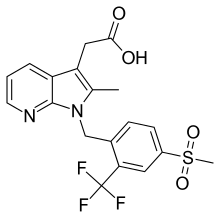
Fevipiprant
Appearance
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unaffected by food[1] |
| Metabolism | Hepatic glucuronidation |
| Elimination half-life | ~20 hours |
| Excretion | Renal (≤30%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.243.911 |
| Chemical and physical data | |
| Formula | C19H17F3N2O4S |
| Molar mass | 426.41 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fevipiprant (INN; code name QAW039) is a drug being developed by Novartis which acts as a selective, orally available antagonist of the prostaglandin D2 receptor 2 (DP2 or CRTh2).[1][2][3]
As of 2016[update], it is in phase III[4] clinical trials for the treatment of asthma.[5]
On Monday, December 16, 2019, Switzerland-based Novartis officially announced that it was jettisoning fevipiprant from its development program, given that the medicine has failed in two additional clinical trials in patients with moderate-to-severe asthma. The firm said that it had hoped fevipiprant would be a billion-dollar-selling asthma drug.[6]
See also
References
- ^ a b Erpenbeck VJ, Vets E, Gheyle L, Osuntokun W, Larbig M, Neelakantham S, et al. (2016). "Pharmacokinetics, Safety, and Tolerability of Fevipiprant (QAW039), a Novel CRTh2 Receptor Antagonist: Results From 2 Randomized, Phase 1, Placebo-Controlled Studies in Healthy Volunteers". Clinical Pharmacology in Drug Development. 5 (4): 306–13. doi:10.1002/cpdd.244. PMC 5071756. PMID 27310331.
- ^ Sykes DA, Bradley ME, Riddy DM, Willard E, Reilly J, Miah A, Bauer C, Watson SJ, Sandham DA, Dubois G, Charlton SJ (May 2016). "Fevipiprant (QAW039), a Slowly Dissociating CRTh2 Antagonist with the Potential for Improved Clinical Efficacy". Mol Pharmacol. 89 (5): 593–605. doi:10.1124/mol.115.101832. PMID 26916831.
- ^ Erpenbeck VJ, Popov TA, Miller D, Weinstein SF, Spector S, Magnusson B, et al. (2016). "The oral CRTh2 antagonist QAW039 (fevipiprant): A phase II study in uncontrolled allergic asthma". Pulm Pharmacol Ther. 39: 54–63. doi:10.1016/j.pupt.2016.06.005. PMID 27354118.
- ^ https://clinicaltrials.gov/ct2/show/NCT02555683
- ^ Gonem S, Berair R, Singapuri A, Hartley R, Laurencin M, Bacher G, et al. (2016). "Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial". Lancet Respir Med. 4 (9): 699–707. doi:10.1016/S2213-2600(16)30179-5. hdl:2381/38430. PMID 27503237.
- ^ Novartis drops asthma drug fevipiprant after trial failures. Reuters News. https://www.reuters.com/article/us-novartis-asthma/novartis-drops-asthma-drug-fevipiprant-after-trial-failures-idUSKBN1YK0DR - Accessed Sunday, December 15, 2019, from North America.
