Tropone: Difference between revisions
m Task 18 (cosmetic): eval 3 templates: del empty params (1×); |
added a new section on tropone derivatives |
||
| Line 78: | Line 78: | ||
:[[Image:TroponeAnnulationReaction.png|400px|Tropone annulation reaction]]{{clear-left}} |
:[[Image:TroponeAnnulationReaction.png|400px|Tropone annulation reaction]]{{clear-left}} |
||
==Derivatives== |
|||
{| class="wikitable sortable" |
|||
|- |
|||
! Name |
|||
! Chemical structure |
|||
! Natural sources |
|||
|- |
|||
| [[Tropolone]] |
|||
| [[File:Tropolone.png|70px|center]] |
|||
| ''Pseudomonas lindbergii'', ''[[Pseudomonas plantarii]]''<ref>{{cite journal |last1=Liu |first1=Na |last2=Song |first2=Wangze |last3=Schienebeck |first3=Casi M. |last4=Zhang |first4=Min |last5=Tang |first5=Weiping |title=Synthesis of naturally occurring tropones and tropolones |journal=Tetrahedron |date=December 2014 |volume=70 |issue=49 |pages=9281–9305 |doi=10.1016/j.tet.2014.07.065}}</ref> |
|||
|- |
|||
| [[Hinokitiol]] |
|||
| [[File:Gamma-thujaplicin.png|70px|center]] |
|||
| ''[[Cupressaceae]]'' trees<ref>{{cite journal |last1=Saniewski |first1=Marian |last2=Horbowicz |first2=Marcin |last3=Kanlayanarat |first3=Sirichai |title=The Biological Activities of Troponoids and Their Use in Agriculture A Review |journal=Journal of Horticultural Research |date=10 September 2014 |volume=22 |issue=1 |pages=5–19 |doi=10.2478/johr-2014-0001}}</ref> |
|||
|- |
|||
| [[Stipitatic acid]] |
|||
| [[File:Stipitatic acid.png|110px|center]] |
|||
| ''[[Talaromyces stipitatus]]''<ref>{{cite journal |last1=Davison |first1=J. |last2=al Fahad |first2=A. |last3=Cai |first3=M. |last4=Song |first4=Z. |last5=Yehia |first5=S. Y. |last6=Lazarus |first6=C. M. |last7=Bailey |first7=A. M. |last8=Simpson |first8=T. J. |last9=Cox |first9=R. J. |title=Genetic, molecular, and biochemical basis of fungal tropolone biosynthesis |journal=Proceedings of the National Academy of Sciences |date=15 May 2012 |volume=109 |issue=20 |pages=7642–7647 |doi=10.1073/pnas.1201469109}}</ref> |
|||
|- |
|||
| [[Tropodithietic acid]] |
|||
| [[File:Tropodithietic acid.svg|90px|center]] |
|||
| ''[[Phaeobacter piscinae]]'', ''[[Phaeobacter inhibens]]'', ''[[Phaeobacter gallaeciensis]]''<ref>{{cite journal |last1=Rabe |first1=Patrick |last2=Klapschinski |first2=Tim A |last3=Brock |first3=Nelson L |last4=Citron |first4=Christian A |last5=D’Alvise |first5=Paul |last6=Gram |first6=Lone |last7=Dickschat |first7=Jeroen S |title=Synthesis and bioactivity of analogues of the marine antibiotic tropodithietic acid |journal=Beilstein Journal of Organic Chemistry |date=6 August 2014 |volume=10 |pages=1796–1801 |doi=10.3762/bjoc.10.188}}</ref><ref>{{cite journal |last1=Beyersmann |first1=Paul G. |last2=Tomasch |first2=Jürgen |last3=Son |first3=Kwangmin |last4=Stocker |first4=Roman |last5=Göker |first5=Markus |last6=Wagner-Döbler |first6=Irene |last7=Simon |first7=Meinhard |last8=Brinkhoff |first8=Thorsten |title=Dual function of tropodithietic acid as antibiotic and signaling molecule in global gene regulation of the probiotic bacterium Phaeobacter inhibens |journal=Scientific Reports |date=December 2017 |volume=7 |issue=1 |pages=730 |doi=10.1038/s41598-017-00784-7}}</ref> |
|||
|- |
|||
| [[Colchicine]] |
|||
| [[File:Colchicin.svg|180px|center]] |
|||
| ''[[Colchicum autumnale]]'', ''[[Gloriosa superba]]''<ref>{{cite journal |last1=Keith |first1=Michael P. |last2=Gilliland |first2=William R. |last3=Uhl |first3=Kathleen |title=GOUT |journal=Pharmacology and Therapeutics |date=2009 |pages=1039–1046 |doi=10.1016/B978-1-4160-3291-5.50079-2}}</ref> |
|||
|} |
|||
Other tropone derivatives include puberulonic and puberulic acids, roseobacticides, pernambucone, crototropone, orobanone.<ref>{{cite journal |last1=Thiel |first1=Verena |last2=Brinkhoff |first2=Thorsten |last3=Dickschat |first3=Jeroen S. |last4=Wickel |first4=Susanne |last5=Grunenberg |first5=Jörg |last6=Wagner-Döbler |first6=Irene |last7=Simon |first7=Meinhard |last8=Schulz |first8=Stefan |title=Identification and biosynthesis of tropone derivatives and sulfur volatiles produced by bacteria of the marine Roseobacter clade |journal=Organic & Biomolecular Chemistry |date=10 December 2009 |volume=8 |issue=1 |pages=234–246 |doi=10.1039/B909133E#}}</ref><ref>{{cite journal |last1=Duan |first1=Ying |last2=Petzold |first2=Melanie |last3=Saleem‐Batcha |first3=Raspudin |last4=Teufel |first4=Robin |title=Bacterial Tropone Natural Products and Derivatives: Overview of their Biosynthesis, Bioactivities, Ecological Role and Biotechnological Potential |journal=ChemBioChem |date=September 2020 |volume=21 |issue=17 |pages=2384–2407 |doi=10.1002/cbic.201900786}}</ref><ref>{{cite journal |last1=Randau |first1=K. P. |last2=Sproll |first2=S. |last3=Lerche |first3=H. |last4=Bracher |first4=F. |title=Pernambucone, a new tropone derivative from Croton argyroglossum |journal=Die Pharmazie - An International Journal of Pharmaceutical Sciences |date=1 May 2009 |volume=64 |issue=5 |pages=350–351 |doi=10.1691/ph.2009.7592#}}</ref><ref>{{cite journal |last1=Bracher |first1=Franz |last2=Randau |first2=Karina P. |last3=Lerche |first3=Holger |title=Crototropone, a new tropone derivative from Croton zehntneri |journal=Fitoterapia |date=1 April 2008 |volume=79 |issue=3 |pages=236–237 |doi=10.1016/j.fitote.2007.12.001#}}</ref><ref>{{cite journal |last1=Fruchier |first1=Alain |last2=Rascol |first2=Jean-Pierre |last3=Andary |first3=Claude |last4=Privatt |first4=Guy |title=A tropone derivative from orobanche rapum-genistae |journal=Phytochemistry |date=1 January 1981 |volume=20 |issue=4 |pages=777–779 |doi=10.1016/0031-9422(81)85173-4#}}</ref> |
|||
==References== |
==References== |
||
Revision as of 20:01, 7 January 2021
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclohepta-2,4,6-trien-1-one | |||
| Other names
Cyclohepta-2,4,6-trienone
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.007.933 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H6O | |||
| Molar mass | 106.12 g/mol | ||
| Density | 1.094 g/mL | ||
| Boiling point | 113 °C (235 °F; 386 K) (15 mmHg) | ||
| Hazards | |||
| Flash point | > 113 °C (235 °F; 386 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tropone or 2,4,6-cycloheptatrien-1-one is an organic compound with some importance in organic chemistry as a non-benzenoid aromatic.[2] The compound consists of a ring of seven carbon atoms with three conjugated alkene groups and a ketone group. The related compound tropolone (2-hydroxy-2,4,6-cycloheptatrien-1-one) has an additional alcohol (or an enol including the double bond) group next to the ketone.
The tropone moiety can be found in biomolecules such as colchicine, stipitatic acid and hinokitiol.
Tropone has been known since 1951 and is also called cycloheptatrienylium oxide. The name tropolone was coined by M. J. S. Dewar in 1945 in connection to perceived aromatic properties.[3]
Properties
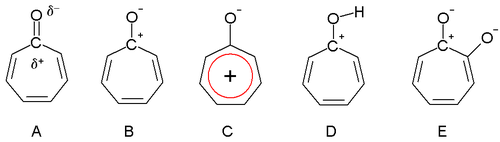
Dewar in 1945 proposed that tropones could have aromatic properties. The carbonyl group is polarized with a partial positive charge on the carbon atom (A) and a partial negative charge on oxygen. In an extreme case the carbon atom has a full positive charge (B) forming a tropylium ion ring which is an aromatic 6 electron system (C).
Tropolone is acidic (conjugate base shown, E) with a pKa of 7 which is in between that of phenol (10) and benzoic acid (4). The increased acidity compared to phenol is due to regular resonance stabilization. Tropones and to a lesser extent tropolones are also basic (D) and this is very much due to aromatic stabilization. This property can be observed in the ease of salt formation with acids. The dipole moment for tropone is 4.17 D compared to a value of only 3.04 D for cycloheptanone, which can also be taken as evidence for aromaticity.
Synthesis
Numerous methods exist for the organic synthesis of tropones and its derivatives. Two selected methods for the synthesis of tropone are by selenium dioxide oxidation of cycloheptatriene[4] and indirectly from tropinone by a Hofmann elimination and a bromination.[2]
Two methods for the synthesis of tropolone are by bromination of 1,2-cycloheptanedione with N-bromosuccinimide followed by dehydrohalogenation at elevated temperatures and by acyloin condensation of the ethyl ester of pimelic acid the acyloin again followed by oxidation by bromine.[2]
Reactions
- Tropone undergoes ring contraction to benzoic acid with potassium hydroxide at elevated temperature. Many derivatives also contract to the corresponding arene compounds.[2]
- Tropone reacts in electrophilic substitution, for instance with bromine, but the reaction proceeds through the 1,2-addition product and is not an electrophilic aromatic substitution.[2]
- Tropone derivatives also react in nucleophilic substitution very much like in nucleophilic aromatic substitution.[2]
- Tropone is a diene in a Diels-Alder reaction, for instance with maleic anhydride.[2]
- Tropone is also found to react in an [8+3]annulation with a cinnamic aldehyde[5]
Derivatives
| Name | Chemical structure | Natural sources |
|---|---|---|
| Tropolone |  |
Pseudomonas lindbergii, Pseudomonas plantarii[6] |
| Hinokitiol |  |
Cupressaceae trees[7] |
| Stipitatic acid |  |
Talaromyces stipitatus[8] |
| Tropodithietic acid |  |
Phaeobacter piscinae, Phaeobacter inhibens, Phaeobacter gallaeciensis[9][10] |
| Colchicine |  |
Colchicum autumnale, Gloriosa superba[11] |
Other tropone derivatives include puberulonic and puberulic acids, roseobacticides, pernambucone, crototropone, orobanone.[12][13][14][15][16]
References
- ^ Tropone at Sigma-Aldrich
- ^ a b c d e f g Pauson, Peter L. (1955). "Tropones and Tropolones". Chem. Rev. 55 (1): 9–136. doi:10.1021/cr50001a002.
- ^ M. J. S. Dewar (1945). "Structure of Stipitatic Acid". Nature. 155 (3924): 50–51. doi:10.1038/155050b0.
- ^ Dahnke, Karl R.; Paquette, Leo A. (1993). "Inverse Electron-Demand Diels-Alder Cycloaddition of a Ketene Dithioacetal. Copper Hydride-Promoted Reduction of a Conjugated Enone. 9-Dithiolanobicyclo[3.2.2]non-6-en-2-one". Org. Synth. 71: 181.
- ^ An N-Heterocyclic Carbene-Catalyzed [8 + 3] Annulation of Tropone and Enals via Homoenolate Vijay Nair, Manojkumar Poonoth, Sreekumar Vellalath, Eringathodi Suresh, and Rajasekaran Thirumalai J. Org. Chem.; 2006; 71(23) pp 8964 - 8965; (Note) doi:10.1021/jo0615706
- ^ Liu, Na; Song, Wangze; Schienebeck, Casi M.; Zhang, Min; Tang, Weiping (December 2014). "Synthesis of naturally occurring tropones and tropolones". Tetrahedron. 70 (49): 9281–9305. doi:10.1016/j.tet.2014.07.065.
- ^ Saniewski, Marian; Horbowicz, Marcin; Kanlayanarat, Sirichai (10 September 2014). "The Biological Activities of Troponoids and Their Use in Agriculture A Review". Journal of Horticultural Research. 22 (1): 5–19. doi:10.2478/johr-2014-0001.
- ^ Davison, J.; al Fahad, A.; Cai, M.; Song, Z.; Yehia, S. Y.; Lazarus, C. M.; Bailey, A. M.; Simpson, T. J.; Cox, R. J. (15 May 2012). "Genetic, molecular, and biochemical basis of fungal tropolone biosynthesis". Proceedings of the National Academy of Sciences. 109 (20): 7642–7647. doi:10.1073/pnas.1201469109.
- ^ Rabe, Patrick; Klapschinski, Tim A; Brock, Nelson L; Citron, Christian A; D’Alvise, Paul; Gram, Lone; Dickschat, Jeroen S (6 August 2014). "Synthesis and bioactivity of analogues of the marine antibiotic tropodithietic acid". Beilstein Journal of Organic Chemistry. 10: 1796–1801. doi:10.3762/bjoc.10.188.
- ^ Beyersmann, Paul G.; Tomasch, Jürgen; Son, Kwangmin; Stocker, Roman; Göker, Markus; Wagner-Döbler, Irene; Simon, Meinhard; Brinkhoff, Thorsten (December 2017). "Dual function of tropodithietic acid as antibiotic and signaling molecule in global gene regulation of the probiotic bacterium Phaeobacter inhibens". Scientific Reports. 7 (1): 730. doi:10.1038/s41598-017-00784-7.
- ^ Keith, Michael P.; Gilliland, William R.; Uhl, Kathleen (2009). "GOUT". Pharmacology and Therapeutics: 1039–1046. doi:10.1016/B978-1-4160-3291-5.50079-2.
- ^ Thiel, Verena; Brinkhoff, Thorsten; Dickschat, Jeroen S.; Wickel, Susanne; Grunenberg, Jörg; Wagner-Döbler, Irene; Simon, Meinhard; Schulz, Stefan (10 December 2009). "Identification and biosynthesis of tropone derivatives and sulfur volatiles produced by bacteria of the marine Roseobacter clade". Organic & Biomolecular Chemistry. 8 (1): 234–246. doi:10.1039/B909133E#.
- ^ Duan, Ying; Petzold, Melanie; Saleem‐Batcha, Raspudin; Teufel, Robin (September 2020). "Bacterial Tropone Natural Products and Derivatives: Overview of their Biosynthesis, Bioactivities, Ecological Role and Biotechnological Potential". ChemBioChem. 21 (17): 2384–2407. doi:10.1002/cbic.201900786.
- ^ Randau, K. P.; Sproll, S.; Lerche, H.; Bracher, F. (1 May 2009). "Pernambucone, a new tropone derivative from Croton argyroglossum". Die Pharmazie - An International Journal of Pharmaceutical Sciences. 64 (5): 350–351. doi:10.1691/ph.2009.7592#.
- ^ Bracher, Franz; Randau, Karina P.; Lerche, Holger (1 April 2008). "Crototropone, a new tropone derivative from Croton zehntneri". Fitoterapia. 79 (3): 236–237. doi:10.1016/j.fitote.2007.12.001#.
- ^ Fruchier, Alain; Rascol, Jean-Pierre; Andary, Claude; Privatt, Guy (1 January 1981). "A tropone derivative from orobanche rapum-genistae". Phytochemistry. 20 (4): 777–779. doi:10.1016/0031-9422(81)85173-4#.