UGT2B7: Difference between revisions
morphine-6-glucuronide is more potent , not less potent than morphine Klimas R, Mikus G. Morphine-6-glucuronide is responsible for the analgesic effect after morphine administration: a quantitative review of morphine, morphine-6-glucuronide, and morphine- |
Added section on structure |
||
| Line 10: | Line 10: | ||
Together with [[UGT2B4]], UGT2B7 is capable of glucosidation of [[hyodesoxycholic acid]] in the liver, but, unlike the 2B4 isoform, 2B7 is also able to glucuronidate various [[steroid hormones]] ([[androsterone]], [[epitestosterone]]) and [[fatty acids]].<ref name="pmid12527334">{{cite journal | vauthors = Mackenzie P, Little JM, Radominska-Pandya A | title = Glucosidation of hyodeoxycholic acid by UDP-glucuronosyltransferase 2B7 | journal = Biochem. Pharmacol. | volume = 65 | issue = 3 | pages = 417–21 | date = February 2003 | pmid = 12527334 | doi = 10.1016/S0006-2952(02)01522-8 | url = }}</ref><ref name="pmid17263731">{{cite journal | vauthors = Barre L, Fournel-Gigleux S, Finel M, Netter P, Magdalou J, Ouzzine M | title = Substrate specificity of the human UDP-glucuronosyltransferase UGT2B4 and UGT2B7. Identification of a critical aromatic amino acid residue at position 33 | journal = FEBS J. | volume = 274 | issue = 5 | pages = 1256–64 | date = March 2007 | pmid = 17263731 | doi = 10.1111/j.1742-4658.2007.05670.x }}</ref> It is also able to conjugate major classes of drugs such as analgesics ([[morphine]]), carboxylic nonsteroidal anti-inflammatory drugs ([[ketoprofen]]), and anticarcinogens (''all-trans'' [[retinoic acid]]).<ref name="pmid17263731"/> UGT2B7 is the major enzyme isoform for the metabolism of [[morphine]] to the main metabolites, [[morphine-3-glucuronide]] (M3G) which has no analgesic effect and [[morphine-6-glucuronide]] (M6G),<ref>{{cite journal | vauthors = Coffman BL, Rios GR, King CD, Tephly TR | title = Human UGT2B7 catalyzes morphine glucuronidation | journal = Drug Metab. Dispos. | volume = 25 | issue = 1 | pages = 1–4 | date = 1 January 1997 | pmid = 9010622 | url = http://dmd.aspetjournals.org/cgi/content/abstract/25/1/1 }}</ref> which has analgesic effects more potent than morphine.<ref name="van Dorp">{{cite journal | vauthors = van Dorp EL, Romberg R, Sarton E, Bovill JG, Dahan A | title = Morphine-6-glucuronide: morphine's successor for postoperative pain relief? | journal = Anesthesia and Analgesia | volume = 102 | issue = 6 | pages = 1789–1797 | year = 2006 | pmid = 16717327 | doi = 10.1213/01.ane.0000217197.96784.c3 | url = http://www.anesthesia-analgesia.org/cgi/content/full/102/6/1789 }}</ref> As a consequence, altered UGT2B7 activity can significantly affect both the effectiveness and side-effects of morphine, as well as some related opiate drugs.<ref>{{cite journal | vauthors = Coller JK, Christrup LL, Somogyi AA | title = Role of active metabolites in the use of opioids. | journal = European journal of clinical pharmacology | volume = 65 | issue = 2 | pages = 121–39 | year = 2009 | pmid = 18958460 | doi = 10.1007/s00228-008-0570-y }}</ref><ref>{{cite journal | vauthors = Fujita K, Ando Y, Yamamoto W, Miya T, Endo H, Sunakawa Y, Araki K, Kodama K, Nagashima F, Ichikawa W, Narabayashi M, Akiyama Y, Kawara K, Shiomi M, Ogata H, Iwasa H, Okazaki Y, Hirose T, Sasaki Y | title = Association of UGT2B7 and ABCB1 genotypes with morphine-induced adverse drug reactions in Japanese patients with cancer | journal = Cancer chemotherapy and pharmacology | volume = 65 | issue = 2 | pages = 251–8 | year = 2009 | pmid = 19466410 | doi = 10.1007/s00280-009-1029-2 }}</ref><ref>{{cite journal | vauthors = Abildskov K, Weldy P, Garland M | title = Molecular Cloning of the Baboon UDP-Glucuronosyltransferase 2B Gene Family and Their Activity in Conjugating Morphine | journal = Drug metabolism and disposition: the biological fate of chemicals | volume = 38 | issue = 4 | pages = 545–53 | year = 2010 | pmid = 20071451 | pmc = 2845934 | doi = 10.1124/dmd.109.030635 }}</ref><ref>{{cite journal | vauthors = Pergolizzi JV, Raffa RB, Gould E | title = Considerations on the use of oxymorphone in geriatric patients | journal = Expert opinion on drug safety | volume = 8 | issue = 5 | pages = 603–13 | year = 2009 | pmid = 19614559 | doi = 10.1517/14740330903153854 }}</ref><ref>{{cite journal | vauthors = Rouguieg K, Picard N, Sauvage FL, Gaulier JM, Marquet P | title = Contribution of the different UDP-glucuronosyltransferase (UGT) isoforms to buprenorphine and norbuprenorphine metabolism and relationship with the main UGT polymorphisms in a bank of human liver microsomes | journal = Drug metabolism and disposition: the biological fate of chemicals | volume = 38 | issue = 1 | pages = 40–5 | year = 2010 | pmid = 19841060 | doi = 10.1124/dmd.109.029546 }}</ref> |
Together with [[UGT2B4]], UGT2B7 is capable of glucosidation of [[hyodesoxycholic acid]] in the liver, but, unlike the 2B4 isoform, 2B7 is also able to glucuronidate various [[steroid hormones]] ([[androsterone]], [[epitestosterone]]) and [[fatty acids]].<ref name="pmid12527334">{{cite journal | vauthors = Mackenzie P, Little JM, Radominska-Pandya A | title = Glucosidation of hyodeoxycholic acid by UDP-glucuronosyltransferase 2B7 | journal = Biochem. Pharmacol. | volume = 65 | issue = 3 | pages = 417–21 | date = February 2003 | pmid = 12527334 | doi = 10.1016/S0006-2952(02)01522-8 | url = }}</ref><ref name="pmid17263731">{{cite journal | vauthors = Barre L, Fournel-Gigleux S, Finel M, Netter P, Magdalou J, Ouzzine M | title = Substrate specificity of the human UDP-glucuronosyltransferase UGT2B4 and UGT2B7. Identification of a critical aromatic amino acid residue at position 33 | journal = FEBS J. | volume = 274 | issue = 5 | pages = 1256–64 | date = March 2007 | pmid = 17263731 | doi = 10.1111/j.1742-4658.2007.05670.x }}</ref> It is also able to conjugate major classes of drugs such as analgesics ([[morphine]]), carboxylic nonsteroidal anti-inflammatory drugs ([[ketoprofen]]), and anticarcinogens (''all-trans'' [[retinoic acid]]).<ref name="pmid17263731"/> UGT2B7 is the major enzyme isoform for the metabolism of [[morphine]] to the main metabolites, [[morphine-3-glucuronide]] (M3G) which has no analgesic effect and [[morphine-6-glucuronide]] (M6G),<ref>{{cite journal | vauthors = Coffman BL, Rios GR, King CD, Tephly TR | title = Human UGT2B7 catalyzes morphine glucuronidation | journal = Drug Metab. Dispos. | volume = 25 | issue = 1 | pages = 1–4 | date = 1 January 1997 | pmid = 9010622 | url = http://dmd.aspetjournals.org/cgi/content/abstract/25/1/1 }}</ref> which has analgesic effects more potent than morphine.<ref name="van Dorp">{{cite journal | vauthors = van Dorp EL, Romberg R, Sarton E, Bovill JG, Dahan A | title = Morphine-6-glucuronide: morphine's successor for postoperative pain relief? | journal = Anesthesia and Analgesia | volume = 102 | issue = 6 | pages = 1789–1797 | year = 2006 | pmid = 16717327 | doi = 10.1213/01.ane.0000217197.96784.c3 | url = http://www.anesthesia-analgesia.org/cgi/content/full/102/6/1789 }}</ref> As a consequence, altered UGT2B7 activity can significantly affect both the effectiveness and side-effects of morphine, as well as some related opiate drugs.<ref>{{cite journal | vauthors = Coller JK, Christrup LL, Somogyi AA | title = Role of active metabolites in the use of opioids. | journal = European journal of clinical pharmacology | volume = 65 | issue = 2 | pages = 121–39 | year = 2009 | pmid = 18958460 | doi = 10.1007/s00228-008-0570-y }}</ref><ref>{{cite journal | vauthors = Fujita K, Ando Y, Yamamoto W, Miya T, Endo H, Sunakawa Y, Araki K, Kodama K, Nagashima F, Ichikawa W, Narabayashi M, Akiyama Y, Kawara K, Shiomi M, Ogata H, Iwasa H, Okazaki Y, Hirose T, Sasaki Y | title = Association of UGT2B7 and ABCB1 genotypes with morphine-induced adverse drug reactions in Japanese patients with cancer | journal = Cancer chemotherapy and pharmacology | volume = 65 | issue = 2 | pages = 251–8 | year = 2009 | pmid = 19466410 | doi = 10.1007/s00280-009-1029-2 }}</ref><ref>{{cite journal | vauthors = Abildskov K, Weldy P, Garland M | title = Molecular Cloning of the Baboon UDP-Glucuronosyltransferase 2B Gene Family and Their Activity in Conjugating Morphine | journal = Drug metabolism and disposition: the biological fate of chemicals | volume = 38 | issue = 4 | pages = 545–53 | year = 2010 | pmid = 20071451 | pmc = 2845934 | doi = 10.1124/dmd.109.030635 }}</ref><ref>{{cite journal | vauthors = Pergolizzi JV, Raffa RB, Gould E | title = Considerations on the use of oxymorphone in geriatric patients | journal = Expert opinion on drug safety | volume = 8 | issue = 5 | pages = 603–13 | year = 2009 | pmid = 19614559 | doi = 10.1517/14740330903153854 }}</ref><ref>{{cite journal | vauthors = Rouguieg K, Picard N, Sauvage FL, Gaulier JM, Marquet P | title = Contribution of the different UDP-glucuronosyltransferase (UGT) isoforms to buprenorphine and norbuprenorphine metabolism and relationship with the main UGT polymorphisms in a bank of human liver microsomes | journal = Drug metabolism and disposition: the biological fate of chemicals | volume = 38 | issue = 1 | pages = 40–5 | year = 2010 | pmid = 19841060 | doi = 10.1124/dmd.109.029546 }}</ref> |
||
== Structure == |
|||
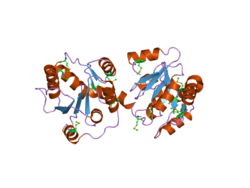
No structure of a full human UGT enzyme has been determined yet, however Miley et al resolved a partial UGT2B7 structure showing two dimeric domains with Rossman-like folds in complex.<ref>{{Cite journal|last=Lampe|first=Jed N.|date=2017|title=Advances in the Understanding of Protein-Protein Interactions in Drug Metabolizing Enzymes through the Use of Biophysical Techniques|url=https://www.ncbi.nlm.nih.gov/pubmed/28848438|journal=Frontiers in Pharmacology|volume=8|pages=521|doi=10.3389/fphar.2017.00521|issn=1663-9812|pmc=PMC5550701|pmid=28848438}}</ref><ref>{{Cite journal|last=Miley|first=Michael J.|last2=Zielinska|first2=Agnieszka K.|last3=Keenan|first3=Jeffrey E.|last4=Bratton|first4=Stacie M.|last5=Radominska-Pandya|first5=Anna|last6=Redinbo|first6=Matthew R.|date=2007-06-01|title=Crystal structure of the cofactor-binding domain of the human phase II drug-metabolism enzyme UDP-glucuronosyltransferase 2B7|url=https://www.ncbi.nlm.nih.gov/pubmed/17442341|journal=Journal of Molecular Biology|volume=369|issue=2|pages=498–511|doi=10.1016/j.jmb.2007.03.066|issn=0022-2836|pmc=PMC1976284|pmid=17442341}}</ref> The Rossman fold typically binds nucleotide substrates, in this case the UDP-glucuronic acid cofactor involved in glucuronidation by UGT2B7. Generally, the C-terminus of UGT enzymes is highly conserved and binds the UDP-glucuronic acid cofactor, while the N-terminus is responsible for substrate binding.<ref name=":0">{{Cite journal|last=Yuan|first=Lingmin|last2=Qian|first2=Sainan|last3=Xiao|first3=Yongsheng|last4=Sun|first4=Hongying|last5=Zeng|first5=Su|date=2015-05-01|title=Homo- and hetero-dimerization of human UDP-glucuronosyltransferase 2B7 (UGT2B7) wild type and its allelic variants affect zidovudine glucuronidation activity|url=https://www.ncbi.nlm.nih.gov/pubmed/25770680|journal=Biochemical Pharmacology|volume=95|issue=1|pages=58–70|doi=10.1016/j.bcp.2015.03.002|issn=1873-2968|pmid=25770680}}</ref> This first resolved structure interestingly indicated that the C-terminus of one of the two dimers projected into the UDP-glucuronic acid binding site of the second dimer, thus rendering the second dimer ineffective. |
|||
Further studies have investigated dimerization of UGT enzyme polymorphisms and found both homodimer and heterodimer formation are possible, with some combinations having an effect on enzyme activity.<ref name=":0" /> |
|||
== References == |
== References == |
||
Revision as of 02:05, 8 March 2018
| UGT2B7 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | UGT2B7, UDPGT 2B9, UDPGT2B7, UDPGTH2, UGT2B9, UDP glucuronosyltransferase family 2 member B7, UDPGT 2B7, UDPGTh-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 600068; MGI: 3576103; HomoloGene: 128251; GeneCards: UGT2B7; OMA:UGT2B7 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
UGT2B7 (UDP-Glucuronosyltransferase-2B7) is a phase II metabolism isoenzyme found to be active in the liver, kidneys, epithelial cells of the lower gastrointestinal tract and also has been reported in the brain. In humans, UDP-Glucuronosyltransferase-2B7 is encoded by the UGT2B7 gene.[5][6]
Function
The UGTs serve a major role in the conjugation and subsequent elimination of potentially toxic xenobiotics and endogenous compounds. UGT2B7 has unique specificity for 3,4-catechol estrogens and estriol, suggesting that it may play an important role in regulating the level and activity of these potent estrogen metabolites.
This enzyme is located on the endoplasmic reticulum and nuclear membranes of cells. Its function is to catalyse the conjugation of a wide variety of lipophilic aglycon substrates with glucuronic acid, using uridine diphosphate glucuronic acid.
Together with UGT2B4, UGT2B7 is capable of glucosidation of hyodesoxycholic acid in the liver, but, unlike the 2B4 isoform, 2B7 is also able to glucuronidate various steroid hormones (androsterone, epitestosterone) and fatty acids.[7][8] It is also able to conjugate major classes of drugs such as analgesics (morphine), carboxylic nonsteroidal anti-inflammatory drugs (ketoprofen), and anticarcinogens (all-trans retinoic acid).[8] UGT2B7 is the major enzyme isoform for the metabolism of morphine to the main metabolites, morphine-3-glucuronide (M3G) which has no analgesic effect and morphine-6-glucuronide (M6G),[9] which has analgesic effects more potent than morphine.[10] As a consequence, altered UGT2B7 activity can significantly affect both the effectiveness and side-effects of morphine, as well as some related opiate drugs.[11][12][13][14][15]
Structure
No structure of a full human UGT enzyme has been determined yet, however Miley et al resolved a partial UGT2B7 structure showing two dimeric domains with Rossman-like folds in complex.[16][17] The Rossman fold typically binds nucleotide substrates, in this case the UDP-glucuronic acid cofactor involved in glucuronidation by UGT2B7. Generally, the C-terminus of UGT enzymes is highly conserved and binds the UDP-glucuronic acid cofactor, while the N-terminus is responsible for substrate binding.[18] This first resolved structure interestingly indicated that the C-terminus of one of the two dimers projected into the UDP-glucuronic acid binding site of the second dimer, thus rendering the second dimer ineffective.
Further studies have investigated dimerization of UGT enzyme polymorphisms and found both homodimer and heterodimer formation are possible, with some combinations having an effect on enzyme activity.[18]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000171234 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000070704 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Ritter JK, Sheen YY, Owens IS (May 1990). "Cloning and expression of human liver UDP-glucuronosyltransferase in COS-1 cells. 3,4-catechol estrogens and estriol as primary substrates". J. Biol. Chem. 265 (14): 7900–6. PMID 2159463.
- ^ Monaghan G, Clarke DJ, Povey S, See CG, Boxer M, Burchell B (September 1994). "Isolation of a human YAC contig encompassing a cluster of UGT2 genes and its regional localization to chromosome 4q13". Genomics. 23 (2): 496–9. doi:10.1006/geno.1994.1531. PMID 7835904.
- ^ Mackenzie P, Little JM, Radominska-Pandya A (February 2003). "Glucosidation of hyodeoxycholic acid by UDP-glucuronosyltransferase 2B7". Biochem. Pharmacol. 65 (3): 417–21. doi:10.1016/S0006-2952(02)01522-8. PMID 12527334.
- ^ a b Barre L, Fournel-Gigleux S, Finel M, Netter P, Magdalou J, Ouzzine M (March 2007). "Substrate specificity of the human UDP-glucuronosyltransferase UGT2B4 and UGT2B7. Identification of a critical aromatic amino acid residue at position 33". FEBS J. 274 (5): 1256–64. doi:10.1111/j.1742-4658.2007.05670.x. PMID 17263731.
- ^ Coffman BL, Rios GR, King CD, Tephly TR (1 January 1997). "Human UGT2B7 catalyzes morphine glucuronidation". Drug Metab. Dispos. 25 (1): 1–4. PMID 9010622.
- ^ van Dorp EL, Romberg R, Sarton E, Bovill JG, Dahan A (2006). "Morphine-6-glucuronide: morphine's successor for postoperative pain relief?". Anesthesia and Analgesia. 102 (6): 1789–1797. doi:10.1213/01.ane.0000217197.96784.c3. PMID 16717327.
- ^ Coller JK, Christrup LL, Somogyi AA (2009). "Role of active metabolites in the use of opioids". European journal of clinical pharmacology. 65 (2): 121–39. doi:10.1007/s00228-008-0570-y. PMID 18958460.
- ^ Fujita K, Ando Y, Yamamoto W, Miya T, Endo H, Sunakawa Y, Araki K, Kodama K, Nagashima F, Ichikawa W, Narabayashi M, Akiyama Y, Kawara K, Shiomi M, Ogata H, Iwasa H, Okazaki Y, Hirose T, Sasaki Y (2009). "Association of UGT2B7 and ABCB1 genotypes with morphine-induced adverse drug reactions in Japanese patients with cancer". Cancer chemotherapy and pharmacology. 65 (2): 251–8. doi:10.1007/s00280-009-1029-2. PMID 19466410.
- ^ Abildskov K, Weldy P, Garland M (2010). "Molecular Cloning of the Baboon UDP-Glucuronosyltransferase 2B Gene Family and Their Activity in Conjugating Morphine". Drug metabolism and disposition: the biological fate of chemicals. 38 (4): 545–53. doi:10.1124/dmd.109.030635. PMC 2845934. PMID 20071451.
- ^ Pergolizzi JV, Raffa RB, Gould E (2009). "Considerations on the use of oxymorphone in geriatric patients". Expert opinion on drug safety. 8 (5): 603–13. doi:10.1517/14740330903153854. PMID 19614559.
- ^ Rouguieg K, Picard N, Sauvage FL, Gaulier JM, Marquet P (2010). "Contribution of the different UDP-glucuronosyltransferase (UGT) isoforms to buprenorphine and norbuprenorphine metabolism and relationship with the main UGT polymorphisms in a bank of human liver microsomes". Drug metabolism and disposition: the biological fate of chemicals. 38 (1): 40–5. doi:10.1124/dmd.109.029546. PMID 19841060.
- ^ Lampe, Jed N. (2017). "Advances in the Understanding of Protein-Protein Interactions in Drug Metabolizing Enzymes through the Use of Biophysical Techniques". Frontiers in Pharmacology. 8: 521. doi:10.3389/fphar.2017.00521. ISSN 1663-9812. PMC 5550701. PMID 28848438.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Miley, Michael J.; Zielinska, Agnieszka K.; Keenan, Jeffrey E.; Bratton, Stacie M.; Radominska-Pandya, Anna; Redinbo, Matthew R. (2007-06-01). "Crystal structure of the cofactor-binding domain of the human phase II drug-metabolism enzyme UDP-glucuronosyltransferase 2B7". Journal of Molecular Biology. 369 (2): 498–511. doi:10.1016/j.jmb.2007.03.066. ISSN 0022-2836. PMC 1976284. PMID 17442341.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b Yuan, Lingmin; Qian, Sainan; Xiao, Yongsheng; Sun, Hongying; Zeng, Su (2015-05-01). "Homo- and hetero-dimerization of human UDP-glucuronosyltransferase 2B7 (UGT2B7) wild type and its allelic variants affect zidovudine glucuronidation activity". Biochemical Pharmacology. 95 (1): 58–70. doi:10.1016/j.bcp.2015.03.002. ISSN 1873-2968. PMID 25770680.
Further reading
- Kwara A, Lartey M, Boamah I, Rezk NL, Oliver-Commey J, Kenu E, Kashuba AD, Court MH (2009). "Interindividual Variability in Pharmacokinetics of Generic Nucleoside Reverse Transcriptase Inhibitors in TB/HIV Co-infected Ghanaian Patients: UGT2B7*1C is Associated with Faster Zidovudine Clearance and Glucuronidation". J Clin Pharmacol. 49 (9): 1079–90. doi:10.1177/0091270009338482. PMC 2749505. PMID 19628728.
- Canzian F, Cox DG, Setiawan VW, Stram DO, Ziegler RG, Dossus L, Beckmann L, Blanché H, Barricarte A, Berg CD, Bingham S, Buring J, Buys SS, Calle EE, Chanock SJ, Clavel-Chapelon F, DeLancey JO, Diver WR, Dorronsoro M, Haiman CA, Hallmans G, Hankinson SE, Hunter DJ, Hüsing A, Isaacs C, Khaw KT, Kolonel LN, Kraft P, Le Marchand L, Lund E, Overvad K, Panico S, Peeters PH, Pollak M, Thun MJ, Tjønneland A, Trichopoulos D, Tumino R, Yeager M, Hoover RN, Riboli E, Thomas G, Henderson BE, Kaaks R, Feigelson HS (2010). "Comprehensive analysis of common genetic variation in 61 genes related to steroid hormone and insulin-like growth factor-I metabolism and breast cancer risk in the NCI breast and prostate cancer cohort consortium". Hum. Mol. Genet. 19 (19): 3873–84. doi:10.1093/hmg/ddq291. PMC 2935856. PMID 20634197.
- Hwang MS, Lee SJ, Jeong HE, Lee S, Yoo MA, Shin JG (2010). "Genetic variations in UDP-glucuronosyltransferase 2B7 gene (UGT2B7) in a Korean population". Drug Metab. Pharmacokinet. 25 (4): 398–402. doi:10.2133/dmpk.DMPK-10-SC-021. PMID 20814162.
- Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR (2010). "Personalized Smoking Cessation: Interactions between Nicotine Dose, Dependence and Quit-Success Genotype Score". Mol. Med. 16 (7–8): 247–53. doi:10.2119/molmed.2009.00159. PMC 2896464. PMID 20379614.
- Holmes MV, Shah T, Vickery C, Smeeth L, Hingorani AD, Casas JP (2009). "Fulfilling the Promise of Personalized Medicine? Systematic Review and Field Synopsis of Pharmacogenetic Studies". PLoS ONE. 4 (12): e7960. doi:10.1371/journal.pone.0007960. PMC 2778625. PMID 19956635.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Kwara A, Lartey M, Sagoe KW, Kenu E, Court MH (2009). "CYP2B6, CYP2A6 and UGT2B7 Genetic Polymorphisms are Predictors of Efavirenz Mid-dose Concentration in HIV-infected Patients". AIDS. 23 (16): 2101–6. doi:10.1097/QAD.0b013e3283319908. PMC 2875867. PMID 19779319.
- Setlur SR, Chen CX, Hossain RR, Ha JS, Van Doren VE, Stenzel B, Steiner E, Oldridge D, Kitabayashi N, Banerjee S, Chen JY, Schäfer G, Horninger W, Lee C, Rubin MA, Klocker H, Demichelis F (2010). "Genetic variation of genes involved in dihydrotestosterone metabolism and the risk of prostate cancer". Cancer Epidemiol. Biomarkers Prev. 19 (1): 229–39. doi:10.1158/1055-9965.EPI-09-1018. PMID 20056642.
- Sánchez MB, Herranz JL, Leno C, Arteaga R, Oterino A, Valdizán EM, Nicolás JM, Adín J, Armijo JA (2010). "Genetic factors associated with drug-resistance of epilepsy: relevance of stratification by patient age and aetiology of epilepsy". Seizure. 19 (2): 93–101. doi:10.1016/j.seizure.2009.12.004. PMID 20064729.
- Chen M, LeDuc B, Kerr S, Howe D, Williams DA (2010). "Identification of human UGT2B7 as the major isoform involved in the O-glucuronidation of chloramphenicol". Drug Metab. Dispos. 38 (3): 368–75. doi:10.1124/dmd.109.029900. PMID 20008037.
- Ross CJ, Katzov-Eckert H, Dubé MP, Brooks B, Rassekh SR, Barhdadi A, Feroz-Zada Y, Visscher H, Brown AM, Rieder MJ, Rogers PC, Phillips MS, Carleton BC, Hayden MR (2009). "Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy". Nat. Genet. 41 (12): 1345–9. doi:10.1038/ng.478. PMID 19898482.
- Tang L, Ye L, Singh R, Wu B, Lv C, Zhao J, Liu Z, Hu M (2010). "Use of Glucuronidation Fingerprinting to Describe and Predict mono- and di-Hydroxyflavone Metabolism by Recombinant UGT Isoforms and Human Intestinal and Liver Microsomes". Mol. Pharm. 7 (3): 664–79. doi:10.1021/mp900223c. PMC 2941766. PMID 20297805.
- Woillard JB, Rerolle JP, Picard N, Rousseau A, Drouet M, Munteanu E, Essig M, Marquet P, Le Meur Y (2010). "Risk of diarrhoea in a long-term cohort of renal transplant patients given mycophenolate mofetil: the significant role of the UGT1A8*2 variant allele". Br J Clin Pharmacol. 69 (6): 675–83. doi:10.1111/j.1365-2125.2010.03625.x. PMC 2883760. PMID 20565459.
- Yu L, Qian M, Liu Y, Yao T, Zeng S (2010). "Stereoselective metabolism of propranolol glucuronidation by human UDP-glucuronosyltransferases 2B7 and 1A9". Chirality. 22 (4): 456–61. doi:10.1002/chir.20765. PMID 19644937.
- Yang JW, Lee PH, Hutchinson IV, Pravica V, Shah T, Min DI (2009). "Genetic polymorphisms of MRP2 and UGT2B7 and gastrointestinal symptoms in renal transplant recipients taking mycophenolic acid". Ther Drug Monit. 31 (5): 542–8. doi:10.1097/FTD.0b013e3181b1dd5e. PMID 19730281.
- Ahn J, Schumacher FR, Berndt SI, Pfeiffer R, Albanes D, Andriole GL, Ardanaz E, Boeing H, Bueno-de-Mesquita B, Chanock SJ, Clavel-Chapelon F, Diver WR, Feigelson HS, Gaziano JM, Giovannucci E, Haiman CA, Henderson BE, Hoover RN, Kolonel LN, Kraft P, Ma J, Le Marchand L, Overvad K, Palli D, Stattin P, Stampfer M, Stram DO, Thomas G, Thun MJ, Travis RC, Trichopoulos D, Virtamo J, Weinstein SJ, Yeager M, Kaaks R, Hunter DJ, Hayes RB (2009). "Quantitative trait loci predicting circulating sex steroid hormones in men from the NCI-Breast and Prostate Cancer Cohort Consortium (BPC3)". Hum. Mol. Genet. 18 (19): 3749–57. doi:10.1093/hmg/ddp302. PMC 2742399. PMID 19574343.
- Hu M, Lui SS, Mak VW, Chu TT, Lee VW, Poon EW, Tsui TK, Ko GT, Baum L, Tam LS, Li EK, Tomlinson B (2010). "Pharmacogenetic analysis of lipid responses to rosuvastatin in Chinese patients". Pharmacogenet. Genomics. 20 (10): 634–7. doi:10.1097/FPC.0b013e32833de489. PMID 20679960.
- Zhao W, Fakhoury M, Deschênes G, Roussey G, Brochard K, Niaudet P, Tsimaratos M, André JL, Cloarec S, Cochat P, Bensman A, Azougagh S, Jacqz-Aigrain E (2010). "Population Pharmacokinetics and Pharmacogenetics of Mycophenolic Acid Following Administration of Mycophenolate Mofetil in De Novo Pediatric Renal-Transplant Patients". Journal of clinical pharmacology. 50 (11): 1280–91. doi:10.1177/0091270009357429. PMID 20147615.
- Blanca Sánchez M, Herranz JL, Leno C, Arteaga R, Oterino A, Valdizán EM, Nicolas JM, Adín J, Shushtarian M, Armijo JA (2010). "UGT2B7_-161C>T polymorphism is associated with lamotrigine concentration-to-dose ratio in a multivariate study". Ther Drug Monit. 32 (2): 177–84. doi:10.1097/FTD.0b013e3181ceecc6. PMID 20216122.
- Yong M, Schwartz SM, Atkinson C, Makar KW, Thomas SS, Newton KM, Aiello Bowles EJ, Holt VL, Leisenring WM, Lampe JW (2010). "Associations between polymorphisms in glucuronidation and sulfation enzymes and mammographic breast density in premenopausal women in the United States". Cancer Epidemiol. Biomarkers Prev. 19 (2): 537–46. doi:10.1158/1055-9965.EPI-09-0898. PMC 2820123. PMID 20142249.
- Joy MS, Boyette T, Hu Y, Wang J, La M, Hogan SL, Stewart PW, Falk RJ, Dooley MA, Smith PC (2010). "Effects of uridine diphosphate glucuronosyltransferase 2B7 and 1A7 pharmacogenomics and patient clinical parameters on steady-state mycophenolic acid pharmacokinetics in glomerulonephritis". European journal of clinical pharmacology. 66 (11): 1119–30. doi:10.1007/s00228-010-0846-x. PMID 20567810.
External links
- human+UGT2B7 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- UGT2B7+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- "GT2B7". PharmGKB. PharmGKB. Retrieved 2009-01-13.




