the incidence of germ cell malignancy to be as low as 0.8% before puberty [1].
Vaginal hypoplasia, a relatively frequent finding in CAIS and some forms of PAIS [22][24], is associated with sexual difficulties including vaginal penetration difficulties and dyspareunia [20][24].
PAIS is associated with a 50% risk of germ cell malignancy when the testes are undescended [32]. If the testes are located intrascrotally, there may still be significant risk of germ cell malignancy; studies have not yet been published to assess this risk [32]. Some men with PAIS may experience sexual dysfunction including impotence and anejaculation [50][19][55][56][57]. A few AR mutations that cause PAIS are also associated with prostate [8][88] and breast [59][89] cancers.
At least one study indicates that individuals with an intersex condition may be more prone to psychological difficulties, due at least in part to parental attitudes and behaviors [90], and concludes that preventative long-term psychological counseling for parents as well as for affected individuals should be initiated at the time of diagnosis.
Lifespan is not thought to be affected by AIS [1].
Genetics

The human androgen receptor (AR) is a protein encoded by a gene located on the proximal long arm of the X chromosome (locus Xq11-Xq12) [91]. The protein coding region consists of approximately 2,757 nucleotides (919 codons) spanning eight exons, designated 1-8 or A-H [1][3]. Introns vary in size between 0.7 and 26 kb [3]. Like other nuclear receptors, the androgen receptor protein consists of several functional domains: the transactivation domain (also called the transcription-regulation domain or the amino / NH2-terminal domain), the DNA-binding domain, the hinge region, and the steroid-binding domain (also called the carboxyl-terminal ligand-binding domain) [1][2][3][12]. The transactivation domain is encoded by exon 1, and makes up more than half of the AR protein [3]. Exons 2 and 3 encode the DNA-binding domain, while the 5' portion of exon 4 encodes the hinge region [3]. The remainder of exon 4 through exon 8 encodes the ligand binding domain [3].
Trinucleotide Satellite Lengths and AR Transcriptional Activity
The androgen receptor gene contains two polymorphic trinucleotide microsatellites in exon 1 [2]. The first microsatellite (nearest the 5' end) contains 8 [92] to 60 [71][74] repetitions of the glutamine codon "CAG" and is thus known as the polyglutamine tract [3]. The second microsatellite contains 4 [93] to 31 [94] repetitions of the glycine codon "GGC" and is known as the polyglycine tract [95]. The average number of repetitions varies by ethnicity, with Caucasians exhibiting an average of 21 CAG repeats, and Blacks 18 [96]. Disease states are associated with extremes in polyglutamine tract length; prostate cancer [70], hepatocellular carcinoma [97], and mental retardation [92] are associated with too few repetitions, while spinal and bulbar muscular atrophy (SBMA) is associated with a CAG repetition length of 40 or more [72]. Some studies indicate that the length of the polyglutamine tract is inversely correlated with transcriptional activity in the AR protein, and that longer polyglutamine tracts may be associated with infertility [98][99][100] and undermasculinized genitalia [101]. However, other studies have indicated that no such correlation exists [102][103][104][105][106][107]. A comprehensive meta-analysis of the subject published in 2007 supports the existence of the correlation, and concluded that these discrepancies could be resolved when sample size and study design are taken into account [10]. Longer polyglycine tract lengths have also been associated with genital masculinization defects in some [108][109], but not all [110], studies.
AR Mutations
As of 2010, over 400 AR mutations have been reported in the AR mutation database, and the number continues to grow [2]. Inheritance is typically maternal and follows an X-linked recessive pattern [1][111]; individuals with a 46,XY karyotype will always express the mutant gene since they only have one X chromosome, whereas 46,XX carriers will be minimally affected. 30% of the time, the AR mutation is a spontaneous result, and is not inherited [9]. Such de novo mutations are the result of a germ cell mutation or germ cell mosaicism in the gonads of one of the parents, or a mutation in the fertilized egg itself [58]. In one study [112], it was found that 3 out of 8 de novo mutations occurred in the post-zygotic stage, leading to the estimate that up to one third of de novo mutations result in somatic mosaicism [1]. It is worthwhile to note that not every mutation of the AR gene results in androgen insensitivity; one particular mutation occurs in 8 to 14 percent of genetic males [113][114][115][116], and is thought to adversely affect only a small number of individuals when other genetic factors are present [117].
Other Causes
Some individuals with CAIS or PAIS do not have any AR mutations despite clinical, hormonal, and histological features sufficient to warrant an AIS diagnosis; up to 5% of women with CAIS do not have an AR mutation [2], as well as between 27% [5][19] and 72% [118] of individuals with PAIS.
In one patient, it was shown that the underlying cause for presumptive PAIS was a mutant steroidogenic factor-1 (SF-1) protein [119]. In another patient, it was shown that CAIS was the result of a deficit in the transmission of a transactivating signal from the N-terminal region of the normal androgen receptor to the basal transcription machinery of the cell [120]. It was suggested that a coactivator protein interacting with the activation function 1 (AF-1) transactivation domain of the androgen receptor was deficient in this patient [120]. The signal disruption could not be corrected by supplementation with any coactivators known at the time, nor was the absent coactivator protein characterized, which left some in the field unconvinced that a mutant coactivator would explain the mechanism of androgen resistance in CAIS or PAIS patients with a normal AR gene [1].
XY Karyotype
Depending on the mutation, genetic males (46,XY karyotype) can have either a male (MAIS) or female (CAIS) phenotype [121], or may have genitalia that is only partially masculinized (PAIS) [122]. A genetic male will develop testes regardless of a female phenotype due to the influence of the Y chromosome [25][26]. Likewise, a 46,XY female (a genetic male with a female phenotype) does not have ovaries or a uterus [123], and can neither contribute an egg towards conception nor gestate a child.
Several case studies of fertile 46,XY males with androgen insensitivity have been published [63][66][68][77][78], although this group is thought to be a minority [12]. Additionally, some infertile males with MAIS have been able to conceive children after increasing their sperm count through the use of supplementary testosterone [1][124]. A genetic male conceived by a man with androgen insensitivity would not receive his father's X chromosome, and thus would neither inherit nor carry the gene for the syndrome. A genetic female conceived in such a way would receive her father's X chromosome, and would thus become a carrier.
XX Karyotype
Genetic females (46,XX karyotype) have two X chromosomes, and thus have two AR genes. A mutation in one (but not both) of the AR genes results in a minimally affected, fertile, female carrier. Some carriers have been noted to have slightly reduced body hair, delayed puberty, and / or tall stature, presumably due to skewed X-inactivation [3][78]. A female carrier will pass the affected AR gene to her children 50% of the time. If the affected child is a genetic female, she too will be a carrier. An affected 46,XY child will have androgen insensitivity syndrome.
A genetic female with mutations in both AR genes could theoretically result from the union of a fertile man with androgen insensitivity and a female carrier of the gene, or from de novo mutation. However, given the scarcity of fertile androgen insensitive men and low incidence of AR mutation, the chances of this occurrence is small. The phenotype of such an individual is a matter of speculation; as of 2010, no such documented case has been published.
Correlation of Genotype and Phenotype
Individuals with partial androgen insensitivity, unlike those with the complete or mild forms, present at birth with ambiguous genitalia, and the decision to raise the child as male or female is often not obvious [1][55][58]. Unfortunately, it is often the case that little information regarding phenotype can be gleaned from precise knowledge of the AR mutation itself; it is well established that the same AR mutation may cause significant variation in the degree of undermasculinization in different individuals, even among members of the same family [1][50][54][125][111][122][126][127][128][129]. Exactly what causes this variation is not entirely understood, although factors contributing to it could include the lengths of the polyglutamine and polyglycine tracts [130], sensitivity to and variations in the intrauterine endocrine milieu [122], the effect of coregulatory proteins that are active in Sertoli cells [69][95], somatic mosaicism [1], expression of the 5RD2 gene in genital skin fibroblasts [50], reduced AR transcription and translation from factors other than mutations in the AR coding region [131], an unidentified coactivator protein [120], enzyme deficiencies such as 21-hydroxylase deficiency [78], or other genetic variations such as a mutant steroidogenic factor-1 (SF-1) protein [119]. The degree of variation, however, does not appear to be constant across all AR mutations, and is much more extreme in some [1][78][117][122]. Missense mutations that result in a single amino acid substitution are known to produce the most phenotypic diversity [2].
Pathophysiology

Androgens and the androgen receptor
The effects that androgens have on the human body --- virilization, masculinization, anabolism, etc. --- are not brought about by androgens themselves, but rather are the result of androgens bound to androgen receptors; the androgen receptor mediates the effects of androgens in the human body [65]. Likewise, under normal circumstances, the androgen receptor itself is inactive in the cell until androgen binding occurs [3].
The following series of steps illustrates how androgens and the androgen receptor work together to produce androgenic effects [1][2][3][12][74][133][134]:
- Androgen enters the cell.
- Only certain organs in the body, such as the gonads and the adrenal glands, produce the androgen testosterone.
- Testosterone is converted into dihydrotestosterone, a chemically similar androgen, in cells containing the 5 alpha reductase enzyme.
- Both androgens exert their influence through binding with the androgen receptor.
- Androgen binds with the androgen receptor.
- The androgen receptor is expressed ubiquitously throughout the tissues of the human body.
- Before it binds with an androgen, the androgen receptor is bound to heat shock proteins.
- These heat shock proteins are released upon androgen binding.
- Androgen binding induces a stabilizing, conformational change in the androgen receptor.
- The two zinc fingers of the DNA-binding domain are exposed as a result of this new conformation.
- AR stability is thought to be aided by type II coregulators, which modulate protein folding and androgen binding, or facilitate NH2/carboxyl-terminal interaction.
- The hormone-activated androgen receptor is phosphorylated.
- Receptor phosphorylation can occur before androgen binding, although the presence of androgen promotes hyperphosphorylation.
- The biological ramifications of receptor phosphorylation are unknown.
- The hormone-activated androgen receptor translocates to the nucleus.
- Nucleocytoplasmic transport is in part facilitated by an amino acid sequence on the AR called the nuclear localization signal.
- The AR's nuclear localization signal is primarily encoded in the hinge region of the AR gene.
- Homodimerization occurs.
- Dimerization is mediated by the second (nearest the 3' end) zinc finger.
- DNA binding to regulatory androgen response elements occurs.
- Target genes contain (or are flanked by) transcriptional enhancer nucleotide sequences that interact with the first zinc finger.
- These areas are called androgen response elements.
- Coactivators are recruited by the AR.
- Type I coactivators (i.e., coregulators) are thought to influence AR transcriptional activity by facilitating DNA occupancy, chromatin remodeling, or the recruitment of general transcription factors associated with RNA polymerase II holocomplex.
- Target gene transcription ensues.
In this way, androgens bound to androgen receptors regulate the expression of target genes, and thus produce androgenic effects.
It is theoretically possible for certain mutant androgen receptors to function without androgens; in vitro studies have demonstrated that a mutant androgen receptor protein can induce transcription in the absence of androgen if its steroid binding domain is deleted [135][136]. Conversely, the steroid-binding domain may act to repress the AR transactivation domain, perhaps due to the AR's unliganded conformation [3].

Androgens in Fetal Development
Human embryos develop similarly for the first six weeks, regardless of genetic sex (46,XX or 46,XY karyotype); the only way to tell the difference between 46,XX or 46,XY embryos during this time period is to look for Barr bodies or a Y chromosome [137]. The gonads begin as bulges of tissue called the genital ridges at the back of the abdominal cavity, near the midline. By the fifth week, the genital ridges differentiate into an outer cortex and an inner medulla, and are called indifferent gonads [137]. By the sixth week, the indifferent gonads begin to differentiate according to genetic sex. If the karyotype is 46,XY, testes develop due to the influence of the SRY gene on the Y chromosome [25][26]. This process does not require the presence of androgen, nor a functional androgen receptor [25][26].
Until approximately the seventh week of development, the embryo has indifferent sex accessory ducts, which consist of two pairs of ducts: the Müllerian ducts and the Wolffian ducts [137]. The testes secrete anti-Müllerian hormone around this time to suppress the development of the Müllerian ducts, and cause their degeneration [137]. Without this anti-Müllerian hormone, the Müllerian ducts develop into the female internal genitalia (uterus, cervix, fallopian tubes, and upper vaginal barrel) [137]. Unlike the Müllerian ducts, the Wolffian ducts will not continue to develop by default [31]. In the presence of testosterone and functional androgen receptors, the Wolffian ducts develop into the epididymides, vasa deferentia, and seminal vesicles [137]. If the testes fail to secrete testosterone, or the androgen receptors do not function properly, the Wolffian ducts degenerate [33].

Masculinization of the external genitalia (the penis, penile urethra, and scrotum), as well as the prostate, are dependent on the androgen dihydrotestosterone [17][34][35][36]. Testosterone is converted into dihydrotestosterone by the 5-alpha reductase enzyme [80]. If this enzyme is absent or deficient, then dihydrotestosterone will not be created, and the external male genitalia will not develop properly [17][34][35][36][80]. As is the case with the internal male genitalia, a functional androgen receptor is needed in order for dihydrotestosterone to regulate the transcription of target genes involved in development [65].
Pathogenesis of Androgen Insensitivity Syndrome
Mutations in the androgen receptor gene can cause problems with any of the steps involved in androgenization, from the synthesis of the androgen receptor protein itself, through the transcriptional ability of the dimerized, androgen-AR complex [3]. AIS can result if even one of these steps is significantly disrupted, as each step is required in order for androgens to successfully activate the AR and regulate gene expression [3]. Exactly which steps a particular mutation will impair can be predicted, to some extent, by identifying the area of the AR in which the mutation resides. This predictive ability is primarily retrospective in origin; the different functional domains of the AR gene have been elucidated by analyzing the effects of specific mutations in different regions of the AR [3]. For example, mutations in the steroid binding domain have been known to affect androgen binding affinity or retention, mutations in the hinge region have been known to affect nuclear translocation, mutations in the DNA-binding domain have been known to affect dimerization and binding to target DNA, and mutations in the transactivation domain have been known to affect target gene transcription regulation [3][31]. Unfortunately, even when the affected functional domain is known, it is difficult to predict the phenotypical consequences of a particular mutation (see Correlation of Genotype and Phenotype).
Some mutations can adversely impact more than one functional domain. For example, a mutation in one functional domain can have deleterious effects on another by altering the way in which the domains interact [31]. A single mutation can affect all downstream functional domains if a premature stop codon or framing error results; such a mutation can result in a completely unusable (or unsynthesizable) androgen receptor protein [3]. The steroid binding domain is particularly vulnerable to the effects of a premature stop codon or framing error, since it occurs at the end of the gene, and its information is thus more likely to be truncated or misinterpreted than other functional domains [3].
Other, more complex relationships have been observed as a consequence of mutated AR; some mutations have been linked to male breast cancer, prostate cancer, or in the case of spinal and bulbar muscular atrophy, disease of the central nervous system [8][59][70][88][89]. The form of male breast cancer that is associated with androgen insensitivity syndrome is caused by a mutation in the AR's DNA-binding domain [59][89]. It has been hypothesized that this mutation causes a disturbance of the AR's target gene interaction that allows it to act at certain additional targets, possibly in conjunction with the estrogen receptor protein, to cause cancerous growth [3]. The etiology of spinal and bulbar muscular atrophy (SBMA) demonstrates that even the mutant AR protein itself can result in pathology. The trinucleotide repeat expansion of the polyglutamine tract of the AR gene that is associated with SBMA results in the synthesis of a misfolded AR protein that the cell fails to properly proteolyze and disperse [138]. These misfolded AR proteins form aggregates in the cell cytoplasm and nucleus [138]. Over the course of 30 to 50 years, these aggregates accumulate and have a cytotoxic effect, eventually resulting in the neurodegenerative symptoms associated with SBMA [138].
Diagnosis
The phenotypes that result from the insensitivity to androgens are not unique to AIS, and thus the diagnosis of AIS requires thorough exclusion of other causes [13][125]. Clinical findings indicative of AIS include the presence of a short vagina [22] or undermasculinized genitalia [1][17][54], partial or complete regression of Müllerian structures [18], bilateral nondysplastic testes [85], and impaired spermatogenesis and / or virilization [1][4][5][8]. Laboratory findings include a 46,XY karyotype [2] and normal or elevated postpubertal testosterone, luteinizing hormone, and estradiol levels [2][13]. The androgen binding activity of genital skin fibroblasts is typically diminished [3][139], although exceptions have been reported [56]. Conversion of testosterone to dihydrotestosterone may be impaired [3]. The diagnosis of AIS is confirmed if androgen receptor gene sequencing reveals a mutation, although not all individuals with AIS (particularly PAIS) will have an AR mutation (see Other Causes) [2][5][19][118].
Each of the three types of AIS --- complete, partial, and mild --- has a different list of differential diagnoses to consider [1]. Depending on the form of AIS that is suspected, the list of differentials can include [24][25][26][140][141]:
- Chromosomal anomalies:
- Klinefelter syndrome (47,XXY karyotype)
- Turner syndrome (45,XO karyotype)
- Mixed gonadal dysgenesis (45,XO/46,XY karyotype)
- Tetragametic chimerism (46,XX/46,XY karyotype)
- Androgen biosynthetic dysfunction in 46,XY individuals:
- Luteinizing hormone (LH) receptor mutations
- Smith-Lemli-Opitz syndrome (associated with mental retardation)
- Lipoid congenital adrenal hyperplasia
- 3β-hydroxysteroid dehydrogenase 2 deficiency
- 17α-hydroxylase deficiency
- 17,20 lyase deficiency
- 17β-hydroxysteroid dehydrogenase deficiency
- 5α-reductase deficiency
- Androgen excess in 46,XX individuals:
- 21-hydroxylase deficiency
- 3β-hydroxysteroid dehydrogenase 2 deficiency
- Cytochrome P450 oxidoreductase deficiency (disorder in mother causes 46,XX fetal virilization)
- 11β-hydroxylase deficiency
- Aromatase deficiency
- Glucocorticoid receptor mutations
- Maternal virilizing tumor (e.g. luteoma)
- Increased androgen exposure in utero, not otherwise specified (e.g. androgenic drugs)
- Developmental
- Mayer-Rokitansky-Küster-Hauser syndrome (46,XX karyotype)
- Swyer syndrome (46,XY karyotype)
- XX gonadal dysgenesis (46,XX karyotype)
- Leydig cell agenesis or hypoplasia, not otherwise specified (46,XY karyotype)
- Absent (vanishing) testes syndrome
- Ovotesticular DSD
- Testicular DSD (i.e. 46,XX sex reversal)
- Teratogenic causes (e.g. estrogens, antiestrogens)
- Other causes:
- Frasier syndrome (associated with progressive glomerulopathy)
- Denys-Drash syndrome (associated with nephropathy and Wilms tumor)
- WAGR syndrome (associated with Wilms tumor and aniridia)
- McKusick-Kaufman syndrome (associated with postaxial polydactyly)
- Robinow syndrome (associated with dwarfism)
- Aarskog-Scott syndrome (associated with facial anomalies)
- Hand-foot-genital syndrome (associated with limb malformations)
- Popliteal pterygium syndrome (associated with extensive webbing behind knees)
- Kallmann syndrome (often associated with anosmia)
- Hypospadias not otherwise specified
- Cryptorchidism not otherwise specified
- vaginal atresia not otherwise specified
CAIS

CAIS can only be diagnosed in normal phenotypic females [2]. It is not usually suspected unless the menses fail to develop at puberty, or an inguinal hernia presents during premenarche [1][2]. As many as 1-2% of prepubertal girls that present with an inguinal hernia will also have CAIS [1][18].
A diagnosis of CAIS or Swyer syndrome can be made in utero by comparing a karyotype obtained by amniocentesis with the external genitalia of the fetus during a prenatal ultrasound [2][143]. Many infants with CAIS do not experience the normal, spontaneous neonatal testosterone surge, a fact which can be diagnostically exploited by obtaining baseline luteinizing hormone and testosterone measurements, followed by a human chorionic gonadotropin (hGC) stimulation test [1].
The main differentials for CAIS are complete gonadal dysgenesis (Swyer syndrome) and Müllerian agenesis (Mayer-Rokitansky-Kuster-Hauser syndrome or MRKH) [1][24]. Both CAIS and Swyer syndrome are associated with a 46,XY karyotype, whereas MRKH is not; MRKH can thus be ruled out by checking for the presence of a Y chromosome, which can be done either by fluorescence in situ hybridization (FISH) analysis or on full karyotype [1]. Swyer syndrome is distinguished by poor breast development and shorter stature [1]. The diagnosis of CAIS is confirmed when AR gene sequencing reveals a mutation, although up to 5% of individuals with CAIS do not have an AR mutation [2].
Up until the 1990s, a CAIS diagnosis was often hidden from the affected individual and / or family [17]. It is current practice to disclose the genotype at the time of diagnosis, particularly when the affected girl is at least of adolescent age [17]. If the affected individual is a child or infant, it is generally up to the parents, often in conjunction with a psychologist, to decide when to disclose the diagnosis [17].
PAIS
Unfortunately, the number of differentials to consider for PAIS is particularly large [1]. Prompt diagnosis is particularly urgent when a child is born with ambiguous genitalia, as some causes are associated with potentially life-threatening adrenal crises [25]. Determination of testosterone, testosterone precursors and dihydrotestosterone (DHT) at baseline and / or after human chorionic gonadotropin (hCG) stimulation can be used to exclude such defects in androgen biosynthesis [2].
Approximately one half of all 46,XY individuals born with ambiguous genitalia will not receive a definitive diagnosis [144]. Androgen receptor (AR) gene mutations cannot be found in 27% [5][19] to 72% [118] of individuals with PAIS. As a result, genetic analysis can be used to confirm a diagnosis of PAIS, but it cannot be used to rule out PAIS [140]. Evidence of abnormal androgen binding in a genital skin fibroblast study has long been the gold standard for the diagnosis of PAIS [3][139], even when an AR mutation is not present [144]. However, some cases of PAIS, including AR-mutant-positive cases [56], will show normal androgen binding. A family history consistent with X-linked inheritance is more commonly found in AR-mutant-positive cases than AR-mutant-negative cases [140].
The use of dynamic endocrine tests is particularly helpful in isolating a diagnosis of PAIS [1][13]. One such test is the human chorionic gonadotropin (hCG) stimulation test. If the gonads are testes, there will be an increase in the level of serum testosterone in response to the hCG, regardless of testicular descent [1]. The magnitude of the testosterone increase can help differentiate between androgen resistance and gonadal dysgenesis, as does evidence of a uterus on ultrasound examination [1]. Testicular function can also be assessed by measuring serum anti-Müllerian hormone levels, which in turn can further differentiate PAIS from gonadal dysgenesis and bilateral anorchia [1].
Another useful dynamic test involves measuring the response to exogenous steroids; individuals with AIS show a decreased response in serum sex hormone binding globulin (SHBG) after a short term administration of anabolic steroids [21][145]. Two studies [21][145] indicate that measuring the response in SHBG after the administration of stanozolol could help to differentiate individuals with PAIS from those with other causes of ambiguous genitalia, although the response in individuals with predominantly male phenotypes overlaps somewhat with the response in normal males.
MAIS
MAIS is only diagnosed in normal phenotypic males, and is not typically investigated except in cases of male infertility [17]. MAIS has a mild presentation that often goes unnoticed and untreated [63]; even with semenological, clinical and laboratory data, it can be difficult to distinguish between men with and without MAIS, and thus a diagnosis of MAIS is not usually made without confirmation of an AR gene mutation [4]. The androgen sensitivity index (ASI), defined as the product of luteinizing hormone (LH) and testosterone (T), is frequently raised in individuals with all forms of AIS, including MAIS, although many individuals with MAIS have an ASI in the normal range [4]. Testosterone levels may be elevated despite normal levels of luteinizing hormone [63][66][69]. Conversion of testosterone (T) to dihydrotestosterone (DHT) may be impaired, although to a lesser extent than is seen in 5α-reductase deficiency [3]. A high ASI in a normal phenotypic male [102], especially when combined with azoospermia or oligospermia [4][6], decreased secondary terminal hair [71], and / or impaired conversion of T to DHT [3], can be indicative of MAIS, and may warrant genetic testing.
Management
Management of AIS is currently limited to symptomatic management; methods to correct a malfunctioning androgen receptor protein that result from an AR gene mutation are not currently available. Areas of management include sex assignment, genitoplasty, gonadectomy in relation to tumor risk, hormone replacement therapy, and genetic and psychological counseling.
CAIS
Individuals with CAIS are raised as females [1]. They are born phenotypically female and almost always have a heterosexual female gender identity [39][146]; the incidence of homosexuality in women with CAIS is thought to be less than unaffected women [147]. At least one case study exists that describes an individual with CAIS and a male gender identity, although its author suggests that cultural and societal circumstances may have exerted undue influence [146].

Most cases of vaginal hypoplasia associated with CAIS can be corrected using non-surgical pressure dilation methods [22][24]. The elastic nature of vaginal tissue, as demonstrated by its ability to accommodate the differences in size between a tampon, a penis, and a baby's head [148], make dilation possible even in cases when the vaginal depth is significantly compromised [22][24]. Treatment compliance is thought to be critical to achieve satisfactory results [20][22][24]. Dilation can also be achieved via the Vecchietti procedure, which stretches vaginal tissues into a functional vagina using a traction device that is anchored to the abdominal wall, subperitoneal sutures, and a mold that is placed against the vaginal dimple [24]. Vaginal stretching occurs by increasing the tension on the sutures, which is performed daily [24]. The non-operative pressure dilation method is currently recommended as the first choice, since it is non-invasive, and highly successful [24]. Vaginal dilation should not be performed before puberty [32].
While it is recommended that women with CAIS eventually undergo gonadectomy to mitigate cancer risk, there are differing opinions regarding early versus late gonadectomy [1]. The risk of malignant germ cell tumors in women with CAIS increases with age and has been estimated to be 3.6% at 25 years and 33% at 50 years [87]. However, only three cases of malignant germ cell tumors in prepubescent girls with CAIS have been reported in the last 100 years [85]. The youngest of these girls was 14 years old [149]. If gonadectomy is performed early, then puberty must be artificially induced using gradually increasing doses of estrogen [1]. If gonadectomy is performed late, then puberty will occur on its own, due to the aromatization of testosterone into estrogen [1]. Some choose to perform gonadectomy if and when inguinal hernia presents [1]. Postoperative estrogen replacement therapy is critical to minimize bone mineral density deficiencies later in life [81][83].
Some have hypothesized that supraphysiological levels of estrogen may reduce the diminished bone mineral density associated with CAIS [81]. Data has been published that suggests affected women who were not compliant with estrogen replacement therapy, or who had a lapse in estrogen replacement, experienced a more significant loss of bone mineral density [80][81]. Progestin replacement therapy is seldom initiated, due to the absence of a uterus [1]. Androgen replacement has been reported to increase a sense of well-being in gonadectomized women with CAIS, although the mechanism by which this benefit is achieved is not well understood [1].
It is no longer common practice to hide a diagnosis of CAIS from the affected individual or her family [17]. Parents of children with CAIS need considerable support in planning and implementing disclosure for their child once the diagnosis has been established [1][17]. For parents with young children, information disclosure is an ongoing, collaborative process requiring an individualized approach that evolves in concordance with the child's cognitive and psychological development [1]. In all cases, the assistance of a psychologist experienced in the subject is recommended [1][17].
Neovaginal Construction
Many surgical procedures have been developed to create a neovagina, as none of them is ideal [24]. Surgical intervention should only be considered after non-surgical pressure dilation methods have failed to produce a satisfactory result [24]. Neovaginoplasty can be performed using skin grafts, a segment of bowel, ileum, peritoneum, Interceed, buccal mucosa, amnion, or dura mater [24][150][151]. Success of such methods should be determined by sexual function, and not just by vaginal length, as has been done in the past [151]. Ileal or cecal segments may be problematic because of a shorter mesentery, which may produce tension on the neovagina, leading to stenosis [151]. The sigmoid neovagina is thought to be self-lubricating, without the excess mucus production associated with segments of small bowel [151]. Vaginoplasty may create scarring at the introitus (the vaginal opening), which requires additional surgery to correct. Vaginal dilators are required postoperatively to prevent vaginal stenosis from scarring [22][24]. Other complications include bladder and bowel injuries [24]. Yearly exams are required as neovaginoplasty carries a risk of carcinoma [24], although carcinoma of the neovagina is uncommon [150][151]. Neither neovaginoplasty nor vaginal dilation should be performed before puberty [24][32].
PAIS
The decision of whether to raise an individual with PAIS as a boy or a girl may not be obvious; grades 3 and 4 in particular present with a phenotype that may be difficult to classify as primarily male or female, and some will be incapable of virilization at puberty [1][55][58]. Parents of an affected newborn should seek immediate help at a center with an experienced multidisciplinary team, and should avoid gender assignment beforehand [32]. Gender assignment should thereafter be expeditiously decided; current guidelines advise against waiting for the child to decide for his / herself [32]. Key considerations involved in assigning gender include the appearance of the genitalia [32], the extent to which the child can virilize at puberty [2], surgical options and the postoperative sexual function of the genitalia [19][22][152], genitoplasty complexity [32], potential for fertility [32], and the projected gender identity of the child [153]. The majority of individuals with PAIS are raised male [1].
Virilization capacity can be assessed by measuring the response to a trial of exogenous androgens; some studies have measured the growth of the phallus in response to exogenous testosterone [58] or dihydrotestosterone [78], while others have measured the change in sex hormone binding globulin (SHBG) in response to the artificial androgen stanozolol to assess androgen sensitivity [21][145]. Some experts have cautioned that it remains to be proved that a good response to exogenous androgens in neonates is a good predictor of androgen response at puberty [2]. If a mutation in the AR gene is found, it is important to determine if the mutation is inherited or de novo (i.e. a somatic mutation); a certain amount of the wild-type androgen receptor will be present in cases of somatic mutation, which can induce virilization at puberty [58]. A genital skin fibroblast study [3][139] and a human chorionic gonadotropin (hCG) stimulation test [13] may also provide information helpful in the assessment of virilization capacity.
Psychosexual development is influenced by many factors, including the timing, amount, and type of androgen exposure, receptor functionality, and environment, and is thus difficult to predict [152][153][154][155][156][157]. Gender identity begins to develop before 3 years of age [158], although the earliest age at which it can be reliably assessed has yet to be determined [32]. Approximately 25% of individuals with PAIS are dissatisfied with their assigned gender, regardless of being raised as male or female [51]. One study reports that 46,XY individuals born with micropenis and no hypospadias are better off being raised male, despite the success of some being raised female [159]. Studies involving the more ambiguous phenotypic forms of PAIS are less decisive [51][55]. Homosexuality with respect to assigned gender [17] and atypical gender role behavior [32] are known to occur more frequently in individual with PAIS, and may occur with or without gender dysphoria; neither should be interpreted as an indication of incorrect gender assignment [32]. If an affected child does express feelings of gender dysphoria, the opportunity to explore such feelings with a psychologist experienced in treating intersex conditions should be accommodated [32]. If feelings of gender dysphoria persist, gender reassignment should be initiated, possibly with the aid of a specialist in the field [32].

Genitoplasty, unlike gender assignment, can be irreversible [140], and there is no guarantee that adult gender identity will develop as assigned despite surgical intervention [152]. Some aspects of genitoplasty are still being debated; a variety of different opinions have been presented by professionals, self-help groups, and patients over the last few decades [2][160]. Points of consideration include what conditions justify genitoplasty, the extent and type of genitoplasty that should be employed, when genitoplasty should be performed, and what the goals of genitoplasty should be [32][51][152][153][161]. Gender assignment itself does not predicate the need for immediate genitoplasty; in some cases, surgical intervention can be delayed to allow the affected child to reach an age and maturity sufficient to have a role in such decisions [140]. Some studies suggest that early surgeries can still produce satisfactory outcomes [51][162], while others suggest it to be unlikely [161]. Even surgeries that are planned as one-stage procedures often require further major surgery [161]. Scarring and tissue loss that result from repeated surgical procedures are of particular concern, due to the presumed negative impact on sexual function [51].
While it is thought that feminizing genitoplasty typically requires fewer surgeries to achieve an acceptable result and results in fewer urologic difficulties [51], there is no evidence that feminizing surgery results in a better psychosocial outcome [152]. In one study [51], individuals with grade 3 PAIS that were raised male rated their body image and sexual function similarly to those that were raised female, even though they were more likely to have genitalia that was abnormal in size and appearance; more than half of the male participants had a stretched penile length that was below 2.5 standard deviations of the mean, while only 6% of female participants presented with a short vagina in adulthood, and participating physicians gave a lower cosmetic rating to the surgical results of the men than the women. Both male and female participants cited the appearance of their genitalia as being the greatest contributing factor to their dissatisfaction with their body image. In two larger studies [163][164], the common predictor of gender reassignment was stigmatization related to having an intersex condition.
The outcome of masculinizing genitoplasty is dependent on the amount of erectile tissue and the extent of hypospadias [32]. Procedures include correction of penile curvature and chordee, reconstruction of the urethra, hypospadias correction, orchidopexy, and Müllerian remnant removal to prevent infection and pseudo-incontinence [1][165]. Erectile prosthesis may be inserted in cases of successful neophalloplasty in adulthood, although it has a high morbidity [32]. Additional surgeries may be required to correct postsurgical complications such as stenosis of the anastomosis between the native urethra and the graft, urethral fistulas, and posterior displacement of the balanic meatus [165]. Successful masculinizing genitoplasty performed on individuals with grade 3 PAIS often requires multiple surgeries [51].
If feminizing genitoplasty is performed in infancy, the result will need to be refined at puberty through additional surgery [166]. Procedures include clitoral reduction / recession, labiaplasty, repair of the common urogenital sinus, vaginoplasty, and vaginal dilation through non-surgical pressure methods [24][32][152][166]. Clitoral reduction / recession surgery carries with it the risk of necrosis [166] as well as the risk of impairing the sexual function of the genitalia [152], and thus should not be performed for less severe clitoromegaly [32]. Clitoral surgery should be focused on function rather than appearance, with care being taken to spare the erectile function and innervation of the clitoris [32]. If PAIS presents with a common urogenital sinus, the American Academy of Pediatrics currently recommends that surgery to separate the urethra from the vagina be performed at an early age [167]. As is the case for CAIS, vaginal dilation using pressure dilation methods should be attempted before the surgical creation of a neovagina is considered, and neither should be performed before puberty [24][32]. Complications of feminizing genitoplasty can include vaginal stenosis, meatal stenosis, vaginourethral fistula, female hypospadias, urinary tract injuries, and recurrent clitoromegaly [24][151]. Successful feminizing genitoplasty performed on individuals with grade 3 PAIS often requires multiple surgeries, although more surgeries are typically required for successful masculinizing genitoplasty in this population [51].
Gonadectomy at time of diagnosis is the current recommendation for PAIS if presenting with cryptorchidism, due to the high (50%) risk of germ cell malignancy [32]. The risk of malignancy when testes are located intrascrotally is unknown; the current recommendation is to biopsy the testes at puberty, allowing investigation of at least 30 seminiferous tubules, with diagnosis preferably based on OCT3/4 immunohistochemistry, followed by regular examinations [32]. Hormone replacement therapy is required after gonadectomy, and should be modulated over time to replicate the hormone levels naturally present in the body during the various stages of puberty [32]. Artificially induced puberty results in the same, normal development of secondary sexual characteristics, growth spurt, and bone mineral accumulation [32]. Women with PAIS may have a tendency towards bone mineralization deficiency, although this increase is thought to be less than is typically seen in CAIS, and is similarly managed [79].
Testosterone has been used to successfully treat undervirilization in some [46] but not all [168] men with PAIS, despite having supraphysiological levels of testosterone to start with [46][63]. Treatment options include transdermal gels or patches, oral or injectable testosterone undecanoate, other injectable testosterone esters, testosterone pellets, or buccal testosterone systems [169]. Supraphysiological doses may be required to achieve the desired physiological effect [32][46][124], which may be difficult to achieve using non-injectable testosterone preparations. Exogenous testosterone supplementation in unaffected men can produce various unwanted side effects, including prostatic hypertrophy, polycythemia, gynecomastia, hair loss, acne, and the suppression of the hypothalamic-pituitary-gonadal axis, which results in the reduction of gonadotropins (i.e., luteinizing hormone and follicle-stimulating hormone) and spermatogenic defect [170][171]. These effects may not manifest at all in men with AIS, or might only manifest at a much higher concentration of testosterone, depending on the degree of androgen insensitivity [46][63][168]. Those undergoing high dose androgen therapy should be monitored for safety and efficacy of treatment, possibly including regular breast [46] and prostate [170] examinations. Some individuals with PAIS have a sufficiently high sperm count to father children; at least one case report has been published that describes fertile men that fit the criteria for grade 2 PAIS (micropenis, penile hypospadias, and gynecomastia) [62]. Several publications have indicated that testosterone treatment can correct low sperm counts in men with MAIS [1][124]. At least one case report has been published that documents the efficacy of treating a low sperm count with tamoxifen in an individual with PAIS [172].
Depending on phenotypic features, impotence and other sexual problems such as anejaculation or sexual aversion may be fairly common among individuals with PAIS [50][19][55][56][57], but do not necessarily indicate low libido [32][55]. Support groups for individuals with PAIS may help affected individuals discuss their concerns more comfortably [32]. Some individuals with PAIS may try to avoid intimate relationships out of fear of rejection; individual therapy may help some to overcome social anxiety, and restore focus to interpersonal relationships instead of solely on sexual function and activity [32].
MAIS
Due to its mild presentation, MAIS often goes unnoticed and untreated [63]. Treatment for MAIS is limited to surgical correction of mild gynecomastia, minor hypospadias repair, and testosterone supplementation [1][63][124]. Supraphysiological doses of testosterone have been shown to correct diminished secondary sexual characteristics in men with MAIS [63], as well as to reverse infertility due to low sperm count [32][124]. As is the case with PAIS, men with MAIS will experience side effects from androgen therapy (such as the suppression of the hypothalamic-pituitary-gonadal axis) at a higher dosage than unaffected men. Careful monitoring is required to ensure the safety and efficacy of treatment [46][63][168]. Regular breast [46] and prostate [170] examinations may be necessary due to comorbid association with breast and prostate cancers.
Epidemiology
Estimates for the incidence of androgen insensitivity syndrome are based on a relatively small population size, and thus are known to be imprecise [1]. CAIS is estimated to occur in 1 out of every 20,400 46,XY births [173]. A nationwide survey in The Netherlands based on patients with genetic confirmation of the diagnosis estimates that the minimal incidence of CAIS is 1 in 99,000 [50]. The incidence of PAIS is estimated to be 1 in 130,000 [174]. Due to its subtle presentation, MAIS is not typically investigated except in the case of male infertility [17], and thus its true prevalence is unknown [2].
History
Recorded descriptions of the effects of androgen insensitivity syndrome date back for hundreds of years, although significant understanding of its underlying histopathology would not occur until the 1950s [1]. The taxonomy and nomenclature associated with androgen insensitivity went through a significant evolution that paralleled this understanding.
Timeline of Major Milestones
- 1950: Lawson Wilkins administers daily methyltestosterone to a 46,XY female patient, who shows no signs of virilization. His experiment is the first documented demonstration of the pathophysiology of androgen insensitivity syndrome [125][175].
- 1970: Mary F. Lyon and Susan Hawkes report that a gene on the X chromosome caused complete insensitivity to androgens in mice [176][177].
- 1981: Barbara Migeon et al. narrow down the locus of the human androgen receptor gene (or a factor controlling the androgen receptor gene) to somewhere between Xq11 and Xq13 [178][179].
- 1988: The human androgen receptor gene is first cloned and partially analyzed by multiple parties [180][181]. Terry Brown et al. report the first mutations proven to cause AIS [2][179].
- 1989: Terry Brown et al. reports the exact locus of the AR gene (Xq11-Xq12) [91], and Dennis Lubahn et al. publishes its intron-exon boundaries [182].
- 1994: The androgen receptor gene mutations database is created to provide a comprehensive listing of mutations published in journals and conference proceedings [183].
Early Terminology
The first descriptions of the effects of androgen insensitivity appeared in the medical literature as individual case reports or as part of a comprehensive description of intersex physicalities. In 1839, Scottish obstetrician Sir James Young Simpson published one such description [184] in an exhaustive study of intersexuality that has been credited with advancing the medical community's understanding of the subject [185]. Simpson's system of taxonomy, however, was far from the first; taxonomies / descriptions for the classification of intersexuality were developed by Italian physician and physicist Fortuné Affaitati in 1549 [186][187], French surgeon Ambroise Paré in 1573 [185][188], French physician and sexology pioneer Nicolas Venette in 1687 (under the pseudonym Vénitien Salocini) [189][190], and French Zoologist Isidore Geoffroy St. Hilaire in 1832 [191]. All five of the aforementioned authors used the colloquial term "hermaphrodite" as the foundation of their taxonomies, although Simpson himself questioned the propriety of the word in his publication [184]. Use of the word "hermaphrodite" in the medical literature has persisted to this day [192][193], although its propriety is still in question. An alternative system of nomenclature has been recently suggested [32], but the subject of exactly which word or words should be used in its place still one of much debate [140][194][195][196][197].

Pseudohermaphroditism
"Pseudohermaphroditism" has, until very recently [32], been the term used in the medical literature to describe the condition of an individual whose gonads and karyotype do not match the external genitalia in the gender binary sense. For example, 46,XY individuals that have a female phenotype, but also have testes instead of ovaries --- a group that includes all individuals with complete androgen insensitivity (CAIS), as well as some individuals with partial androgen insensitivity (PAIS) --- are classified as having "male pseudohermaphroditism," while individuals with both an ovary and a testis (or at least one ovotestis) are classified as having "true hermaphroditism." [32][193]. Usage of the word in the medical literature predates the discovery of the chromosome, and thus its definition has not always taken karyotype into account when determining an individual's sex. Previous definitions of "pseudohermaphroditism" relied on perceived inconsistencies between the internal and external organs; the "true" sex of an individual was determined by the internal organs, and the external organs determined the "perceived" sex of an individual [184][191].
German-Swiss pathologist Edwin Klebs is sometimes noted for using the word "pseudohermaphroditism" in his taxonomy of intersexuality in 1876 [199], although the word is clearly not his invention as is sometimes reported; the history of the word "pseudohermaphrodite," and the corresponding desire to separate "true" hermaphrodites from "false," "spurious," or "pseudo" hermaphrodites, dates back to at least 1709, when Dutch anatomist Frederik Ruysch used it in a publication describing a subject with testes and a mostly female phenotype [198]. "Pseudohermaphrodite" also appeared in the Acta Eruditorum later that same year, in a review of Ruysch's work [200]. There is also evidence that the word was already being used by the German and French medical community long before Klebs used it; German physiologist Johannes Peter Müller equated "pseudohermaphroditism" with a sub-class of hermaphroditism from St. Hilaire's taxonomy in a publication dated 1834 [201], and by the 1840s "pseudo-hermaphroditism" was appearing in several French and German publications, including dictionaries [202][203][204][205].
Testicular Feminization
In 1953, American gynecologist John Morris provided the first full description of what he called "testicular feminization syndrome" based on 82 cases compiled from the medical literature, including 2 of his own patients [1][3][28]. The term "testicular feminization" was coined to reflect Morris' observation that the testicles in these patients produced a hormone that had a feminizing effect on the body, a phenomenon that is now understood to be due to the inaction of androgens, and subsequent aromatization of testosterone into estrogen [1]. A few years before Morris published his landmark paper, Lawson Wilkins had shown through his own experiments that unresponsiveness of the target cell to the action of androgenic hormones was a cause of "male pseudohermaphroditism" [125][175]. Wilkins' work, which clearly demonstrated the lack of a therapeutic effect when 46,XY women were treated with androgens, caused a gradual shift in nomenclature from "testicular feminization" to "androgen resistance" [17].
Other Names
A distinct name has been given to many of the various presentations of androgen insensitivity syndrome, such as Reifenstein syndrome (1947) [206], Goldberg-Maxwell syndrome (1948) [207], Morris' syndrome (1953) [28], Gilbert-Dreyfus syndrome (1957) [208], Lub's syndrome (1959) [209], "incomplete testicular feminization" (1963) [210], Rosewater syndrome (1965) [211], and Aiman's syndrome (1979) [212]. Since it was not understood that these different presentations were all caused by the same set of mutations in the androgen receptor gene, a unique name was given to each new combination of symptoms, resulting in a complicated stratification of seemingly disparate disorders [125][213].
Over the last 60 years, as reports of strikingly different phenotypes were reported to occur even among members of the same family, and as steady progress was made towards the understanding of the underlying molecular pathogenesis of AIS, it has been demonstrated that these disorders are different phenotypic expressions of one syndrome caused by molecular defects in the androgen receptor gene [1][12][125][213].
Androgen insensitivity syndrome (AIS) is now the accepted terminology for the syndromes resulting from unresponsiveness of the target cell to the action of androgenic hormones [1]. AIS is broken down into three classes based on phenotype: complete androgen insensitivity syndrome (CAIS), partial androgen insensitivity syndrome (PAIS), and mild androgen insensitivity syndrome (MAIS) [1][2][4][5][6][9][10][11][12]. CAIS encompasses the phenotypes previously described by "testicular feminization," Morris' syndrome, and Goldberg-Maxwell syndrome [1][214]; PAIS includes Reifenstein syndrome, Gilbert-Dreyfus syndrome, Lub's syndrome, "incomplete testicular feminization," and Rosewater syndrome [213][215][216]; and MAIS includes Aiman's syndrome [43].
The more virilized phenotypes of AIS have sometimes been described as "undervirilized male syndrome," "infertile male syndrome," "undervirilized fertile male syndrome," etc., before evidence was reported that these conditions were caused by mutations in the androgen receptor gene [68]. These diagnoses were used to describe a variety of mild defects in virilization; as a result, the phenotypes of some men that have been diagnosed as such are better described by PAIS (e.g. micropenis, hypospadias and undescended testes), while others are better described by MAIS (e.g. isolated infertility or gynecomastia) [1][62][68][77][216][217].
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx Hughes IA, Deeb A (2006). "Androgen resistance". Best Pract. Res. Clin. Endocrinol. Metab. 20 (4): 577–98. doi:10.1016/j.beem.2006.11.003. PMID 17161333.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae Galani A, Kitsiou-Tzeli S, Sofokleous C, Kanavakis E, Kalpini-Mavrou A (2008). "Androgen insensitivity syndrome: clinical features and molecular defects". Hormones (Athens). 7 (3): 217–29. PMID 18694860.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS (1995). "Androgen receptor defects: historical, clinical, and molecular perspectives". Endocr. Rev. 16 (3): 271–321. PMID 7671849.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l m Zuccarello D, Ferlin A, Vinanzi C, Prana E, Garolla A, Callewaert L, Claessens F, Brinkmann AO, Foresta C. Detailed functional studies on androgen receptor mild mutations demonstrate their association with male infertility. Clin Endocrionol. 2008;68:580-588.
- ^ a b c d e f g h i j k l m n Ferlin A, Vinanzi C, Garolla A, Selice R, Zuccarello D, Cazzadore C, Foresta C (2006). "Male infertility and androgen receptor gene mutations: clinical features and identification of seven novel mutations". Clin. Endocrinol. (Oxf). 65 (5): 606–10. doi:10.1111/j.1365-2265.2006.02635.x. PMID 17054461.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Stouffs K, Tournaye H, Liebaers I, Lissens W (2009). "Male infertility and the involvement of the X chromosome". Hum. Reprod. Update. 15 (6): 623–37. doi:10.1093/humupd/dmp023. PMID 19515807.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Giwercman YL, Nikoshkov A, Byström B, Pousette A, Arver S, Wedell A (2001). "A novel mutation (N233K) in the transactivating domain and the N756S mutation in the ligand binding domain of the androgen receptor gene are associated with male infertility". Clin. Endocrinol. (Oxf). 54 (6): 827–34. PMID 11422119.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f Lund A, Juvonen V, Lähdetie J, Aittomäki K, Tapanainen JS, Savontaus ML (2003). "A novel sequence variation in the transactivation regulating domain of the androgen receptor in two infertile Finnish men". Fertil. Steril. 79 Suppl 3: 1647–8. PMID 12801573.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Ozülker T, Ozpaçaci T, Ozülker F, Ozekici U, Bilgiç R, Mert M (2010). "Incidental detection of Sertoli-Leydig cell tumor by FDG PET/CT imaging in a patient with androgen insensitivity syndrome". Ann Nucl Med. 24 (1): 35–9. doi:10.1007/s12149-009-0321-x. PMID 19957213.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Davis-Dao CA, Tuazon ED, Sokol RZ, Cortessis VK (2007). "Male infertility and variation in CAG repeat length in the androgen receptor gene: a meta-analysis". J. Clin. Endocrinol. Metab. 92 (11): 4319–26. doi:10.1210/jc.2007-1110. PMID 17684052.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Kawate H, Wu Y, Ohnaka K, Tao RH, Nakamura K, Okabe T, Yanase T, Nawata H, Takayanagi R (2005). "Impaired nuclear translocation, nuclear matrix targeting, and intranuclear mobility of mutant androgen receptors carrying amino acid substitutions in the deoxyribonucleic acid-binding domain derived from androgen insensitivity syndrome patients". J. Clin. Endocrinol. Metab. 90 (11): 6162–9. doi:10.1210/jc.2005-0179. PMID 16118342.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i Gottlieb B, Lombroso R, Beitel LK, Trifiro MA (2005). "Molecular pathology of the androgen receptor in male (in)fertility". Reprod. Biomed. Online. 10 (1): 42–8. PMID 15705293.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Ahmed SF, Cheng A, Hughes IA (1999). "Assessment of the gonadotrophin-gonadal axis in androgen insensitivity syndrome". Arch. Dis. Child. 80 (4): 324–9. PMC 1717906. PMID 10086936.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sultan C, Paris F, Terouanne B, Balaguer P, Georget V, Poujol N, Jeandel C, Lumbroso S, Nicolas JC (2001). "Disorders linked to insufficient androgen action in male children". Hum. Reprod. Update. 7 (3): 314–22. PMID 11392378.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cite error: The named reference
1976 simpsonwas invoked but never defined (see the help page). - ^ a b c Gilbert SF (2000). Developmental biology. Sunderland, Mass: Sinauer Associates. ISBN 0-87893-243-7.
- ^ a b c d e f g h i j k l m n o p q r Oakes MB, Eyvazzadeh AD, Quint E, Smith YR (2008). "Complete androgen insensitivity syndrome--a review". J Pediatr Adolesc Gynecol. 21 (6): 305–10. doi:10.1016/j.jpag.2007.09.006. PMID 19064222.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Nichols JL, Bieber EJ, Gell JS. Case of sisters with complete androgen insensitivity syndrome and discordant Müllerian remnants. Fertil Steril. 2009;91:932e15-e18.
- ^ a b c d e f g h Melo KF, Mendonca BB, Billerbeck AE, Costa EM, Inácio M, Silva FA, Leal AM, Latronico AC, Arnhold IJ (2003). "Clinical, hormonal, behavioral, and genetic characteristics of androgen insensitivity syndrome in a Brazilian cohort: five novel mutations in the androgen receptor gene". J. Clin. Endocrinol. Metab. 88 (7): 3241–50. PMID 12843171.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Minto CL, Liao KL, Conway GS, Creighton SM (2003). "Sexual function in women with complete androgen insensitivity syndrome". Fertil. Steril. 80 (1): 157–64. PMID 12849818.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Sinnecker GH, Hiort O, Nitsche EM, Holterhus PM, Kruse K (1997). "Functional assessment and clinical classification of androgen sensitivity in patients with mutations of the androgen receptor gene. German Collaborative Intersex Study Group". Eur. J. Pediatr. 156 (1): 7–14. PMID 9007482.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i Ismail-Pratt IS, Bikoo M, Liao LM, Conway GS, Creighton SM (2007). "Normalization of the vagina by dilator treatment alone in Complete Androgen Insensitivity Syndrome and Mayer-Rokitansky-Kuster-Hauser Syndrome". Hum. Reprod. 22 (7): 2020–4. doi:10.1093/humrep/dem074. PMID 17449508.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Weber AM, Walters MD, Schover LR, Mitchinson A. Vaginal anatomy and sexual function. Obstet Gynaecol. 1995;86:946–949.
- ^ a b c d e f g h i j k l m n o p q r s t u Quint EH, McCarthy JD, Smith YR (2010). "Vaginal surgery for congenital anomalies". Clin Obstet Gynecol. 53 (1): 115–24. doi:10.1097/GRF.0b013e3181cd4128. PMID 20142648.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Achermann JC, Jameson JL (2006). "Disorders of sexual differentiation". In Hauser SL, Kasper DL, Fauci AS, Braunwald E, Longo DL (ed.). Harrison's endocrinology. New York: McGraw-Hill Medical Pub. Division. pp. 161–172. ISBN 0-07-145744-5.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ a b c d e f Simpson JL, Rebar RW (2002). Hung, Wellington; Becker, Kenneth L.; Bilezikian, John P.; William J Bremner (ed.). Principles and Practice of Endocrinology and Metabolism. Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 852–885. ISBN 0-7817-4245-5.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ a b Decaestecker K, Philibert P, De Baere E, Hoebeke P, Kaufman JM, Sultan C, T'Sjoen G (2008). "A novel mutation c.118delA in exon 1 of the androgen receptor gene resulting in complete androgen insensitivity syndrome within a large family". Fertil. Steril. 89 (5): 1260.e3–7. doi:10.1016/j.fertnstert.2007.04.057. PMID 17714709.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Morris JM (1953). "The syndrome of testicular feminization in male pseudohermaphrodites". Am. J. Obstet. Gynecol. 65 (6): 1192–1211. PMID 13057950.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Müller J (1984). "Morphometry and histology of gonads from twelve children and adolescents with the androgen insensitivity (testicular feminization) syndrome". J. Clin. Endocrinol. Metab. 59 (4): 785–9. PMID 6480805.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Johnston DS, Russell LD, Friel PJ, Griswold MD (2001). "Murine germ cells do not require functional androgen receptors to complete spermatogenesis following spermatogonial stem cell transplantation". Endocrinology. 142 (6): 2405–8. PMID 11356688.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Yong EL, Loy CJ, Sim KS. Androgen receptor gene and male infertility. Hum Reprod Update. 2003;9:1-7.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak Hughes IA, Houk C, Ahmed SF, Lee PA (2006). "Consensus statement on management of intersex disorders". Arch. Dis. Child. 91 (7): 554–63. doi:10.1136/adc.2006.098319. PMC 2082839. PMID 16624884.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Hannema SE, Scott IS, Hodapp J, Martin H, Coleman N, Schwabe JW, Hughes IA (2004). "Residual activity of mutant androgen receptors explains wolffian duct development in the complete androgen insensitivity syndrome". J. Clin. Endocrinol. Metab. 89 (11): 5815–22. doi:10.1210/jc.2004-0709. PMID 15531547.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B (1999). "Regulation of androgen action". Vitam. Horm. 55: 309–52. PMID 9949684.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Kokontis JM, Liao S (1999). "Molecular action of androgen in the normal and neoplastic prostate". Vitam. Horm. 55: 219–307. PMID 9949683.
- ^ a b c d Rajender S, Gupta NJ, Chakrabarty B, Singh L, Thangaraj K (2009). "Ala 586 Asp mutation in androgen receptor disrupts transactivation function without affecting androgen binding". Fertil. Steril. 91 (3): 933.e23–8. doi:10.1016/j.fertnstert.2008.10.041. PMID 19062009.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Chen CP, Chen SR, Wang TY, Wang W, Hwu YM (1999). "A frame shift mutation in the DNA-binding domain of the androgen receptor gene associated with complete androgen insensitivity, persistent müllerian structures, and germ cell tumors in dysgenetic gonads". Fertil. Steril. 72 (1): 170–3. PMID 10428170.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Papadimitriou DT, Linglart A, Morel Y, Chaussain JL (2006). "Puberty in subjects with complete androgen insensitivity syndrome". Horm. Res. 65 (3): 126–31. doi:10.1159/000091592. PMID 16491011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Wisniewski AB, Migeon CJ, Meyer-Bahlburg HFL, Gearhart JP, Berkovitz GD, Brown TR, Money J. Complete androgen insensitivity syndrome: long-term medical, surgical, and psychosexual outcome. J Clin Endocrinol Metab. 2000;85;2664-2669.
- ^ Varrela J, Alvesalo L, Vinkka H. Body size and shape in 46,XY females with complete testicular feminization. Ann Hum Biol. 1984;11:291-301.
- ^ Alvesalo L, Varrela J (1980). "Permanent tooth sizes in 46,XY females". Am. J. Hum. Genet. 32 (5): 736–42. PMC 1686090. PMID 7424913.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Pietilä K, Grön M, Alvesalo L (1997). "The craniofacial complex in karyotype 46,XY females". Eur J Orthod. 19 (4): 383–9. PMID 9308259.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Sultan C, Lumbroso S, Paris F, Jeandel C, Terouanne B, Belon C, Audran F, Poujol N, Georget V, Gobinet J, Jalaguier S, Auzou G, Nicolas JC (2002). "Disorders of androgen action". Semin. Reprod. Med. 20 (3): 217–28. doi:10.1055/s-2002-35386. PMID 12428202.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Zachman M, Prader A, Sobel EH, Crigler JF Jr, Ritzén EM, Atarés M, Fernandez A. Pubertal growth in patients with androgen insensitivity: indirect evidence for the importance of estrogens in pubertal growth of girls. J Pediatr. 1986;108:694-697.
- ^ Cermak JM, Krenzer KL, Sullivan RM, Dana MR, Sullivan DA (2003). "Is complete androgen insensitivity syndrome associated with alterations in the meibomian gland and ocular surface?". Cornea. 22 (6): 516–21. PMID 12883343.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h Weidemann W, Peters B, Romalo G, Spindler KD, Schweikert HU. Response to androgen treatment in a patient with partial androgen insensitivity and a mutation in the deoxyribonucleic acid-binding domain of the androgen receptor. J Clin Endocrinol Metab. 1998;83:1173-1176.
- ^ Lee PA, Brown TR, LaTorre HA (1986). "Diagnosis of the partial androgen insensitivity syndrome during infancy". JAMA. 255 (16): 2207–9. PMID 3959303.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bhagabath B, Bradshaw KD (2008). "Non-surgical management of Müllerian anomalies". In Emre S, Aydin A (ed.). Non-Invasive Management of Gynecologic Disorders. Informa Healthcare. pp. 193–202. ISBN 0-415-41742-2.
- ^ a b Shkolny DL, Beitel LK, Ginsberg J, Pekeles G, Arbour L, Pinsky L, Trifiro MA (1999). "Discordant measures of androgen-binding kinetics in two mutant androgen receptors causing mild or partial androgen insensitivity, respectively". J. Clin. Endocrinol. Metab. 84 (2): 805–10. PMID 10022458.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Boehmer AL, Brinkmann O, Brüggenwirth H, van Assendelft C, Otten BJ, Verleun-Mooijman MC, Niermeijer MF, Brunner HG, Rouwé CW, Waelkens JJ, Oostdijk W, Kleijer WJ, van der Kwast TH, de Vroede MA, Drop SL (2001). "Genotype versus phenotype in families with androgen insensitivity syndrome". J. Clin. Endocrinol. Metab. 86 (9): 4151–60. PMID 11549642.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j Migeon CJ, Wisniewski AB, Gearhart JP, Meyer-Bahlburg HF, Rock JA, Brown TR, Casella SJ, Maret A, Ngai KM, Money J, Berkovitz GD (2002). "Ambiguous genitalia with perineoscrotal hypospadias in 46,XY individuals: long-term medical, surgical, and psychosexual outcome". Pediatrics. 110 (3): e31. PMID 12205281.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tanaka Y, Matsuo N, Aya M, et al. Persistent Müllerian duct remnants in three siblings with partial androgen insensitivity. Horumon To Rinsho 1995;43:3-8.
- ^ Mazur T (2005). "Gender dysphoria and gender change in androgen insensitivity or micropenis". Arch Sex Behav. 34 (4): 411–21. doi:10.1007/s10508-005-4341-x. PMID 16010464.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Evans BA, Hughes IA, Bevan CL, Patterson MN, Gregory JW (1997). "Phenotypic diversity in siblings with partial androgen insensitivity syndrome". Arch. Dis. Child. 76 (6): 529–31. PMC 1717223. PMID 9245853.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h Bouvattier C, Mignot B, Lefèvre H, Morel Y, Bougnères P (2006). "Impaired sexual activity in male adults with partial androgen insensitivity". J. Clin. Endocrinol. Metab. 91 (9): 3310–5. doi:10.1210/jc.2006-0218. PMID 16757528.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Deeb A, Jääskeläinen J, Dattani M, Whitaker HC, Costigan C, Hughes IA (2008). "A novel mutation in the human androgen receptor suggests a regulatory role for the hinge region in amino-terminal and carboxy-terminal interactions". J. Clin. Endocrinol. Metab. 93 (10): 3691–6. doi:10.1210/jc.2008-0737. PMID 18697867.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Miller MA, Grant DB (1997). "Severe hypospadias with genital ambiguity: adult outcome after staged hypospadias repair". Br J Urol. 80 (3): 485–8. PMID 9313674.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e f Köhler B, Lumbroso S, Leger J, Audran F, Grau ES, Kurtz F, Pinto G, Salerno M, Semitcheva T, Czernichow P, Sultan C (2005). "Androgen insensitivity syndrome: somatic mosaicism of the androgen receptor in seven families and consequences for sex assignment and genetic counseling". J. Clin. Endocrinol. Metab. 90 (1): 106–11. doi:10.1210/jc.2004-0462. PMID 15522944.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Wooster R, Mangion J, Eeles R, Smith S, Dowsett M, Averill D, Barrett-Lee P, Easton DF, Ponder BA, Stratton MR. A germline mutation in the androgen receptor gene in two brothers with breast cancer and Reifenstein syndrome. Nat Genet. 1992;2:132-134.
- ^ Bhangoo A, Paris F, Philibert P, Audran F, Ten S, Sultan C (2010). "Isolated micropenis reveals partial androgen insensitivity syndrome confirmed by molecular analysis". Asian J. Androl. 12 (4): 561–6. doi:10.1038/aja.2010.6. PMID 20305676.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Aguilar-Ponce J, Chilaca Rosas F, Molina Calzada C, Granados García M, Jiménez Ríos MA, De la Garza Salazar J (2008). "Testicular cancer in androgen insensitivity syndrome in a Mexican population". Clin Transl Oncol. 10 (12): 840–3. PMID 19068456.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Chu J, Zhang R, Zhao Z, Zou W, Han Y, Qi Q, Zhang H, Wang JC, Tao S, Liu X, Luo Z (2002). "Male fertility is compatible with an Arg(840)Cys substitution in the AR in a large Chinese family affected with divergent phenotypes of AR insensitivity syndrome". J. Clin. Endocrinol. Metab. 87 (1): 347–51. PMID 11788673.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k Pinsky L, Kaufman M, Killinger DW (1989). "Impaired spermatogenesis is not an obligate expression of receptor-defective androgen resistance". Am. J. Med. Genet. 32 (1): 100–4. doi:10.1002/ajmg.1320320121. PMID 2705470.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wang QI, Ghadessy FJ, Yong EL. Analysis of the transactivation domain of the androgen receptor in patients with male infertility. Clin Genet. 1998;54:185-192.
- ^ a b c Wang QI, Ghadessy FJ, Trounson A, De Kretser D, McLachlan R, Ng SC, Yong EL. Azoospermia associated with a mutation in the ligand-binding domain of an androgen receptor displaying normal ligand binding, but defective trans-activation. J Clin Endocrinol Metab. 1998;83:4303-4309.
- ^ a b c Grino PB, Griffin JE, Cushard WG, Wilson JD (1988). "A mutation of the androgen receptor associated with partial androgen resistance, familial gynecomastia, and fertility". J. Clin. Endocrinol. Metab. 66 (4): 754–61. PMID 3346354.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pinsky L, Kaufman M, Killinger DW, Burko B, Shatz D, Volpé R (1984). "Human minimal androgen insensitivity with normal dihydrotestosterone-binding capacity in cultured genital skin fibroblasts: evidence for an androgen-selective qualitative abnormality of the receptor". Am. J. Hum. Genet. 36 (5): 965–78. PMC 1684524. PMID 6333813.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Tsukada T, Inoue M, Tachibana S, Nakai Y, Takebe H. An androgen receptor mutation causing androgen resistance in undervirilized male syndrome. J Clin Endocrinol Metab. 1994;79:1202-1207.
- ^ a b c Zenteno JC, Chávez B, Vilchis F, Kofman-Alfaro S. Phenotypic heterogeneity associated with identical mutations in residue 870 of the androgen receptor. Horm Res. 2002;57:90-93.
- ^ a b c d e f Casella R, Maduro MR, Lipshultz LI, Lamb DJ (2001). "Significance of the polyglutamine tract polymorphism in the androgen receptor". Urology. 58 (5): 651–6. PMID 11711330.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Dejager S, Bry-Gauillard H, Bruckert E, Eymard B, Salachas F, LeGuern E, Tardieu S, Chadarevian R, Giral P, Turpin G (2002). "A comprehensive endocrine description of Kennedy's disease revealing androgen insensitivity linked to CAG repeat length". J. Clin. Endocrinol. Metab. 87 (8): 3893–901. PMID 12161529.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH (1991). "Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy". Nature. 352 (6330): 77–9. doi:10.1038/352077a0. PMID 2062380.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Arbizu T, Santamaría J, Gomez JM, Quílez A, Serra JP (1983). "A family with adult spinal and bulbar muscular atrophy, X-linked inheritance and associated testicular failure". J. Neurol. Sci. 59 (3): 371–82. PMID 6683750.
{{cite journal}}: Cite has empty unknown parameter:|1=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Choong CS, Wilson EM (1998). "Trinucleotide repeats in the human androgen receptor: a molecular basis for disease". J. Mol. Endocrinol. 21 (3): 235–57. PMID 9845666.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Biancalana V, Serville F, Pommier J, Julien J, Hanauer A, Mandel JL (1992). "Moderate instability of the trinucleotide repeat in spino bulbar muscular atrophy". Hum. Mol. Genet. 1 (4): 255–8. PMID 1303195.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Menakaya UA, Aligbe J, Iribhogbe P, Agoreyo F, Okonofua FE (2005). "Complete androgen insensitivity syndrome with persistent Mullerian derivatives: a case report". J Obstet Gynaecol. 25 (4): 403–5. doi:10.1080/01443610500143226. PMID 16091340.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Giwercman A, Kledal T, Schwartz M, Giwercman YL, Leffers H, Zazzi H, Wedell A, Skakkebaek NE (2000). "Preserved male fertility despite decreased androgen sensitivity caused by a mutation in the ligand-binding domain of the androgen receptor gene". J. Clin. Endocrinol. Metab. 85 (6): 2253–9. PMID 10852459.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f Giwercman YL, Nordenskjöld A, Ritzén EM, Nilsson KO, Ivarsson SA, Grandell U, Wedell A (2002). "An androgen receptor gene mutation (E653K) in a family with congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency as well as in partial androgen insensitivity". J. Clin. Endocrinol. Metab. 87 (6): 2623–8. PMID 12050225.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Danilovic DL, Correa PH, Costa EM, Melo KF, Mendonca BB, Arnhold IJ (2007). "Height and bone mineral density in androgen insensitivity syndrome with mutations in the androgen receptor gene". Osteoporos Int. 18 (3): 369–74. doi:10.1007/s00198-006-0243-6. PMID 17077943.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Sobel V, Schwartz B, Zhu YS, Cordero JJ, Imperato-McGinley J (2006). "Bone mineral density in the complete androgen insensitivity and 5alpha-reductase-2 deficiency syndromes". J. Clin. Endocrinol. Metab. 91 (8): 3017–23. doi:10.1210/jc.2005-2809. PMID 16735493.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Marcus R, Leary D, Schneider DL, Shane E, Favus M, Quigley CA (2000). "The contribution of testosterone to skeletal development and maintenance: lessons from the androgen insensitivity syndrome". J. Clin. Endocrinol. Metab. 85 (3): 1032–7. PMID 10720035.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Bertelloni S, Baroncelli GI, Federico G, Cappa M, Lala R, Saggese G (1998). "Altered bone mineral density in patients with complete androgen insensitivity syndrome". Horm. Res. 50 (6): 309–14. PMID 9973670.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Soule SG, Conway G, Prelevic GM, Prentice M, Ginsburg J, Jacobs HS (1995). "Osteopenia as a feature of the androgen insensitivity syndrome". Clin. Endocrinol. (Oxf). 43 (6): 671–5. PMID 8736267.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Muñoz-Torres M, Jódar E, Quesada M, Escobar-Jiménez F (1995). "Bone mass in androgen-insensitivity syndrome: response to hormonal replacement therapy". Calcif. Tissue Int. 57 (2): 94–6. PMID 7584881.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Hannema SE, Scott IS, Rajpert-De Meyts E, Skakkebaek NE, Coleman N, Hughes IA (2006). "Testicular development in the complete androgen insensitivity syndrome". J. Pathol. 208 (4): 518–27. doi:10.1002/path.1890. PMID 16400621.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Rutgers JL, Scully RE (1991). "The androgen insensitivity syndrome (testicular feminization): a clinicopathologic study of 43 cases". Int. J. Gynecol. Pathol. 10 (2): 126–44. PMID 2032766.
- ^ a b c Manuel M, Katayama PK, Jones HW (1976). "The age of occurrence of gonadal tumors in intersex patients with a Y chromosome". Am. J. Obstet. Gynecol. 124 (3): 293–300. PMID 1247071.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Evans BA, Harper ME, Daniells CE, Watts CE, Matenhelia S, Green J, Griffiths K (1996). "Low incidence of androgen receptor gene mutations in human prostatic tumors using single strand conformation polymorphism analysis". Prostate. 28 (3): 162–71. doi:10.1002/(SICI)1097-0045(199603)28:3<162::AID-PROS3>3.0.CO;2-H. PMID 8628719.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Lobaccaro JM, Lumbroso S, Belon C, Galtier-Dereure F, Bringer J, Lesimple T, Namer M, Cutuli BF, Pujol H, Sultan C (1993). "Androgen receptor gene mutation in male breast cancer". Hum. Mol. Genet. 2 (11): 1799–802. PMID 8281139.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Slijper FM, Drop SL, Molenaar JC, de Muinck Keizer-Schrama SM (1998). "Long-term psychological evaluation of intersex children". Arch Sex Behav. 27 (2): 125–44. PMID 9562897.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Brown CJ, Goss SJ, Lubahn DB, Joseph DR, Wilson EM, French FS, Willard HF (1989). "Androgen receptor locus on the human X chromosome: regional localization to Xq11-12 and description of a DNA polymorphism". Am. J. Hum. Genet. 44 (2): 264–9. PMC 1715398. PMID 2563196.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Kooy RF, Reyniers E, Storm K, Vits L, van Velzen D, de Ruiter PE, Brinkmann AO, de Paepe A, Willems PJ (1999). "CAG repeat contraction in the androgen receptor gene in three brothers with mental retardation". Am. J. Med. Genet. 85 (3): 209–13. PMID 10398229.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Audi L, Fernández-Cancio M, Carrascosa A; et al. (2010). "Novel (60%) and recurrent (40%) androgen receptor gene mutations in a series of 59 patients with a 46,XY disorder of sex development". J. Clin. Endocrinol. Metab. 95 (4): 1876–88. doi:10.1210/jc.2009-2146. PMID 20150575.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lumbroso R, Beitel LK, Vasiliou DM, Trifiro MA, Pinsky L (1997). "Codon-usage variants in the polymorphic (GGN)n trinucleotide repeat of the human androgen receptor gene". Hum. Genet. 101 (1): 43–6. PMID 9385367.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Gottlieb B, Pinsky L, Beitel LK, Trifiro M (1999). "Androgen insensitivity". Am. J. Med. Genet. 89 (4): 210–7. PMID 10727996.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Edwards A, Hammond HA, Jin L, Caskey CT, Chakraborty R (1992). "Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups". Genomics. 12 (2): 241–53. PMID 1740333.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Yeh SH, Chiu CM, Chen CL, Lu SF, Hsu HC, Chen DS, Chen PJ. Somatic mutations at the trinucleotide repeats of androgen receptor gene in male hepatocellular carcinoma. Int J Cancer. 2007;120:1610-1617.
- ^ Casella R, Maduro MR, Misfud A, Lipshultz LI, Yong EL, Lamb DJ (2003). "Androgen receptor gene polyglutamine length is associated with testicular histology in infertile patients". J. Urol. 169 (1): 224–7. doi:10.1097/01.ju.0000035361.18870.6e. PMID 12478141.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dowsing AT, Yong EL, Clark M, McLachlan RI, de Kretser DM, Trounson AO (1999). "Linkage between male infertility and trinucleotide repeat expansion in the androgen-receptor gene". Lancet. 354 (9179): 640–3. PMID 10466666.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tut TG, Ghadessy FJ, Trifiro MA, Pinsky L, Yong EL. Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab. 1997;82:3777-3782.
- ^ Lim HN, Chen H, McBride S, Dunning AM, Nixon RM, Hughes IA, Hawkins JR (2000). "Longer polyglutamine tracts in the androgen receptor are associated with moderate to severe undermasculinized genitalia in XY males". Hum. Mol. Genet. 9 (5): 829–34. PMID 10749991.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Hiort O, Holterhus PM, Horter T, Schulze W, Kremke B, Bals-Pratsch M, Sinnecker GH, Kruse K (2000). "Significance of mutations in the androgen receptor gene in males with idiopathic infertility". J. Clin. Endocrinol. Metab. 85 (8): 2810–5. PMID 10946887.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kukuvitis A, Georgiou I, Bouba I, Tsirka A, Giannouli CH, Yapijakis C, Tarlatzis B, Bontis J, Lolis D, Sofikitis N, Papadimas J (2002). "Association of oestrogen receptor alpha polymorphisms and androgen receptor CAG trinucleotide repeats with male infertility: a study in 109 Greek infertile men". Int. J. Androl. 25 (3): 149–52. PMID 12031042.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ von Eckardstein S, Syska A, Gromoll J, Kamischke A, Simoni M, Nieschlag E. Inverse correlation between sperm concentration and number of androgen receptor CAG repeats in normal men. J Clin Endocrinol Metab. 2001;86:2585-2590.
- ^ Rajpert-De Meyts E, Leffers H, Petersen JH, Andersen AG, Carlsen E, Jørgensen N, Skakkebaek NE (2002). "CAG repeat length in androgen-receptor gene and reproductive variables in fertile and infertile men". Lancet. 359 (9300): 44–6. PMID 11809188.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hiort O, Horter T, Schulze W, Kremke B, Sinnecker GH (1999). "Male infertility and increased risk of diseases in future generations". Lancet. 354 (9193): 1907–8. PMID 10584751.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Muroya K, Sasagawa I, Suzuki Y, Nakada T, Ishii T, Ogata T (2001). "Hypospadias and the androgen receptor gene: mutation screening and CAG repeat length analysis". Mol. Hum. Reprod. 7 (5): 409–13. PMID 11331662.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Radpour R, Rezaee M, Tavasoly A, Solati S, Saleki A (2007). "Association of long polyglycine tracts (GGN repeats) in exon 1 of the androgen receptor gene with cryptorchidism and penile hypospadias in Iranian patients". J. Androl. 28 (1): 164–9. doi:10.2164/jandrol.106.000927. PMID 16957138.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Aschim EL, Nordenskjöld A, Giwercman A, Lundin KB, Ruhayel Y, Haugen TB, Grotmol T, Giwercman YL (2004). "Linkage between cryptorchidism, hypospadias, and GGN repeat length in the androgen receptor gene". J. Clin. Endocrinol. Metab. 89 (10): 5105–9. doi:10.1210/jc.2004-0293. PMID 15472213.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Rajender S, Rajani V, Gupta NJ, Chakravarty B, Singh L, Thangaraj K (2006). "No association of androgen receptor GGN repeat length polymorphism with infertility in Indian men". J. Androl. 27 (6): 785–9. doi:10.2164/jandrol.106.000166. PMID 16809273.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Gottlieb B, Beitel LK, Trifiro MA (2001). "Variable expressivity and mutation databases: The androgen receptor gene mutations database". Hum. Mutat. 17 (5): 382–8. doi:10.1002/humu.1113. PMID 11317353.
{{cite journal}}: Cite has empty unknown parameter:|1=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hiort O, Sinnecker GH, Holterhus PM, Nitsche EM, Kruse K (1998). "Inherited and de novo androgen receptor gene mutations: investigation of single-case families". J. Pediatr. 132 (6): 939–43. PMID 9627582.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Batch JA, Williams DM, Davies HR, Brown BD, Evans BA, Hughes IA, Patterson MN (1992). "Androgen receptor gene mutations identified by SSCP in fourteen subjects with androgen insensitivity syndrome". Hum. Mol. Genet. 1 (7): 497–503. PMID 1307250.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hiort O, Klauber G, Cendron M, Sinnecker GH, Keim L, Schwinger E, Wolfe HJ, Yandell DW (1994). "Molecular characterization of the androgen receptor gene in boys with hypospadias". Eur. J. Pediatr. 153 (5): 317–21. PMID 8033918.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lu J, Danielsen M (1996). "A Stu I polymorphism in the human androgen receptor gene (AR)". Clin. Genet. 49 (6): 323–4. PMID 8884086.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Macke JP, Hu N, Hu S, Bailey M, King VL, Brown T, Hamer D, Nathans J (1993). "Sequence variation in the androgen receptor gene is not a common determinant of male sexual orientation". Am. J. Hum. Genet. 53 (4): 844–52. PMC 1682384. PMID 8213813.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Gottlieb B, Vasiliou DM, Lumbroso R, Beitel LK, Pinsky L, Trifiro MA (1999). "Analysis of exon 1 mutations in the androgen receptor gene". Hum. Mutat. 14 (6): 527–39. doi:10.1002/(SICI)1098-1004(199912)14:6<527::AID-HUMU12>3.0.CO;2-X. PMID 10571951.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Ahmed SF, Cheng A, Dovey L, Hawkins JR, Martin H, Rowland J, Shimura N, Tait AD, Hughes IA (2000). "Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome". J. Clin. Endocrinol. Metab. 85 (2): 658–65. PMID 10690872.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Coutant R, Mallet D, Lahlou N, Bouhours-Nouet N, Guichet A, Coupris L, Croué A, Morel Y (2007). "Heterozygous mutation of steroidogenic factor-1 in 46,XY subjects may mimic partial androgen insensitivity syndrome". J. Clin. Endocrinol. Metab. 92 (8): 2868–73. doi:10.1210/jc.2007-0024. PMID 17488792.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Adachi M, Takayanagi R, Tomura A, Imasaki K, Kato S, Goto K, Yanase T, Ikuyama S, Nawata H (2000). "Androgen-insensitivity syndrome as a possible coactivator disease". N. Engl. J. Med. 343 (12): 856–62. doi:10.1056/NEJM200009213431205. PMID 10995865.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ghadessy FJ, Lim J, Abdullah AA, Panet-Raymond V, Choo CK, Lumbroso R, Tut TG, Gottlieb B, Pinsky L, Trifiro MA, Yong EL (1999). "Oligospermic infertility associated with an androgen receptor mutation that disrupts interdomain and coactivator (TIF2) interactions". J. Clin. Invest. 103 (11): 1517–25. doi:10.1172/JCI4289. PMC 408364. PMID 10359561.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Giwercman YL, Ivarsson SA, Richthoff J, Lundin KB, Giwercman A (2004). "A novel mutation in the D-box of the androgen receptor gene (S597R) in two unrelated individuals Is associated with both normal phenotype and severe PAIS". Horm. Res. 61 (2): 58–62. doi:10.1159/000075240. PMID 14646391.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brinkmann A, Jenster G, Ris-Stalpers C, van der Korput H, Brüggenwirth H, Boehmer A, Trapman J (1996). "Molecular basis of androgen insensitivity". Steroids. 61 (4): 172–5. PMID 8732995.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Yong EL, Ng SC, Roy AC, Yun G, Ratnam SS. Pregnancy after hormonal correction of severe spermatogenic defect due to mutation in androgen receptor gene. Lancet 1994;344:826-827.
- ^ a b c d e f Pérez-Palacios G, Chávez B, Méndez JP, McGinley JI, Ulloa-Aguirre A (1987). "The syndromes of androgen resistance revisited". J. Steroid Biochem. 27 (4–6): 1101–8. PMID 3320547.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Radmayr C, Culig Z, Glatzl J, Neuschmid-Kaspar F, Bartsch G, Klocker H (1997). "Androgen receptor point mutations as the underlying molecular defect in 2 patients with androgen insensitivity syndrome". J. Urol. 158 (4): 1553–6. PMID 9302173.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Deeb A, Mason C, Lee YS, Hughes IA (2005). "Correlation between genotype, phenotype and sex of rearing in 111 patients with partial androgen insensitivity syndrome". Clin. Endocrinol. (Oxf). 63 (1): 56–62. doi:10.1111/j.1365-2265.2005.02298.x. PMID 15963062.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Rodien P, Mebarki F, Mowszowicz I, Chaussain JL, Young J, Morel Y, Schaison G (1996). "Different phenotypes in a family with androgen insensitivity caused by the same M780I point mutation in the androgen receptor gene". J. Clin. Endocrinol. Metab. 81 (8): 2994–8. PMID 8768864.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nordenskjöld A, Söderhäll S (1998). "An androgen receptor gene mutation (A645D) in a boy with a normal phenotype". Hum. Mutat. 11 (4): 339. PMID 9554755.
- ^ Werner R, Holterhus PM, Binder G, Schwarz HP, Morlot M, Struve D, Marschke C, Hiort O. The A645D mutation in the hinge region of the human androgen receptor (AR) gene modulates AR activity, depending on the context of the polymorphic glutamine and glycine repeats. J Clin Endocrinol Metab. 2006;91:3515-3520. Epub 2006 Jun 27.
- ^ Holterhus PM, Werner R, Hoppe U, Bassler J, Korsch E, Ranke MB, Dörr HG, Hiort O. Molecular features and clinical phenotypes in androgen insensitivity syndrome in the absence and presence of androgen receptor gene mutations. J Mol Med. 2005;83:1005-1113.
- ^ Meehan KL, Sadar MD (2003). "Androgens and androgen receptor in prostate and ovarian malignancies". Front. Biosci. 8: d780–800. PMID 12700055.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Taneja SS, Ha S, Swenson NK, Huang HY, Lee P, Melamed J, Shapiro E, Garabedian MJ, Logan SK. Cell-specific regulation of androgen receptor phosphorylation in vivo. J Biol Chem. 2005;280:40916-40924.
- ^ Heinlein CA, Chang C (2002). "Androgen receptor (AR) coregulators: an overview". Endocr. Rev. 23 (2): 175–200. PMID 11943742.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Jenster G, van der Korput HA, van Vroonhoven C, van der Kwast TH, Trapman J, Brinkmann AO (1991). "Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization". Mol. Endocrinol. 5 (10): 1396–404. PMID 1775129.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Simental JA, Sar M, Lane MV, French FS, Wilson EM (1991). "Transcriptional activation and nuclear targeting signals of the human androgen receptor". J. Biol. Chem. 266 (1): 510–8. PMID 1985913.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f Jones RE, Lopez KH (2006). "Chapter 5: Sexual differentiation". Human reproductive biology. Amsterdam: Elsevier Academic Press. pp. 127–148. ISBN 0-12-088465-8.
- ^ a b c Stenoien DL, Cummings CJ, Adams HP, Mancini MG, Patel K, DeMartino GN, Marcelli M, Weigel NL, Mancini MA (1999). "Polyglutamine-expanded androgen receptors form aggregates that sequester heat shock proteins, proteasome components and SRC-1, and are suppressed by the HDJ-2 chaperone". Hum. Mol. Genet. 8 (5): 731–41. PMID 10196362.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Weidemann W, Linck B, Haupt H, Mentrup B, Romalo G, Stockklauser K, Brinkmann AO, Schweikert HU, Spindler KD. Clinical and biochemical investigations and molecular analysis of subjects with mutations in the androgen receptor gene. Clin Endocrinol (Oxf). 1996;45:733-739.
- ^ a b c d e f Hughes IA (2008). "Disorders of sex development: a new definition and classification". Best Pract. Res. Clin. Endocrinol. Metab. 22 (1): 119–34. doi:10.1016/j.beem.2007.11.001. PMID 18279784.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kim KR, Kwon Y, Joung JY, Kim KS, Ayala AG, Ro JY (2002). "True hermaphroditism and mixed gonadal dysgenesis in young children: a clinicopathologic study of 10 cases". Mod. Pathol. 15 (10): 1013–9. doi:10.1097/01.MP.0000027623.23885.0D. PMID 12379746.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Borisa AD, Puri Y, Wakade V, Alagappan C, Agarkhedkar N. Complete Androgen Insensitivity Syndrome Presenting as Bilateral Inguinal Hernia. Bombay Hosp J. 2006;48:668:673.
- ^ Michailidis GD, Papageorgiou P, Morris RW, Economides DL (2003). "The use of three-dimensional ultrasound for fetal gender determination in the first trimester". Br J Radiol. 76 (907): 448–51. PMID 12857703.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Morel Y, Rey R, Teinturier C, Nicolino M, Michel-Calemard L, Mowszowicz I, Jaubert F, Fellous M, Chaussain JL, Chatelain P, David M, Nihoul-Fékété C, Forest MG, Josso N (2002). "Aetiological diagnosis of male sex ambiguity: a collaborative study". Eur. J. Pediatr. 161 (1): 49–59. PMID 11808880.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Sinnecker G, Köhler S (1989). "Sex hormone-binding globulin response to the anabolic steroid stanozolol: evidence for its suitability as a biological androgen sensitivity test". J. Clin. Endocrinol. Metab. 68 (6): 1195–200. PMID 2723028.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Kulshreshtha B, Philibert P, Eunice M, Khandelwal SK, Mehta M, Audran F, Paris F, Sultan C, Ammini AC (2009). "Apparent male gender identity in a patient with complete androgen insensitivity syndrome". Arch Sex Behav. 38 (6): 873–5. doi:10.1007/s10508-009-9526-2. PMID 19636694.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Money J, Schwartz M, Lewis VG (1984). "Adult erotosexual status and fetal hormonal masculinization and demasculinization: 46,XX congenital virilizing adrenal hyperplasia and 46,XY androgen-insensitivity syndrome compared". Psychoneuroendocrinology. 9 (4): 405–14. PMID 6514935.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Grover, S. Stretch Yourself. Alias 1996;1:76.
- ^ Hurt WG, Bodurtha JN, McCall JB, Ali MM (1989). "Seminoma in pubertal patient with androgen insensitivity syndrome". Am. J. Obstet. Gynecol. 161 (3): 530–1. PMID 2782332.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Steiner E, Woernle F, Kuhn W, Beckmann K, Schmidt M, Pilch H, Knapstein PG (2002). "Carcinoma of the neovagina: case report and review of the literature". Gynecol. Oncol. 84 (1): 171–5. doi:10.1006/gyno.2001.6417. PMID 11748997.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Breech LL (2008). "Complications of vaginoplasty and clitoroplasty". In Teich S, Caniano DA (ed.). Reoperative pediatric surgery. Totowa, N.J: Humana. pp. 499–514. ISBN 1-58829-761-6.
- ^ a b c d e f g Minto CL, Liao LM, Woodhouse CR, Ransley PG, Creighton SM (2003). "The effect of clitoral surgery on sexual outcome in individuals who have intersex conditions with ambiguous genitalia: a cross-sectional study". Lancet. 361 (9365): 1252–7. doi:10.1016/S0140-6736(03)12980-7. PMID 12699952.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Meyer-Bahlburg HF (1999). "Gender assignment and reassignment in 46,XY pseudohermaphroditism and related conditions". J. Clin. Endocrinol. Metab. 84 (10): 3455–8. PMID 10522979.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Goy RW, Bercovitch FB, McBrair MC (1988). "Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques". Horm Behav. 22 (4): 552–71. PMID 3235069.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7-26.
- ^ Moore CL (1992). "The role of maternal stimulation in the development of sexual behavior and its neural basis". Ann. N. Y. Acad. Sci. 662: 160–77. PMID 1456637.
- ^ Wallen K. Nature needs nurture: the interaction of hormonal and social influences on the development of behavioral sex differences in rhesus monkeys. Horm Behav. 1996;30:364-378.
- ^ Martin CL, Ruble DN, Szkrybalo J (2002). "Cognitive theories of early gender development". Psychol Bull. 128 (6): 903–33. PMID 12405137.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wisniewski AB, Migeon CJ, Gearhart JP, Rock JA, Berkovitz GD, Plotnick LP, Meyer-Bahlburg HF, Money J. Congenital micropenis: long-term medical, surgical and psychosexual follow-up of individuals raised male or female. Horm Res. 2001;56:3-11.
- ^ Zucker KJ. Intersexuality and gender identity differentiation. J Pediatr Adolesc Gynecol. 2002;15:3–13.
- ^ a b c Creighton SM, Minto CL, Steele SJ (2001). "Objective cosmetic and anatomical outcomes at adolescence of feminising surgery for ambiguous genitalia done in childhood". Lancet. 358 (9276): 124–5. doi:10.1016/S0140-6736(01)05343-0. PMID 11463417.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Warne G, Grover S, Hutson J, Sinclair A, Metcalfe S, Northam E, Freeman J; Murdoch Childrens Research Institute Sex Study Group. A long-term outcome study of intersex conditions. J Pediatr Endocrinol Metab. 2005;18:555-567.
- ^ Money J, Devore H, Norman BF (1986). "Gender identity and gender transposition: longitudinal outcome study of 32 male hermaphrodites assigned as girls". J Sex Marital Ther. 12 (3): 165–81. PMID 3761370.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Money J, Norman BF (1987). "Gender identity and gender transposition: longitudinal outcome study of 24 male hermaphrodites assigned as boys". J Sex Marital Ther. 13 (2): 75–92. PMID 3612827.
- ^ a b Nihoul-Fékété C, Thibaud E, Lortat-Jacob S, Josso N (2006). "Long-term surgical results and patient satisfaction with male pseudohermaphroditism or true hermaphroditism: a cohort of 63 patients". J. Urol. 175 (5): 1878–84. doi:10.1016/S0022-5347(05)00934-1. PMID 16600787.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Alizai NK, Thomas DF, Lilford RJ, Batchelor AG, Johnson N (1999). "Feminizing genitoplasty for congenital adrenal hyperplasia: what happens at puberty?". J. Urol. 161 (5): 1588–91. PMID 10210421.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ American Academy of Pediatrics (1996). "Timing of elective surgery on the genitalia of male children with particular reference to the risks, benefits, and psychological effects of surgery and anesthesia". Pediatrics. 97 (4): 590–4. PMID 8632952.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Tincello DG, Saunders PT, Hodgins MB, Simpson NB, Edwards CR, Hargreaves TB, Wu FC. Correlation of clinical, endocrine and molecular abnormalities with in vivo responses to high-dose testosterone in patients with partial androgen insensitivity syndrome. Clin Endocrinol. 1997;46:497-506.
- ^ Leichtnam ML, Rolland H, Wüthrich P, Guy RH (2006). "Testosterone hormone replacement therapy: state-of-the-art and emerging technologies". Pharm. Res. 23 (6): 1117–32. doi:10.1007/s11095-006-0072-5. PMID 16755346.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Nieschlag E (2006). "Testosterone treatment comes of age: new options for hypogonadal men". Clin. Endocrinol. (Oxf). 65 (3): 275–81. doi:10.1111/j.1365-2265.2006.02618.x. PMID 16918944.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Handelsman DJ, Conway AJ, Boylan LM (1992). "Suppression of human spermatogenesis by testosterone implants". J. Clin. Endocrinol. Metab. 75 (5): 1326–32. PMID 1430094.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Gooren L (1989). "Improvement of spermatogenesis after treatment with the antiestrogen tamoxifen in a man with the incomplete androgen insensitivity syndrome". J. Clin. Endocrinol. Metab. 68 (6): 1207–10. PMID 2566621.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bangsbøll S, Qvist I, Lebech PE, Lewinsky M (1992). "Testicular feminization syndrome and associated gonadal tumors in Denmark". Acta Obstet Gynecol Scand. 71 (1): 63–6. PMID 1315102.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mazen I, El-Ruby M, Kamal R, El-Nekhely I, El-Ghandour M, Tantawy S, El-Gammal M (2010). "Screening of genital anomalies in newborns and infants in two egyptian governorates". Horm Res Paediatr. 73 (6): 438–42. doi:10.1159/000313588. PMID 20407231.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Wilkins L. Heterosexual development. In: The diagnosis and treatment of endocrine disorders in childhood and adolescence. Springfield, IL: Charles C Thomas, 1950, pp. 256-279.
- ^ Lyon MF, Hawkes SG (1970). "X-linked gene for testicular feminization in the mouse". Nature. 227 (5264): 1217–9. PMID 5452809.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Ohno S, Lyon MF (1970). "X-Linked testicular feminization in the mouse as a non-inducible regulatory mutation of the Jacob-Monod type". Clinical Genetics. 1 (3–4): 121–127. doi:10.1111/j.1399-0004.1970.tb01627.x.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Migeon BR, Brown TR, Axelman J, Migeon CJ (1981). "Studies of the locus for androgen receptor: localization on the human X chromosome and evidence for homology with the Tfm locus in the mouse". Proc. Natl. Acad. Sci. U.S.A. 78 (10): 6339–43. PMC 349034. PMID 6947233.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Brown TR, Lubahn DB, Wilson EM, Joseph DR, French FS, Migeon CJ (1988). "Deletion of the steroid-binding domain of the human androgen receptor gene in one family with complete androgen insensitivity syndrome: evidence for further genetic heterogeneity in this syndrome". Proc. Natl. Acad. Sci. U.S.A. 85 (21): 8151–5. PMC 282385. PMID 3186717.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM (1988). "Cloning of human androgen receptor complementary DNA and localization to the X chromosome". Science. 240 (4850): 327–30. PMID 3353727.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Chang CS, Kokontis J, Liao ST (1988). "Molecular cloning of human and rat complementary DNA encoding androgen receptors". Science. 240 (4850): 324–6. PMID 3353726.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lubahn DB, Brown TR, Simental JA, Higgs HN, Migeon CJ, Wilson EM, French FS (1989). "Sequence of the intron/exon junctions of the coding region of the human androgen receptor gene and identification of a point mutation in a family with complete androgen insensitivity". Proc. Natl. Acad. Sci. U.S.A. 86 (23): 9534–8. PMC 298531. PMID 2594783.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Patterson MN, Hughes IA, Gottlieb B, Pinsky L (1994). "The androgen receptor gene mutations database". Nucleic Acids Res. 22 (17): 3560–2. PMC 308319. PMID 7937057.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Simpson JY. Hermaphroditism. In: Todd RB, ed. Cyclopaedia of Anatomy and Physiology, Vol II. London: Longman, Brown, Green, Longmans, & Roberts 1839;2:684-738.
- ^ a b King H (2007). Midwifery, obstetrics and the rise of gynaecology: the uses of a sixteenth-century compendium. Aldershot, Hants, England: Ashgate Pub. ISBN 0-7546-5396-X.
- ^ Affaitati F [Affaitat]. De hermaphroditis. Venet. 1549.
- ^ Panckoucke CLF, ed. Dictionnaire des sciences médicales - biographie médicale, 1st ed. Paris: Panckoucke 1820;1:59.
- ^ Paré, A. Des monstres et prodiges. Paris: Dupuys 1573.
- ^ Venette N [Vénitien Salocini]. Tableau de l'amour humain considéré dans l'état du mariage. A Parme: Chez Franc d'Amour 1687.
- ^ Jacob G. Tractatus de hermaphroditis. London: E. Curll 1718.
- ^ a b Saint Hilaire IG. Histoire générale et particulière des anomolies de l'organisation. Paris: J.-B. Baillière 1832-1836.
- ^ Dorsey FY, Hsieh MH, Roth DR (2009). "46,XX SRY-negative true hermaphrodite siblings". Urology. 73 (3): 529–31. doi:10.1016/j.urology.2008.09.050. PMID 19038427.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Verkauskas G, Jaubert F, Lortat-Jacob S, Malan V, Thibaud E, Nihoul-Fékété C. The Long-Term Followup of 33 Cases of True Hermaphroditism: A 40-Year Experience With Conservative Gonadal Surgery. J Urol. 2007;177:726-731.
- ^ Simmonds M (2007). "Was "variations of reproductive development" considered?". Arch. Dis. Child. 92 (1): 89. doi:10.1136/adc.2006.107797. PMC 2083124. PMID 17185456.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Zannoni GF, Vellone VG, Cordisco EL, Sangiorgi E, Grimaldi ME, Neri C, Nanni L, Neri G. Morphology and immunophenotyping of a monolateral ovotestis in a 46,XY/45,X mosaic individual with ambiguous genitalia. Int J Gynecol Pathol. 2010;29:33-38.
- ^ Feder EK, Karkazis K (2008). "What's in a name? The controversy over "disorders of sex development"". Hastings Cent Rep. 38 (5): 33–6. PMID 18947138.
- ^ Reis E (2007). "Divergence or disorder?: the politics of naming intersex". Perspect. Biol. Med. 50 (4): 535–43. doi:10.1353/pbm.2007.0054. PMID 17951887.
- ^ a b Ruysch F. Thesaurus anatomicus octavus. Amsterdam: Joannem Wolters 1709. p. 33, Plate II.
- ^ Klebs E. Handbuch der pathologischen anatomie. Berlin: A. Hirschwald 1876;1:718.
- ^ Mencke JB, ed. Acta eruditorum anno mdccix. Leipzig: Joh. Grossii Haeredes, Joh. Frid. Gleditsch, & Frid. Groschuf. 1709;28:272-274.
- ^ Müller JP, ed. Archiv für Anatomie, Physiologie und wissenschaftliche Medicin. Berlin: G. Eichler 1834, p. 171.
- ^ Académie française. Complément du Dictionnaire de l'Académie française. Paris: Chez Firmin Didot Fréres 1843, p. 997.
- ^ Ritter von Raiman JN, Edlen von Rosas A, Fischer SC, Wisgrill J, eds. Medicinische Jahrbücher des kaiserlich-königlichen österreichischen Staates (volume 22). Vienna: Carl Gerold 1840;22:380-384.
- ^ Bertuch FJ, Schütz CG, eds. Allgemeine Literatur-Zeitung Issues 1-97. Leipzig 1815, pp. 257-260.
- ^ Peschier A, Mozin DJ, eds. Supplément au dictionnaire complet des langues française et allemande de l'abbe Mozin. Paris: Stuttgart et Augsbourg 1859, p. 333.
- ^ Reifenstein EC Jr. (1947). "Hereditary familial hypogonadism". Proc Am Fed Clin Res. 3: 86. PMID 18909356.
- ^ oldberg MB, Maxwell A (1948). "Male pseudohermaphroditism proved by surgical exploration and microscopic examination; a case report with speculations concerning pathogenesis". J. Clin. Endocrinol. Metab. 8 (5): 367–79. PMID 18863968.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Gilbert-Dreyfus S, Sabaoun CIA, Belausch J. Etude d'un cas familial d'androgynoidisme avec hypospadias grave, gynecomastie et hyperoestrogenie. Ann Endocr. 1957;18:93-101.
- ^ ubs HA Jr, Vilar O, Bergenstal DM (1959). "Familial male pseudohermaphrodism with labial testes and partial feminization: endocrine studies and genetic aspects". J. Clin. Endocrinol. Metab. 19: 1110–20. PMID 14418653.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Morris JM, Mahesh VB (1963). "Further observations on the syndrome, "testicular feminization."". Am. J. Obstet. Gynecol. 87: 731–48. PMID 14085776.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Rosewater S, Gwinup G, Hamwi JG (1965). "Familial gynecomastia". Ann. Intern. Med. 63: 377–85. PMID 14327504.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Aiman J, Griffin JE, Gazak JM, Wilson JD, MacDonald PC (1979). "Androgen insensitivity as a cause of infertility in otherwise normal men". N. Engl. J. Med. 300 (5): 223–7. doi:10.1056/NEJM197902013000503. PMID 759869.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Simpson JL (2008). "Male Pseudohermaphroditism Due to Androgen Insensitivity or 5α-Reductase Deficiency". Glob. Libr. Women's Med. doi:10.3843/GLOWM.10349.
- ^ Hester JD. Intersex(e) und alternative Heilungsstrategien - Medizin, soziale Imperative und identitatsstiftende Gegengemeinschaften. Ethik Med. 2004;16:48-67.
- ^ McPhaul MJ (1999). "Molecular defects of the androgen receptor". J. Steroid Biochem. Mol. Biol. 69 (1–6): 315–22. PMID 10419008.
- ^ a b Hoff, TA, Fuqua, SAW (2000). "Steroid and nuclear receptor polymorphism variants in hormone resistance and hormone independence". In Miller MS, Cronin MT (ed.). Genetic polymorphisms and susceptibility to disease. Washington, DC: Taylor & Francis. p. 111. ISBN 0-7484-0822-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Meschede D, Horst J (1997). "The molecular genetics of male infertility". Mol. Hum. Reprod. 3 (5): 419–30. PMID 9239727.
{{cite journal}}: Unknown parameter|month=ignored (help)
External links
- Information
- androgen at NIH/UW GeneTests
- An Australian parent/patient booklet on CAIS
- The Secret of My Sex news article
- Women With Male DNA All Female news article at ABCnews.com
- Patient groups
- AIS Support Group AISSG (UK and International)
- AIS Support Group (US)
- AIS Support Group (Australasia)
- Intersex Support Forums (US and International)
This template is no longer used; please see Template:Endocrine pathology for a suitable replacement
