Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
June 15
This element does not exist in our periodic table
Recently many movies have alien ship crashing to earth and the scientists always says this: After our spectrometry analysis we find out that this object element does not exist in our periodic table. My question is, is that possible? Can the alien have finally find the mythical island of stability? Or they shouldn't need that at all because they probably have the tech to make force field? — Preceding unsigned comment added by 118.136.5.235 (talk) 02:23, 15 June 2013 (UTC)
- I think you've answered your own question. Bearing mind that creators of fiction can do whatever they want in their stories, the island of stability could be this, I suppose. Mingmingla (talk) 02:44, 15 June 2013 (UTC)
- Unobtainium may be an interesting read for the OP. --Jayron32 02:54, 15 June 2013 (UTC)
- Wouldn't the spectral data from new, super heavy elements be recognizable as such? I mean, especially if it's ICP-MS...the software wouldn't be able to identify the element, but the charge to mass ratio should just tell you straight off which element it is, even if we never knew that it existed before. 202.155.85.18 (talk) 03:15, 15 June 2013 (UTC)
- Since this is fiction, Clarke's third law bears reading and understanding in relation to this. --Jayron32 03:31, 15 June 2013 (UTC)
- Things past Element 173 are said not to exist, and I doubt the mass spec leaves room in its detector for them. :) It is entirely conceivable that if you're staring at a lump of Element 3501, you'll be seeing some physics you never guessed at. :) Wnt (talk) 04:04, 15 June 2013 (UTC)
- Actually, there is no theoretical limit preventing elements from going past #173. Whoop whoop pull up Bitching Betty | Averted crashes 14:10, 15 June 2013 (UTC)
- Mass spec can certainly detect things of rather large mass, MALDI can get mass spec of proteins and DNA whose mass runs in the tens of thousands of daltons or more. --Jayron32 04:17, 15 June 2013 (UTC)
- I may well be wrong here, but MALDI uses a fairly special time-of-flight mass spectrometry with a large mass range. I would assume that if you have some instrument where you vaporize a bit of metal to see what elements are in it, that you'd be using maybe a sector instrument or a quadrupole mass analyzer with (perhaps) a far more restricted range? I'll admit though, I'm out of my expertise entirely where that's concerned. Wnt (talk) 05:52, 15 June 2013 (UTC)
- MALDI is going to several thousands of u. If you assume a island of stability there you should go for a ToF instrument as you suggest. For me this looks a little bit strange to assume that but if gravity takes over the stabilisation there might be a chemical element very similar to a neutron star. This is a little bit larger than what a ToF can handle.--Stone (talk) 06:10, 15 June 2013 (UTC)
- I may well be wrong here, but MALDI uses a fairly special time-of-flight mass spectrometry with a large mass range. I would assume that if you have some instrument where you vaporize a bit of metal to see what elements are in it, that you'd be using maybe a sector instrument or a quadrupole mass analyzer with (perhaps) a far more restricted range? I'll admit though, I'm out of my expertise entirely where that's concerned. Wnt (talk) 05:52, 15 June 2013 (UTC)
- Things past Element 173 are said not to exist, and I doubt the mass spec leaves room in its detector for them. :) It is entirely conceivable that if you're staring at a lump of Element 3501, you'll be seeing some physics you never guessed at. :) Wnt (talk) 04:04, 15 June 2013 (UTC)
- Since this is fiction, Clarke's third law bears reading and understanding in relation to this. --Jayron32 03:31, 15 June 2013 (UTC)
- Wouldn't the spectral data from new, super heavy elements be recognizable as such? I mean, especially if it's ICP-MS...the software wouldn't be able to identify the element, but the charge to mass ratio should just tell you straight off which element it is, even if we never knew that it existed before. 202.155.85.18 (talk) 03:15, 15 June 2013 (UTC)
- I don't think we can totally rule out the possibility of making exotic atoms out of subatomic particles that go beyond the usual protons, neutrons, and electrons. We don't know any way to make anything exotic that is stable, but I don't think we can absolutely prove that it is impossible. Looie496 (talk) 15:25, 15 June 2013 (UTC)
- Right. The way the periodic table is set up, anything composed of protons, electrons and neutrons would be on it, even if you'd have to extend it for things with lots of protons. However, there are a number of exotic atoms which don't fit the proton+electon(+neutron) model of normal atoms, and as such "would not exist in our periodic table". All the ones we know about (and all the ones we've theorized to exist) are unstable - often rapidly so, so even if you were able to get a sizable chunk of it, it would rapidly disapper. (So unfortunately no super-strong ship hulls, etc.) - That said, when exotic atoms are used in fiction, they're always a plot device. TV Tropes calls it Applied Phlebotinum - it's just a way to pretend to explain off unrealistic or exotic effects: "Why doesn't the blaster melt in lava? It's made of unobtainium - now let's get back to shooting things." -- 71.35.105.42 (talk) 18:59, 15 June 2013 (UTC)
- That's certainly true - "unobtainium" is a way out for lazy authors. But although we're reasonably sure that we know about all of the stable elements - and we've mapped the properties of all of their stable isotopes - we haven't explored all of the compounds that can be made from them - and even more significant, we're just scratching the surface of what can be made from nano-structures and smart materials. We can be pretty sure that there isn't a metal that would withstand our most powerful weapons in thicknesses still light enough to build a spaceship hull from. But we certainly don't know whether there isn't some kind of clever material that could heal up puncture holes or ablate to dissipate energy. It's almost certain that there are no magical new elements - but it's just as certain that a sufficiently advanced civilization could find some means to combine them in ways we've never imagined to produce magical-seeming materials. You only have to look at the strides that material science had made in the last few decades to realize that we've got a long way to go yet.
- SteveBaker (talk) 20:29, 15 June 2013 (UTC)
- Right. The way the periodic table is set up, anything composed of protons, electrons and neutrons would be on it, even if you'd have to extend it for things with lots of protons. However, there are a number of exotic atoms which don't fit the proton+electon(+neutron) model of normal atoms, and as such "would not exist in our periodic table". All the ones we know about (and all the ones we've theorized to exist) are unstable - often rapidly so, so even if you were able to get a sizable chunk of it, it would rapidly disapper. (So unfortunately no super-strong ship hulls, etc.) - That said, when exotic atoms are used in fiction, they're always a plot device. TV Tropes calls it Applied Phlebotinum - it's just a way to pretend to explain off unrealistic or exotic effects: "Why doesn't the blaster melt in lava? It's made of unobtainium - now let's get back to shooting things." -- 71.35.105.42 (talk) 18:59, 15 June 2013 (UTC)
- Strange matter and the stranglets made from it are a "possible" way to get elements that are not in the table. Dauto (talk) 23:06, 15 June 2013 (UTC)
- I vote for hypertungsten. Plasmic Physics (talk) 23:36, 15 June 2013 (UTC)
- Let we speculate a bit now: What properties will the strange matter/hypernucleus/island of stability matter have? Maybe they are sufficiently dense to make its own gravity field that can mess up with projectiles? Maybe because of there is so many atoms between that they become strong? Its just an spaceship why do they need strange stuff while our normal mundane titanium is enough? If they are going FTL a force field would be better because it will make it lighter 118.136.5.235 (talk) 00:27, 16 June 2013 (UTC)
- Strange matter could be more stable than ordinary matter. Iron is then not the most stable nucleus, it is then only meta-stable with an astronomically large lifetime. But strange matter could catalize the transition of ordinary matter to strange matter. So, a piece of strange matter hirtting the Earth could destroy the Earth in a gigantic nuclear explosion where all the matter in the Earth is converted to strange matter. Count Iblis (talk) 01:14, 16 June 2013 (UTC)
- I never understood why strangelets during an early hot epoch of the Big Bang wouldn't have taken everything over then. Wnt (talk) 01:44, 16 June 2013 (UTC)
- Look at a science book published every 5 years back for a few decades and you'll see we are constantly adding new elements and there surely are many more combinations of neutrons protons and electrons that form elements we not only have not included on the periodic table but that might just not exist on earth. Further we always discover elements way before we add them to the PT. My2¢ — Preceding unsigned comment added by 108.212.70.237 (talk) 04:40, 16 June 2013 (UTC)
- No - that's absolutely not true.
- Look at a science book published every 5 years back for a few decades and you'll see we are constantly adding new elements and there surely are many more combinations of neutrons protons and electrons that form elements we not only have not included on the periodic table but that might just not exist on earth. Further we always discover elements way before we add them to the PT. My2¢ — Preceding unsigned comment added by 108.212.70.237 (talk) 04:40, 16 June 2013 (UTC)
- The elements are numbered according to the number of protons they have. We know most of what it's possible to know about every single one of them from Hydrogen (1 proton) to somewhere up elements in the mid 110 range - so no new elements in that range are possible - even in principle. You're correct in saying that new elements are added every few years - but none of them with more protons than Einsteinium (element number 99 - discovered in 1952) is stable with a half life over a year - they all decay into something more mundane after a few days - so you can't build starships out of them. Not one element that has been discovered since 1952 has a half-life longer than a year...and there are no gaps in our table of the elements up to well over element 110 so no new discoveries of stable elements is now likely.
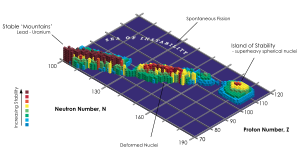
- The plot over on the right here is a graph of number of neutrons versus number of protons. The color and height of each column shows how stable each combination is. The dark brown columns are "stable" elements that would exist for more than a year or two. The red ones are stable for days to weeks and all of the other colors represent things that decay within hours to milliseconds. So only the brown combinations are useful for making physical objects. Viewed as a landscape, there is that island off to the top-right of the table. This is the hypothical "island of stability" - a bunch of elements that have yet to be discovered that might be stable. If any "unobtainium" starship hulls are to be found, they'd have to be made up of elements in that part of the table. Sadly, even the peak of that island is a red column...and that means that those very heavy elements are unlikely to be stable for more than hours to days. Only a very few "fringe" scientists believe that the peak on that island will be tall enough for elements found in that region to be stable for more than a few days...and nobody who knows what they're talking about believes that there will be other islands of stability in the further realms of that chart.
- Altering the number of neutrons does generate new isotopes - and some of those are moderately stable - but because the majority of the chemistry of atoms comes from the number of electrons (which equals the number of protons), these isotopes are unlikely to have much in the way of interesting properties - and, again, they are likely to be much less stable than the form of the element that we already know.
- Summary: Our current knowledge strongly suggests that the 99 elements up to Einsteinium (along with a handful of isotopes of each of them) are the only ones that will ever be sufficiently stable to be useful - and we know of every single one of them in wonderful detail. There are no "new elements" or "new isotopes" that could possibly be used to make a starship hull.
- The problem with that is that nobody has ever managed to make isotopes with lots and lots of neutrons - nothing to make them out of - so when you look at the tables, the cutoff of what is known is at the most stable point known. See Isotopes of livermorium, Isotopes of flerovium, etc. Wnt (talk) 18:30, 16 June 2013 (UTC)
- Now that would be just totally ignoring my suggestion. Plasmic Physics (talk) 11:30, 17 June 2013 (UTC)
- That is like LHC doomsday scenario so creepy because a single strange matter could destroy entire Earth... so if we have those how do we contain it? 118.136.5.235 (talk) 10:38, 16 June 2013 (UTC)
- You can't, strange matter is electrically neutral. Only gravity can keep it at bay, and last time I checked, we can't artificially cause gravity. Plasmic Physics (talk) 11:05, 16 June 2013 (UTC)
Structural steel bolts
What type of bot provides the most clearance? machine, high strength, rib, spline? — Preceding unsigned comment added by 67.101.160.118 (talk) 02:37, 15 June 2013 (UTC)
- Welcome to the Wikipedia Reference Desk. Your question appears to be a homework question. I apologize if this is a misinterpretation, but it is our aim here not to do people's homework for them, but to merely aid them in doing it themselves. Letting someone else do your homework does not help you learn nearly as much as doing it yourself. Please attempt to solve the problem or answer the question yourself first. If you need help with a specific part of your homework, feel free to tell us where you are stuck and ask for help. If you need help grasping the concept of a problem, by all means let us know. And if I may add a personal comment, this is the type of "learn the answer by rote without understanding the subject" question that Feynman so robustly decries in Surely You're Joking, Mr. Feynman!. Our articles Screw and Bolted joint may be useful. Tevildo (talk) 17:37, 15 June 2013 (UTC)
Cicadas in NJ?
Can anyone recommend a good location, centered on Camden and Gloucester Counties, NJ, for me to look for cicadas this weekend? I missed them in 1996, and only found one molting in 1979. Thanks! μηδείς (talk) 03:10, 15 June 2013 (UTC)
- No personal experience, but these maps [1], [2] are fairly reliable, from what I can tell. If you are interested, many people also enjoy eating them: [3]. SemanticMantis (talk) 04:05, 15 June 2013 (UTC)
- The maps are cool, but the dots range up to covering areas of hundreds of square miles. I would really need a source that says "spotted in Cooper River Park" or something like that to be helpful. Thanks. μηδείς (talk) 19:00, 15 June 2013 (UTC)
- This map depends on reports from the public, so it's not likely to be complete; but the closest locations to your area of interest that it currently shows are Franklinville and Pittsgrove. (Note that it shows only the 500 most recent reports, so there may have been earlier reports from the area.) Deor (talk) 09:41, 16 June 2013 (UTC)
- The maps are cool, but the dots range up to covering areas of hundreds of square miles. I would really need a source that says "spotted in Cooper River Park" or something like that to be helpful. Thanks. μηδείς (talk) 19:00, 15 June 2013 (UTC)
Reverse osmosis
My understanding of the process of reverse osmosis is that it is essentially molecular level filtering, where the solution is pumped through a membrane that allows the solvent molecules through, while excluding dissolved ions and molecules that are too large to pass through. If I understand the process correctly, then it seems that fluoride, with its small radius, should pass through a membrane more easily than water, so RO should be ineffective for its removal. The Wikipedia article on RO seems to say that only molecules are able to pass the membrane, and ions are always rejected irrespective of size, but it doesn't say that clearly, or explain why that would be the case. A Google search shows many commercial sources that say RO units are effective for home removal of fluoride from drinking water [4], [5], [6]. I also found this opinion that isn't obviously commercial and claims to be from a PhD [7]. But there are some sources that agree with my reasoning that it is ineffective [8]. So if RO is effective, what the flaw in my reasoning? 202.155.85.18 (talk) 03:11, 15 June 2013 (UTC)
- Your most obvious mistake is the assumption that the fluoride ion can somehow pass through the membrane while leaving its positively charged counterion behind -- an impossible situation in the absence of a strong electric field such as is used in electrolysis. So even if the RO membrane doesn't block the fluoride ion itself (and I don't see why it shouldn't), it could easily block the fluoride ion indirectly by blocking the counterions (sodium, potassium, etc.) Also, even though the fluoride ion is small, its high electronegativity means that it's much more likely to form ion pairs (which would be too big to pass through the membrane), and also to stick to the membrane itself. These are just 2 reasons why RO can remove fluoride from the water -- I'm sure there are others, but these are the first two I can think of. 24.23.196.85 (talk) 03:51, 15 June 2013 (UTC)
According to ionic radius, the ionic radius of Na+ is only 116pm compared to 119pm for F-. Oxygen as a neutral atom is 73pm and water's bond length is 95pm according to water (data page), so if you negate the contribution of the hydrogen, the width is minimum width of a water molecule is 130pm at its equilibrium bond length and angle (this is using the trig to work out the minimum distance between one hydrgogen and the axis formed by a line running from the oxygen to the other hydrogen, and adding that value to half the neutral oxygen atom's radius). Even if the NaF is present as an ionic pair, the two can orient themselves along the axis of the membranes hole's and pass through with the minimum width of the largest ion. So, again, as I see it, NaF can pass through a membrane hole with a minimum width of 119pm whereas water requires a minimum width of 130pm. This same analysis results in the conclusion that Cl-, Br-, K+ and I- are all removed from the solution by RO, which I know from experience to be the case. 202.155.85.18 (talk) 04:23, 15 June 2013 (UTC)Oooops...I was conflating radius and diameter. The water's minimum width is 166pm whereas F- is 238pm. 182.6.227.104 (talk) 05:50, 15 June 2013 (UTC)
- A different perspective on 24.23.196.85's view above. While I don't believe strongly dissociating ionic solutions can be characterized as forming ion pairs (in that the counterion is anywhere nearby), each ion has a strongly polarized cloud of solute molecules around it that in a sense displaces the charge of the ion towards the outside of the cloud. If a flouride ion were to fit into a the channels of a membrane, and the membrane material were not as readily polarized as the solute (and water is exceptional amongst most materials in this regard), stripping most of this cloud to allow it through would be energetically unfavourable. Ions with their polarized clouds of water molecules, therefore, will behave like much larger "balls" that will not fit into the small channels of the membrane – it should have the effect of an effective repulsive force that keeps the ion itself at a distance from the membrane surface. — Quondum 17:38, 15 June 2013 (UTC)
- Very true for all ions in aqueous solutions; but in addition, in the case of the fluoride ion specifically (and unlike most other ions), ion-pair formation is also significant. (BTW, can you please explain what is a "flouride" ion -- is it an ionized form of flour?) 24.23.196.85 (talk) 18:59, 15 June 2013 (UTC)
- Point taken about my misspelling – I'll watch for that particular case in future. My explanation is not applicable to ion pairs, though a dipole variant of the argument may apply to extremely polar molecules (though I would not expect a species such as biflouride to be polar). — Quondum 12:57, 16 June 2013 (UTC)
- Very true for all ions in aqueous solutions; but in addition, in the case of the fluoride ion specifically (and unlike most other ions), ion-pair formation is also significant. (BTW, can you please explain what is a "flouride" ion -- is it an ionized form of flour?) 24.23.196.85 (talk) 18:59, 15 June 2013 (UTC)
Celibacy vs. female sexual desire
If female sex hormones are estrogen and progesterone which are produced by the ovaries, could a celibate adult female have her ovaries removed to end her natural occasional desire for sex? What noticeable changes would this have on her mind and body? And I read today that women have a small amount of testosterone as well - can a woman have that removed? — Preceding unsigned comment added by 174.65.51.113 (talk) 16:15, 15 June 2013 (UTC)
- Yes, removing a woman's ovaries would end her desire for sex -- but it will also have some VERY noticeable changes on her mind and body (like the effects of menopause, but MUCH more severe -- see Oophorectomy#Risks and adverse effects). 24.23.196.85 (talk) 19:12, 15 June 2013 (UTC)
- I can't find any backup for the contention that it ends a woman's desire for sex, but rather that there is an increased risk that it will. (See the abstract of the article referenced in the linked section above.) And it is not so much the loss of estrogen and progesterone -unless I am misreading the report- as it is the reduction in testosterone. Bielle (talk) 19:23, 15 June 2013 (UTC)
- Libido isn't just moderated by the obvious female hormones. There is some evidence that hypothyroidism also affects libido. I really wouldn't recommend thyroid removal as a means of managing the female sex drive! --TammyMoet (talk) 10:22, 16 June 2013 (UTC)
- Did you doubt it? We have an article (or at least a section of an article) about that. Oophorectomy#Adverse effect on sexuality. Thincat (talk) 11:20, 16 June 2013 (UTC)
- "as a means of managing the female sex drive"? While we can comment on the possible effects of oophorectomy, we most certainly cannot comment on it as a means of managing anything, and it does not appear that we have any material on that subject. The OP's original question does carry with it an implication of inquiring about it as a means rather than its effects, which pretty much puts it out of line of permissibility for this page as being a request for medical advice. BTW, the bald statement that "removing a woman's ovaries would end her desire for sex" is not even correct, even though there is apparently a correlation. — Quondum 18:17, 16 June 2013 (UTC)
- History tells us that celibate orders came up with far more restrained methods of managing sex drive, such as the use of lettuce opium,[9] common rue and various other reputed anaphrodisiacs. The level of evidence for such things varies, but surgical methods would need to prove not merely some possibility of effectiveness, but superiority to herbal approaches. (There is also a question here that science can't answer, namely whether religious orders would view such methods as "cheating" rather than fighting an inner jihad against what they perceive to be sinful temptations) Wnt (talk) 19:17, 16 June 2013 (UTC)
Melting point of calcium acetate
Not just a reference desk question but an article issue. At the help desk, here, a user asked for help fixing the display of {{chembox}} in calcium acetate, which was displaying an error upon his change from listing the melting point in it from 160 °C to 400 °C. I fixed the template error, but then seeing such a radical difference in the information, went to check myself, and the sources seemed to verify the earlier number. I thus changed it back to 160 °C and added a source I found. I don't have access to the paper the user had cited when he made his change. He has followed-up. I am posting below the conversation to this point, and hope that someone here with a chemistry background can speak authoritatively on this issue, where I certainly cannot.--Fuhghettaboutit (talk) 16:56, 15 June 2013 (UTC)
- (Originally posted at help desk)
- Hi Fuhghettaboutit. Thanks for solving the formatting problem. I would be grateful if you could confirm that you read the paper I cited before your latest edit. The TGA curve and Results and Discussion section showed 400°C, and the XRD data supported this. I also have several other published papers that give this figure. Please could you provide a TGA curve, or some other actual data (ie not just a number), that proves that calcium acetate melts at 160°C - I have not been able to find this data on the net. I was curious, so actually heated calcium acetate in the lab, but all that happened at 160°C was that it lost the water of hydration and became calcium acetate anhydrous. TIA Taikobeat (talk) 14:50, 15 June 2013 (UTC)
- Taikobeat - I'm sure you know far more than I do about chemistry. I only know that the article previously said 160, and after fixing the chembox display, I went to look at what sources say because 400 °C and 160 °C are so radically different—it's not like you were correcting a rounding error to something more precise. So upon looking I found that everything seemed to confirm the original number. I accordingly added what appears to me to be a very reliable source ([10]) upon reverting back to 160.
Now I note that this and other sources qualify "melting" to decomposition to acetone and CaCO3 I don't precisely understand that distinction. Are they using "melting" loosely; that we are not talking about a change from the solid to liquid state but that "melting" is used as a term of art in chemistry to denote the decomposition point into another substances, even if there is no state shift? I don't know. I certainly can't argue from a knowledge standpoint with you on this topic and have no stake but to keep the encyclopedia proper. But on Wikipedia we follow the sources. Since you cite a source (which I don't have access to), I think it's best at this point to turn to people who actually know this stuff. Let me go post to the science section of the reference desk, laying out the issue, so that people with a background can hopefully comment.--Fuhghettaboutit (talk) 16:39, 15 June 2013 (UTC)
- Taikobeat - I'm sure you know far more than I do about chemistry. I only know that the article previously said 160, and after fixing the chembox display, I went to look at what sources say because 400 °C and 160 °C are so radically different—it's not like you were correcting a rounding error to something more precise. So upon looking I found that everything seemed to confirm the original number. I accordingly added what appears to me to be a very reliable source ([10]) upon reverting back to 160.
I see values in the mid-100s from various refs based on MSDS, NIOSH, etc. and much higher values from some journal articles. The journal articles suggest several origins of confusion in figuring out the "right" value. First, various hydrate salts loses water at mid-100s, which is technically decomposition of the chemical...if the chemical is the hydrate rather than the anhydrous form. It's the anhydrous form that decomposes to form the carbonate via loss acetone. But there appear to be two different crystalline forms: one seems to be described as one O from each acetate binding to the Ca2+ ion, whereas the other specifically has two acetates each binding by both oxygens (chelation). I have no idea which (or both) of these would decompose how and at what T. DMacks (talk) 19:13, 15 June 2013 (UTC)
I think this] is the article used to reference 400 °C. So far as I can see it is publicly available. Thincat (talk) 19:25, 15 June 2013 (UTC)
Thincat, Yes, Bilton (2012) is the open access article I linked to as a reference. Perhaps the best solution is to expand the information included to make the entry easier to understand. The information currently displayed is incorrect as decomposition to CaCO3 + acetone occurs at 400°C. Taikobeat (talk) 11:06, 16 June 2013 (UTC)
- I'm not qualified to express an opinion on the temperature. However yesterday, when looking into the matter, I found a fascinating discussion about completely different chemicals but along similar lines. see Talk:Cyclohexanone#Cyclohexanone melting point. Thincat (talk) 12:10, 16 June 2013 (UTC)
Advantage of smoking when it comes down to getting the substance
I don't see any advantage in burning and inhaling the resulting smoke, I wonder why people don't administer the drug through a more efficient way. Specially if you are taking a scarce drug, you don't want to partially waste it. Is there any regulated drug that was ever smoked? I know that some are inhaled, but that's a different scenario, more efficient. OsmanRF34 (talk) 18:43, 15 June 2013 (UTC)
- Smoking gives direct access of the drug to the bloodstream, similar to injection, which is even more unpleasant to most people, and snorting. The point in smoking is not to burn the active ingredient, but to volatilize it. That's the benefit of such things as e-cigarettes. μηδείς (talk) 18:57, 15 June 2013 (UTC)
- Indeed, that's the benefit of e-cigarettes, pipes, bongs or whatever you got. But why would any one smoke a plain cigarette, given that it's either a highly taxed legal substance or a expensive due scarcity illegal substance? Lots of the substance gets lost through this inefficient way. OsmanRF34 (talk) 19:06, 15 June 2013 (UTC)
- Smoking was never about efficiency. There was probably originally an element of "proving your bravery" by having something on fire in your mouth. Later on it became a "mark of sophistication" since only the wealthy could afford tobacco. Then it became "a sign of rebellion", especially after it was found to be unhealthy. So, various social aspects explain smoking, more than delivering nicotine efficiently. Indeed, trying to get people to quit smoking by giving them nicotine patches or gum isn't all that successful, precisely because the social aspects are lacking with such a delivery system: "What do you say we go out on the balcony and chew some nicotine gum together ?". :-) StuRat (talk) 19:39, 15 June 2013 (UTC)
- Also, smoking not only volatilizes the drug, but in some cases also partly ionizes it, thus increasing its solubility and making it easier for it to diffuse into the bloodstream. 24.23.196.85 (talk) 19:04, 15 June 2013 (UTC)
- Smoking is rarely used with prescribed drugs because it is very difficult to precisely control the dose. For nicotine that doesn't matter so much, and consuming nicotine by mouth is not a good option, because in the stomach it causes nausea and vomiting. In fact if you consume poison and need to make yourself vomit quickly, eating a cigarette is one of the standard suggestions for how to make it happen. Looie496 (talk) 23:57, 15 June 2013 (UTC)
- Well, not only that but there are huge numbers of nasty side-effects from all of the other ingredients present in the smoke. But there are other "drugs" that are volatilized and inhaled - I well recall as a child having to lean over a bowl of hot water with a towel over my head to allow me to inhale some kind of menthol-based decongestant (although I suspect that the high humidity levels that resulted did most of the work!). Transdermal_patch#Vapour_Patch describes a device that releases vapors to treat various conditions. SteveBaker (talk) 13:34, 16 June 2013 (UTC)
- Whether combustion or vaporization (or both) occurs, the lung alveoli are a huge absorption surface, and they provide a rapid delivery to the rest of the body, and in the case of psychoactive drugs, the brain. It also avoids first pass metabolism that occurs in ingested drugs. IV injection is similar, although different. Shadowjams (talk) 00:54, 17 June 2013 (UTC)
Explosives from creosote
Is it feasible to make homemade high explosives from creosote, or are the necessary separations/purifications simply not worth the effort? Has anyone actually made high explosives from creosote (I'm asking about the French Resistance, in particular)? DISCLAIMER: I'm NOT trying to blow anything up, so please don't give me any recipes, I don't CARE about the recipes -- all I want to know is, is it feasible in practice, and has it been done in real life, either in the French Resistance or elsewhere? (For the record, I'm asking because I've read in Nancy Wake's biography that she was trained to make explosives from "everyday ingredients", and it seems to me that creosote would be particularly suitable for this purpose -- and also, because I'm writing a military thriller about the French Resistance where one of the good guys works as a chimney sweep, and another as a pharmacist, which suggests how they can collaborate on making explosives.) 24.23.196.85 (talk) 19:35, 15 June 2013 (UTC)
- One comment: Creosote stinks. That makes it less than ideal for secret use, as a French resistance member trying to bicycle past some Nazi guards with a basket full of the stuff would give the show away. StuRat (talk) 19:43, 15 June 2013 (UTC)
- I know it does (I've worked with the stuff personally) -- but, if the person bicycling past the guards with the stuff has a legitimate reason for having it with him, wouldn't that make the odor a moot point? 24.23.196.85 (talk) 19:46, 15 June 2013 (UTC)
- And, just so we're clear, I wasn't envisioning the Maquis demolitionists nitrating raw creosote (which would in any case be very dangerous due to the risk of runaway side reactions, etc.), but rather, separating it into its individual components by distillation, liquid-liquid extraction, etc., to make picric acid, TNT and other suchlike compounds. 24.23.196.85 (talk) 20:06, 15 June 2013 (UTC)
- My understanding is that high explosives generally have lots of loosely bound nitrate groups. Looking at our article for the composition of a typical creosote, I can't see even a single nitrogen atom in there. So it doesn't seem likely to me. It would probably be relatively easy to get stuff that would burn like gasoline, but I doubt you'll be able to get something that makes a bang. Looie496 (talk) 20:12, 15 June 2013 (UTC)
- Creosote consists of about 20% cresols. These can be converted to toluene, which can be used to produce TNT, a high explosive. The other chemicals used in the process are easily acquired. As for "homemade", not exactly. Making significant quantities of high explosives requires considerable expertise and a good facility. However, the resistance movement had access to these and it is entirely feasible that they covertly produced high explosives from creosote. Whether they actually did so is another matter.
- More likely is using creosote in improvised low explosives and incendiary devices. Creosote lends itself well to such purposes, as do most flammable fluids. Dominus Vobisdu (talk) 20:29, 15 June 2013 (UTC)
- Also, as I suspected, creosote has been used to make high explosives, including before the war: [[11]]. It's a cheap and easily available source of raw material. Dominus Vobisdu (talk) 20:39, 15 June 2013 (UTC)
- I can't help but think of Mr Creosote now. -- Jack of Oz [Talk] 20:59, 15 June 2013 (UTC)
- Why do you want to know this 24.23.196.85 ? One can even make explosives form oxidants in denture cleaners mixed with any suitable fuel. Why, oh why, do you think your life will be enriched by this information? And to have the answer posted 'here' so that others can see and maybe miss use the chemical technology? I am aware that I can be accused of being paternalistic here, but if you don't already know then you need not to know. --Aspro (talk) 22:34, 15 June 2013 (UTC)
- Aspro, read the OP's post more carefully. The answer to your question is there. HiLo48 (talk) 23:50, 15 June 2013 (UTC)
- Indeed, our OP has a long and interesting history of asking the weirdest questions in the process of writing this book. It's quite clear that no ulterior motive is present here. This is a shining example of why the Reference desk does not have a rule about not giving out information about dangerous activities such as making high explosives. SteveBaker (talk) 13:28, 16 June 2013 (UTC)
- You're missing the point! OK, so 24.23.196.85 is researching for a book. Have you ever noticed the artist licence taken by Hollywood films? You could go through the footage of similar scenarios frame by frame and try to duplicate it and it wont work. There is also creatures commonly referred to as teenagers. Now you may have come into this world for all I know, equipped with false dentures, hearing aid, wrinkly skin and knowing all the irregular Latin verbs already. Some teenagers don't have your advantage. You say: Reference desk does not have a rule about not giving out information. I also agree with that but would you agree that with that freedom comes responsibility. One of my flatmates told me how as a school kid he badly got the back of his hand burnt trying to make gunpowder. He was woefully ill informed about the correct process. Now lets get back to 24.23.196.85 question. Yes, creosote would be a suitable fuel and in occupied France probable more obtainable than paraffin (kerosine). Yet, creosote is far from a pure hydrocarbon. It is a complex mixture. Why does that matter you might well ask. Well, mixing fuel with an oxidiser can be a risky process. Pure paraffin mixed with say con nitric will just sit there … unless you introduces a suitable catalyst. Then it reacts. It may not exploded but the reaction is so quick that one hasn’t the time to take one or two steps back until its finished. The heat pulse is such, that one cloths can just spontaneously ignite in an instant. Now, I have never attempted to make an explosive with creosote and I'm pretty sure that posters here haven't but I do know that it is a dirty chemical. It may well react whilst mixing in the oxidant (ie it contains it own catalysts). So back to the OP question and to help him with his book. “Is it feasible to make homemade high explosives from creosote”. My gut reaction is that creosote mixed with any oxidant won't make a good high explosive, it will be too unstable and the Nazi's will laugh themselves silly that all the French Resistance can do is blow themselves up. However, a low explosive mix of creosote with a booster (to achieve true detonation throughout the charge) could well have all the neighbours with in earshot asking c'est quoi ce bordel que c'était? So finally, to 24.23.196.85. Use your artistic licence to the full, be vague in the details (after all its fiction and only a small percentage will relize that like all Holywood films it too cantains a lot of nonsence ) and good luck with your book.--Aspro (talk) 22:15, 16 June 2013 (UTC)
- AND Don't forget to add in the intro that Wikipedia aided (hindered ?) you in your research.--Aspro (talk) 22:23, 16 June 2013 (UTC)
- You're missing the point! OK, so 24.23.196.85 is researching for a book. Have you ever noticed the artist licence taken by Hollywood films? You could go through the footage of similar scenarios frame by frame and try to duplicate it and it wont work. There is also creatures commonly referred to as teenagers. Now you may have come into this world for all I know, equipped with false dentures, hearing aid, wrinkly skin and knowing all the irregular Latin verbs already. Some teenagers don't have your advantage. You say: Reference desk does not have a rule about not giving out information. I also agree with that but would you agree that with that freedom comes responsibility. One of my flatmates told me how as a school kid he badly got the back of his hand burnt trying to make gunpowder. He was woefully ill informed about the correct process. Now lets get back to 24.23.196.85 question. Yes, creosote would be a suitable fuel and in occupied France probable more obtainable than paraffin (kerosine). Yet, creosote is far from a pure hydrocarbon. It is a complex mixture. Why does that matter you might well ask. Well, mixing fuel with an oxidiser can be a risky process. Pure paraffin mixed with say con nitric will just sit there … unless you introduces a suitable catalyst. Then it reacts. It may not exploded but the reaction is so quick that one hasn’t the time to take one or two steps back until its finished. The heat pulse is such, that one cloths can just spontaneously ignite in an instant. Now, I have never attempted to make an explosive with creosote and I'm pretty sure that posters here haven't but I do know that it is a dirty chemical. It may well react whilst mixing in the oxidant (ie it contains it own catalysts). So back to the OP question and to help him with his book. “Is it feasible to make homemade high explosives from creosote”. My gut reaction is that creosote mixed with any oxidant won't make a good high explosive, it will be too unstable and the Nazi's will laugh themselves silly that all the French Resistance can do is blow themselves up. However, a low explosive mix of creosote with a booster (to achieve true detonation throughout the charge) could well have all the neighbours with in earshot asking c'est quoi ce bordel que c'était? So finally, to 24.23.196.85. Use your artistic licence to the full, be vague in the details (after all its fiction and only a small percentage will relize that like all Holywood films it too cantains a lot of nonsence ) and good luck with your book.--Aspro (talk) 22:15, 16 June 2013 (UTC)
- Whilst uploading this reply, someone was looking over my shoulder and asked (as teenagers do) what is a “# ¿ @%** booster”? So for completeness -please also see:Explosive booster. --Aspro (talk) 22:37, 16 June 2013 (UTC)
Creosote is not easily available anywhere in the European Union, due to its carcinogenic properties. Sale to the public was banned on 30 April 2003 and use was prohibited from 30 June 2003. It can only be used commercially with a special licence. [12] Alansplodge (talk) 16:23, 16 June 2013 (UTC)
- We're talking about WWII. Read the original post. Dominus Vobisdu (talk) 16:38, 16 June 2013 (UTC)
- Oops! Alansplodge (talk) 17:42, 16 June 2013 (UTC)
- Hmmm, something smells fishy there. "Creosote and coal tar creosote are complex mixtures of coal tar derivatives..." But the article says there is also a wood tar creosote. Did somebody manage to get wood tar banned as coal tar for financial advantage? (From [13] it kind of sounds like it ... wonder who has the patent?) Wnt (talk) 18:24, 16 June 2013 (UTC)
- Oops! Alansplodge (talk) 17:42, 16 June 2013 (UTC)
- Creosote itself is sticky enough to be an acceptable substitute for napalm, it would probably make a fairly good molotov cocktail filler if petrol or diesel was hard to obtain or too valuable as a vehicle fuel - a likely sitution in the "Resistance v. le Boche" scenario. Roger (Dodger67) (talk) 16:47, 16 June 2013 (UTC)
- By the time that the French resistance were able to truly take offensive action, there was so little of anything available, that any inflammable liquid served the purpose. Used engine oil (sump oil) (remember Nazi aircraft had around ten gallon sumps and the ground crew were not against earning a little on the side by selling the wast oil off), this stuff only needs a little petroleum sprite and alcohol added to make it into a incendiary grenade. It would be interesting to get the input from an member of the French Resistance as to what measure he-himself had to go in-order to to singe the Nazi's hair. All my antidotes are second hand.--Aspro (talk) 23:10, 16 June 2013 (UTC)
Creosote to explosive the safe way: Wood tar creosote -> liquid-liquid extraction or fractional distillation -> get cresol -> distill cresol with zinc dust to get toulene -> I have no idea what to do with it, search it yourself but toulene can be used to make bioplastics or TNT — Preceding unsigned comment added by 118.136.5.235 (talk) 02:18, 17 June 2013 (UTC)
- The obvious next question is whether that chemical pathway would have been known to a civilian chemist in France during WWII...also whether you could have performed those steps with easily available equipment and in a manner that would not attract a lot of attention (eg, Does it produce nasty smells? Is the equipment sufficiently compact that you could run it out of someone's basement?). SteveBaker (talk) 13:21, 17 June 2013 (UTC)
- The reason why this discussion has been foundering is that the OP is actually asking a Humanities question (did anyone ever USE creosote as an explosive) but the people here are more oriented to explain how, which the OP isn't even looking for. I'd suggest re-asking over there. Wnt (talk) 17:09, 17 June 2013 (UTC)
- (un-indent) Thanks for the replies! And no, the discussion has NOT been "foundering" the way I see it -- in fact, I see a lot of useful info here. So, let me answer you one by one:
- -DV and Dodger: Yes, creosote can in fact be used as a thickener for Molotov cocktails (along with some other chemicals to make it self-ignite on impact) -- in fact, that sounds like a good use for the heavier hydrocarbons left over as still residue after the cresols and toluene have been distilled off! I'll have Francois (the pharmacist) make use of this in the "Sub Pens at St. Nazaire" chapter (which I will start writing shortly).
- -SteveBaker: Thanks for helping me make my intentions clear! As I've already said in a previous discussion (that one was about the effects of EMP), I would NEVER seriously consider blowing up my own countrymen for ANY reason (true, I might want to blow up some others, but I wouldn't need to make my own explosives for that, given that those nations are at war with my country). (And in any case, if I was up to anything, I wouldn't be asking this on Wikipedia from my own personal computer -- I'd just get ahold of a copy of the "Terrorist's Handbook" (or is it the "Anarchist's Cookbook"?) ) So yes, in a word, this IS for book research ONLY (as evidenced by the "No Recipes" disclaimer in my original comment) -- and if Homeland Security wants to make sure, they're welcome to look, I got nothing to hide and will even let them see my manuscript if they ask for it.
- -Aspro: Thanks for the technical info in your comment, but I find it VERY hypocritical of you to screech about teenagers "miss using (sic) the chemical technology" when in fact the relevant Wikipedia articles THEMSELVES have some quite detailed info about how to make guncotton, nitroglycerine, TNT, Molotov cocktails, thermite mixture and a whole bunch of other high explosives (believe me, I checked -- see for yourself if you want to know!) So if someone wants to misuse this knowledge, it's ALREADY out here on Wikipedia, REGARDLESS of my question! Also, regarding the nitration process itself: You said that "Pure paraffin mixed with say con nitric will just sit there … unless you introduces a suitable catalyst. Then it reacts." Well, I already know that you DON'T premix the organic material with the nitric acid and then introduce the catalyst (not unless you're competing for a Darwin Award, anyway) -- you FIRST mix the acid with the liquid catalyst (I know what the catalyst is, but I won't say here -- but anyone who's taken college-level o-chem would know this without me telling them, or otherwise they can just look it up in the relevant Wikipedia articles), and THEN add the organic material, SLOWLY, with CAREFUL STIRRING, and in some cases in an ice bath for cooling. And also, I'm well aware that "[creosote] may well react whilst mixing in the oxidant (ie it contains it own catalysts)" -- that's exactly what I meant when I said that nitrating raw creosote would be "very dangerous due to the risk of runaway side reactions", and this is precisely the reason why I specifically said that they WON'T be nitrating raw creosote, but instead will separate it into its individual components and nitrate THOSE. (And one final comment: Yes, I know exactly what is an explosive booster, as well as what is a "f*&%ing booster" ;-) .)
- -118 IP: Yes, I'm aware of the pathway you mentioned for making "toulene" (sic) from creosote (as well as how to convert the "toulene" to TNT by aromatic nitration, which I won't describe in detail either here or in my novel, for obvious reasons). And for the record, this reaction was well known since the late 19th century, so it's very plausible that a French chemist in the 1940s would be aware of it.
- -And finally, regarding SteveBaker's last comment: As I just said, this reaction was well known in the 1940s; as for the necessary equipment, it depends on the quantity to be manufactured, but for small quantities (up to a few pounds per batch), ordinary lab glassware would suffice (or even improvised equipment, provided that it's made of suitably acid/base-resistant material). And as far as attracting attention: The equipment CAN be made compact enough to fit in a basement (for small quantities of explosives, anyway), but the process WILL produce nasty smells -- so in order to conceal this activity, ALL who live in the house upstairs will have to be sworn to secrecy.
- Its toluene herp derp and anyway it is theoretically possible to hide the smell at that time, which is to make an improvised fume hood out of the fireplace chimney and for optional extra protection if they really want to dedicate the chimney to making that stuff put some charcoal inside it to absorb the smell and put a fan on top of it. Well no one in the house will recognize that that is creosote smell because it is coming from the usual place it come from and the authorities wouldn't suspect a secret lab because they probably will think ohh that is a normal fireplace chimney, alright nothing wrong here 118.136.5.235 (talk) 23:19, 17 June 2013 (UTC)
- Thanks for the idea! Yes, this would be a feasible option; however, in my case, I'll have the homeowners be willing to put up with the occasional smells from the basement, and to keep the explosives-making activities of the Maquis team a secret. After all, they want to help drive the Nazis out of France, and this small sacrifice would be well worth it, wouldn't it? 24.23.196.85 (talk) 23:41, 17 June 2013 (UTC)
- Heh, when I suggested that improvised hood I was thinking more of small but dangerous amounts of chlorine gas. Distilling flammable liquids and using a wood-burning stove for draw might be pushing your luck too far. :) But then again... for Maquis safety was definitely a rare luxury, and it certainly adds a little dramatic question when you're never quite sure if it will reach an explosive mixture. Wnt (talk) 01:28, 18 June 2013 (UTC)
- It would strain credulity that anyone who had the requisite expertise to synthesize high explosives would be stupid enough to do it in an occupied residential building. The risk of mishap is pretty high, and they wouldn't want to put innocent lives at risk. Even if it doesn't blow up, your still talking about some pretty nasty chemicals, including phenol and toluene, that you definitely don't want to expose anyone to. Getting rid of the waste products is going to be a major problem, too. These aren't things you can just dump down the sink.
- Making high explosives isn't kitchen chemistry like making Molotov cocktails. The French resistance would most probably convert a remote and isolated barn or abandoned industrial building into a properly equipped facility. The French resistance would have had contacts who could supply the equipment and chemicals. Dominus Vobisdu (talk) 01:59, 18 June 2013 (UTC)
- Thanks! I'll reconsider the basement -- maybe they could convert a horse stable into their explosives lab, or something (which would also keep the smell from becoming an annoyance to the homeowners). But then, there's another difficulty -- how can they rig up a vacuum pump for creosote distillation and for "getting the red out" (an essential precaution to keep them from blowing themselves up during the nitrating process) if they don't have running water? 24.23.196.85 (talk) 06:29, 18 June 2013 (UTC)
- Dealing with phenol and toluene doesn't seem like that big a deal. Yeah, sure, if you get phenol on your hand and you don't wash it off you get a big nasty (but oddly painless, for some people, it seems) blister. Toluene is technically not that good for you but back then I don't think people knew nor cared. I mean, those were the days when the maids scrubbed the floors with carbon tetrachloride! You don't want your Maquis to seem like shrinking violets. As for disposal, it's called a pit. Preferably an outhouse. The Nazis are welcome to search through it all they want. :) Yeah, I know, I know, it's not really so funny because they had the local citizens for that... Wnt (talk) 07:43, 18 June 2013 (UTC)
- They'll have running water. It's a trivial thing to set up a proper water and power supply when converting the barn. Just so that you understand, the resulting facility is going to be pretty sophisticated and rather high tech. It's definitely not going to be a slapdash effort, like the tunnels in Hogan's Heroes, nor you local meth lab, nor even your college chem lab. Even a facilty for "small scale" production is going to be much larger scale than you imagine for it to be worth the undertaking and investment. The smallest feasible operation would be still be a very professional industrial setup, requiring at least a few professional industrial chemists, and a few professional engineers, as well. It would be a VERY BIG project for the Resistance, requiring the coordination of dozens of members and contacts.
- It's far beyond the capabilities of a handful of non-specialists, like your two blokes, one of whom is a "pharmacist". If that's all you have to work with, forget about high explosives altogether and stick with improvised incendiary devices like molotov coctails. If your two blokes really needed high explosives on small amounts, it would be far, far easier simply to steal them from the Germans than to attempt to manufacture them themselves. And a lot less risky. Dominus Vobisdu (talk) 08:13, 18 June 2013 (UTC)
- For someone that says you cannot pour this chemical down the sink, who cares? This is a warfare situation and no one thinks about enviromental stuff now. For the vacuum distillation part, the vacuum pump could be a steam ejector outputting into a radiator that outputs to a open ended pipe under water. For the still part, it could be like your normal moonshine still with all the leak sealed to seperate the cresol from creosote, and run the cresol vapor into a zinc bed to convert it directly into toluene and condense it.
But there is still a question: How do you get enough wood tar creosote? It is more feasible to make a fertilizer explosive in my mind 118.136.5.235 (talk) 07:40, 19 June 2013 (UTC)
- Explosives for demolitions (civil or military) come on two forms: those for steel and those for masonry. Destroying masonry requires large quantities of cheap explosives, set carefully into place beforehand by digging holes and tamping them into place with soil or rubble on top. A vast bulk effect "shoves" the masonry out of the way. It's a lot of work, for a lot of people, for a lot of time. There's a lot of materiel to bring, and a lot of work to do on-site. Even for an occupying army, this is hard work. Many bridges were built with demolition chambers pre-formed during construction to simplify this work. On the other hand, cheap low-velocity explosives like TNT (cheapish) or ammonium nitrate can be used.
- Destroying steelwork is rather easier (assuming good supplies, but a well-guarded target), whether this is high value equipment (factories, electricity distribution, railways, trucks) or just the girders of a steel bridge. A high-speed shock wave is used to cut the steel. This is generated by explosives with a high Vdet, such as plastic explosives. Inertia at these high velocities is such that tamping is less essential (although charge placement is even more so) and a small package of explosives can be placed, tied or held magnetically in place and left uncovered. The explosives used are complex, expensive, but are required in smaller quantities.
- In the French Resistance context, the obvious implications of all this are that they went after the high-value steel targets, using small quantities of high-quality plastic explosives, supplied by the Allies. These could be put in place by small teams, working quickly and quietly. Masonry targets were either ignored, or marked as targets for air bombing. The French Resistance was well-supplied by RAF air drops, including explosives, and shortage of demolition explosives (AFAIK) just wasn't a problem. They had neither the need, nor the capacity to start making their own. The SOE training for "home made" is almost entirely about incendiaries, not blast. It's also notable that the ordnance consumed by infiltrating demolition teams (whether the Resistance or Popski's Private Army) much favoured plastic explosives over the TNT that was the stock in trade of mainstream Engineers performing battlefield demolitions. Andy Dingley (talk) 10:06, 19 June 2013 (UTC)
June 16
Conscious control of breathing
In our aquatic ape hypothesis article is the claim that "humans also have a considerable amount of control over their breathing, which is an involuntary reflex for most terrestrial mammals.[21][27]" I can't access either of the two references, so how do scientists know whether other animals can consciously control their breathing? It's not as if we can talk to a dog and ask him to hold his breath. --50.125.164.7 (talk) 07:07, 16 June 2013 (UTC)
- The sentence you quote doesn't imply that other animals cannot consciously control their breathing. It's an "involuntary reflex" for humans too. Many terrestrial mammals (including dogs as well as the numerous aquatic mammals) are excellent swimmers, which requires control of the beathing.--Shantavira|feed me 07:45, 16 June 2013 (UTC)
- I think the study specifically refers holding one's breath, to enable submersion. Plasmic Physics (talk) 08:00, 16 June 2013 (UTC)
- Even so, I know of diving dogs. Plasmic Physics (talk) 08:00, 16 June 2013 (UTC)
- A few points. It might be partly an involuntary reflex for humans, but not entirely. If I'm in a pool and I know I'm going to put my face in the water, I will consciously hold my breath. Some dog's certainly do it too. But the real marine mammals such as seals, whales and dolphins have a bigger advantage. They can close their nostrils. Kinda wish I could do that. HiLo48 (talk) 08:25, 16 June 2013 (UTC)
- @HiLo48 buy yourself a noseclip. Roger (Dodger67) (talk) 11:05, 16 June 2013 (UTC)
- To parallel what a seal can do I'd need one with telepathic controls. HiLo48 (talk) 12:09, 16 June 2013 (UTC)
- I can close my nose if I inhale hard and quick enough. Of course, inhaling hard underwater isn't smart. An electronic remote controlled noseclip would work nearly as well, I suppose, and more easily than telepathy. InedibleHulk (talk) 21:42, 16 June 2013 (UTC)
- To parallel what a seal can do I'd need one with telepathic controls. HiLo48 (talk) 12:09, 16 June 2013 (UTC)
- @HiLo48 buy yourself a noseclip. Roger (Dodger67) (talk) 11:05, 16 June 2013 (UTC)
- A few points. It might be partly an involuntary reflex for humans, but not entirely. If I'm in a pool and I know I'm going to put my face in the water, I will consciously hold my breath. Some dog's certainly do it too. But the real marine mammals such as seals, whales and dolphins have a bigger advantage. They can close their nostrils. Kinda wish I could do that. HiLo48 (talk) 08:25, 16 June 2013 (UTC)
- Just because animals like dogs can swim, doesn't mean that they have conscious control of breathing - it could just be an involuntary reflex to the face getting wet. Our Mammalian diving reflex article says this explicitly: "Every animal's diving reflex is triggered specifically by cold water contacting the face – water that is warmer than 21 °C (70 °F) does not cause the reflex, and neither does submersion of body parts other than the face. Also, the reflex is always exhibited more dramatically, and thus can grant longer survival, in young individuals." - and our Primitive_reflexes#Swimming_reflex article says that human infants can swim from birth using only reflexive behaviors...and Reflex#Human_reflexes says that the diving reflex is present in all humans.
- Moreover, dogs can easily be observed to inhale sharply before barking - and barking certainly appears to be a conscious decision because you can train a dog to bark on command. You could argue, perhaps, that they make a conscious decision to bark - and that the necessity of taking a breath first is still an involuntary matter - but if you get into the realms of "in order to perform some conscious act, the involuntary systems must first intervene" - then it becomes hard to decide what is truly a conscious act and what isn't - even in humans.
- Worse still, it all becomes a fuzzy line anyway. There is no sharp line between the conscious and the involuntary reflex: I decide (consciously) that I wish to walk across the room - but I don't consciously consider how to shift my balance and move my feet to do that. So walking has become as subconscious as breathing.
- But what about driving to work? I don't consciously think about how to move my feet to operate clutch and gas pedals when shifting gears - I don't think I consciously consider when to shift gears either. I certainly haven't "evolved" to develop special brain circuits for driving a car - and at one time, back when I was learning to drive, this was an entirely conscious activity. So what I choose to have conscious control over - and when - is a learned behavior and being in the right gear for my car to operate optimally can now occur at a subconscious level. When I was teaching my son to drive stick-shift, I found myself unable to explain the precise sequence of pedal actions when shifting gears without first watching myself do it!
- Sure, conscious behavior *can* take over those things - but mostly, my conscious mind can be thinking about something entirely different while my subconscious takes control of the mundane tasks.
- The aquatic ape hypothesis is widely regarded as a load of hogwash - so the things it claims do not have to be rationalized! SteveBaker (talk) 13:20, 16 June 2013 (UTC)
- Note that the sentence quoted by the OP doesn't use the word "conscious" -- for good reason, it's really irrelevant in this context. The practical issue is simply whether various animals are capable of holding their breath, that is, inhibiting breathing while closing off their air passages. Whether they can do it in response to instructions is not relevant to their ability to dive. Looie496 (talk) 13:38, 16 June 2013 (UTC)
- The article may not make this clear - but the original aquatic ape hypothesis uses this "conscious control of breathing" idea to explain all sorts of things - such as our ability to talk. We needed to evolve to swim in order to switch to a fish-based diet, we needed conscious control of breathing in order to do that, we were better able to develop speech with conscious breath control. But that's really nonsense on several levels - firstly that conscious control of breathing is necessary for swimming (it's not - the mammalian dive reflex provides a means to do that reflexively) or that mammals in general don't have this conscious control ability (watch a dog barking to realize that they do). SteveBaker (talk) 00:43, 17 June 2013 (UTC)
- Actually I'm quite fond of the aquatic ape idea in a weaker form. The Okavango Delta is part of an ancient lake in a region with dramatic yearly climate change. Part of the year it is dry desert, part of the year it is a vast area of waist-deep flooding. Chacma Baboons learned to stand upright there. Lechwe antelope developed elongated feet (like ours?). Every year there are massive fires that leave behind cooked meat, for those clever enough to avoid them. The place seems like the mold that humanity could have been stamped from, and part of that is dealing with life largely in the water for part of the year. Wnt (talk) 01:25, 18 June 2013 (UTC)
- The article may not make this clear - but the original aquatic ape hypothesis uses this "conscious control of breathing" idea to explain all sorts of things - such as our ability to talk. We needed to evolve to swim in order to switch to a fish-based diet, we needed conscious control of breathing in order to do that, we were better able to develop speech with conscious breath control. But that's really nonsense on several levels - firstly that conscious control of breathing is necessary for swimming (it's not - the mammalian dive reflex provides a means to do that reflexively) or that mammals in general don't have this conscious control ability (watch a dog barking to realize that they do). SteveBaker (talk) 00:43, 17 June 2013 (UTC)
electron spin reversal
in a direct current circuit that is initiated by a plasma field why does the circuit preform its normal circuit path ,but also operate on a one wire system in the off position and when operating in the one wire off position the batterys in the d c circuit seem to recharge themselves the question is ,are the characteristics of the diode changing or is the a reversal of polarity happening in the battery cell material itself causing this unusual characteristic 50.93.30.1 (talk) 18:46, 16 June 2013 (UTC) or are both conditions of the circuit changing .50.93.30.1 (talk) 19:11, 16 June 2013 (UTC)
- I'm not sure I understand your question, but I think by "electron spin" in the question title, you're not actually referring to the quantum mechanical spin of an electron, but rather an apparent reversal of the flow of electrons within the circuit in question. If I'm understanding you correctly, I think you're asking why the battery appears to become somewhat recharged when you switch off the normal flow of current in a circuit containing a battery and a gas-filled tube acting as a diode. There wouldn't actually be a reversed current flow in such a circumstance, since no current flows around an open circuit. Rather, I think what you are seeing is the voltage recovery effect that some types of batteries have, in which the battery appears to regain a bit of its charge when the battery goes for a little while without a current being drawn from it. The effect is most pronounced after drawing a large current from the battery, as would be the case if you're discharging the battery through a forward-biased diode without using a resistor to limit the current. I unfortunately can't seem to find a Wikipedia article discussing the recovery effect in batteries, but you can read an excerpt from a book that briefly mentions the effect if you click on this link and scroll down a bit to the section entitled "Battery Peculiarities: Voltage Recovery". Red Act (talk) 02:31, 17 June 2013 (UTC)
what makes earths seasons
QBold text — Preceding unsigned comment added by 68.115.48.41 (talk) 22:28, 16 June 2013 (UTC)
- Mainly the axial tilt of Earth which during a year changes the time from sunrise to sunset when you are not on the Equator. See more at Season#Causes and effects. PrimeHunter (talk) 22:39, 16 June 2013 (UTC)
- The angle of the sun to the ground makes a huge difference to how much heat it provides. That's why it's hotter at the equator than at the north and south poles - and why the middle latitudes are somewhere between those extremes. At the equator, the sun is almost vertically overhead around noon - but when you're closer to the poles, it scarcely rises above the horizon. The seasonal variation is caused by the fact that the earth's axis of rotation is tilted. So at some times of year, the northern hemisphere is tilted towards the sun during the day and the southern hemisphere is tilted away. That makes for summer in June/July/August in the northern hemisphere when those are the winter months in the south. Six months later...in December/January/February, the earth has moved around to the opposite side of it's orbit and the positions are reversed making it winter in the north and summer in the south. That axial tilt simply makes the sun be higher or lower in the sky depending on the time of year - which accounts for the temperature variation. Some seasons are rainier or drier than others SteveBaker (talk) 00:37, 17 June 2013 (UTC)
Nosecones on airliners and fighter jets
I suddenly wondered why the difference between nosecone shapes on passenger and fighter jets - the article says the simple cone is easier to construct, but hints that it isn't very aerodynamically efficient - seems odd, since companies that makes fighter jets have money to burn... Is it possible they use the simple cone because it looks more badass? The friendly ellipse or whatever it is of passenger jets is certainly less weaponish in appearance... Adambrowne666 (talk) 22:44, 16 June 2013 (UTC)
- I don't see any fighter aircraft with true "cones" (ie simple, circular cross-section, straight-sided) - they all seem to have curved sides to some degree. The optimum shape is a symmetrical "volume of revolution" - and the exact shape depends on the speeds that the aircraft is expected to travel and whether top speed or best fuel economy is the goal. Nose cone design goes into this in incredible detail. That said, not all aircraft have optimal drag coefficient as a goal - some have to accommodate equipment such as cameras and radar gear in there - others have to be stealthy and have a minimal radar profile. There are dozens, perhaps hundreds of variables determining the best shape, so it should be no surprise that there is a wide variety of shapes out there. However, I'm very sure that "looking badass" isn't one of them - and "ease of construction" is an unlikely goal compared to all of the other things (such as engines and instrumentation) that cost so much to manufacture. SteveBaker (talk) 23:28, 16 June 2013 (UTC)
- I don't think the article suggest this. The shape may deviate from the ideal in order that is protects the avionics but the reasons are never to make the aircraft look badass. It is not the 'look' of a fighter aircraft that makes it effective but its actual in combat effectiveness. Consider the Fairey Swordfish. Saw service in during WWII and it was a biplane. Yet, it could spoil your whole day if it dropped its load on you.--Aspro (talk) 23:31, 16 June 2013 (UTC)
- @Aspro: from the article: A very common nose cone shape is a simple cone. This shape is often chosen for its ease of manufacture, and is also often (mis)chosen for its drag characteristics. - but I was assuming the fighter jet nosecone is a simple cone, which SteveBaker points out it isn't.Adambrowne666 (talk) 23:36, 16 June 2013 (UTC)
- The fundamental reason for different shapes is the intended operating speed. Airliners operate at subsonic speeds, where a 'blunt ellipse' is the optimum shape, whereas jet fighters are designed for transonic and supersonic speeds, where a more pointed shape is better. AndyTheGrump (talk) 23:40, 16 June 2013 (UTC)
- Thanks, Andy - yes, that makes sense - I thought I was onto something - some semiotic reason for the pointy tip on fighters... Adambrowne666 (talk) 23:55, 16 June 2013 (UTC)
- “This shape is often chosen for its ease of manufacture” A defence contractor usually gets a contract based on development cost plus 20% profit. “Ease of manufacture” doesn’t come into it at all. If it is technically feasible they will push to incorporate it. Flight duration and max speed is a very important parameter. The tax payer then pays for it. So it is shaped... however the aircraft designers (defense contractors) think it should be shaped. Passenger aircraft (with exception of Concord) are sub or transonic. So they don't benefit from these type of cones. Don't believe everything you read on Wikipedia. Take it from me, I know some of the editors that contribute!--Aspro (talk) 02:22, 17 June 2013 (UTC)
- The whole "to make them look more badass" idea is probably upside down. They only look like noses of planes which are known to be badass. If the planes which featured this kind of nose had not been successful, we wouldn't associate their shape with speed, nor any other kind of badassitude (is that a word???) - ¡Ouch! (hurt me / more pain) 10:19, 17 June 2013 (UTC)

- If it's not a word, it SHOULD be! How does one nominate a word for "Best New Word of the Year"? 24.23.196.85 (talk) 20:06, 17 June 2013 (UTC)
- It's not even the best I can think of. I should go ahead and post some on my user page - unless a WP: rule says I cannot do that. Then my talk page will have to do. - ¡Ouch! (hurt me / more pain) 07:06, 18 June 2013 (UTC)
- If it's not a word, it SHOULD be! How does one nominate a word for "Best New Word of the Year"? 24.23.196.85 (talk) 20:06, 17 June 2013 (UTC)
- The technology concealed within will often lead to some rather odd shapes. I believe that the Hawker Siddeley Harrier GR3 had a forward looking infrared sensor, while others have air-to-air radar scanners. Alansplodge (talk) 12:53, 17 June 2013 (UTC)
- Our F-106 and F-4 fighters also had FLIR sensors installed along with the normal air-intercept radar, but that didn't influence the shape of their nosecones in any significant way. 24.23.196.85 (talk) 20:09, 17 June 2013 (UTC)

- You can find all sorts of weird nose cones out there - the F117 nose...erm..."wedge" for example is an aerodynamic disaster area - but it keeps the aircraft stealthy - and that's what matters most. Curved surfaces are a very bad thing for keeping a low radar cross-section, and nose cones are amongst the worst offenders. SteveBaker (talk) 13:14, 17 June 2013 (UTC)
June 17
NIR and static
Hello
I had a question about Near Infra Red Spectroscopy. If we were conducting the measurements on a powder sample, is there any potential for the electrosatic forces within the powder to affect the readings in anyway?
Many thanks
114.77.39.141 (talk) 12:44, 17 June 2013 (UTC)
- Yes - in science, almost everything has some effect on almost everything else. I would not consider the presence of an electrostatic field to be a primary contributor to the infrared spectrum for most materials. But, it is detectable - at least in some materials, with some very specialized equipment. For example, a near-field optical microscope can detect Raman scattering, and the spectrum of the Raman-scattered light may be affected by an applied electric field. In fact, I know of a specific professor who specializes in this sort of thing, and you can read his publications-list; and here is Electric field gradient effects in Raman spectroscopy (Physics Review Letters, 2000). I don't think powdered substrates are very good candidates for an NSOM microscope experiment; but in principle, the application of an electric field does have a small and measurable effect on the scattering spectrum. For most cases, in ordinary spectroscopy using ordinary equipment, the DC electric field has no significant effect on the infrared spectrum of a sample. Nimur (talk) 17:41, 17 June 2013 (UTC)
Dinosaur's genders
There was a line in the "Jurassic Park" film that said that dinosaurs could change their gender, or even have offspring by themselves when in a time of need. Just to confirm it: that was a huge artistic license, and in the real world dinosaur's reproduction did not work that way, right? Cambalachero (talk) 14:28, 17 June 2013 (UTC)
- Did they claim that the dinosaurs of old did that? I don't recall them saying it. What I do recall is them saying that the dinosaurs in Jurassic Park had that trait because the bits of DNA that were missing from the archeological samples were replaced from frogs who do, in the present day, show this ability in times of need. Dismas|(talk) 14:35, 17 June 2013 (UTC)
- Of course there's much artistic license there, but it does not seem impossible. Our knowledge about dinosaurs is limited, but the process you describe, which is called Parthenogenesis has been observed in some modern-day reptiles (I'm talking about having offspring by themselves, not changing their gender). - Lindert (talk) 14:37, 17 June 2013 (UTC)
- (EC) Well, we can't really be sure if dinosaurs could change sex or not. As I recall, the book had some "rationale" for this plot device. The idea was the partial genomes recovered were supplemented with frog DNA. And it is true that some frogs (and fishes, and a few other things) can change their sex, even after sexual maturity. The common example of this is the Common Reed Frog, you can find other examples by googling things like /frog fish sex change/. I don't think the book or movie had the dinos selfing, but I could be mistaken. Lastly, not to nitpick too much, but recall that gender is a social identity in humans (and perhaps a few other social animals), while sex is the biological concept. SemanticMantis (talk) 14:38, 17 June 2013 (UTC)
- Temperature-dependent sex determination occurs in reptiles but not birds. It would probably be hard to figure out if it happens dinosaurs, which are generally agreed to occupy the "space" between reptiles and birds. Roger (Dodger67) (talk) 17:23, 17 June 2013 (UTC)
- More specifically, dinosaurs lie between birds and crocodilians, not the much more distant lizards and turtle. Crocodilian eggs are known to beinfluenced in sex by external temperature, but I am unaware of sex change in adults. That usually occurs in species where the female is the larger sex (if the one large female is removed from a group of smaller males, the largest male will turn female), but this is not the case in crocodilians. μηδείς (talk) 18:16, 17 June 2013 (UTC)
- Put another way, birds are dinosaurs, a subgroup of the theropods :) SemanticMantis (talk) 18:32, 17 June 2013 (UTC)
- Yes, but all that proves is that some branches of the dinosaurs can't/couldn't do it. We still don't know whether some could. Actually, birds can kinda/sorta change sex. This article and others indicate that about one in 10,000 female birds will spontaneously change into males towards the end of their lives. Female chickens will grow rooster tails, start crowing and become quite defensive about males trying to muscle in on their hareem. However, they don't completely finish the job - they may change plumage and behavior and in most outward ways appear to be male - but they don't grow primary sexual organs to match - so they can't reproduce as males. There is no particular reason to assume that dinosaurs were unable to do that - so perhaps they did. However, that doesn't fit with the plot point in Jurassic park which was that somehow the dino's were breeding even though they'd all been created as females...so we're left with some very dubious handwaving about frog DNA, yadda, yadda, yadda. SteveBaker (talk) 18:47, 17 June 2013 (UTC)
- Put another way, birds are dinosaurs, a subgroup of the theropods :) SemanticMantis (talk) 18:32, 17 June 2013 (UTC)
- More specifically, dinosaurs lie between birds and crocodilians, not the much more distant lizards and turtle. Crocodilian eggs are known to beinfluenced in sex by external temperature, but I am unaware of sex change in adults. That usually occurs in species where the female is the larger sex (if the one large female is removed from a group of smaller males, the largest male will turn female), but this is not the case in crocodilians. μηδείς (talk) 18:16, 17 June 2013 (UTC)
- As I recall, the book made it pretty clear that the sex change was an accident based on the frog DNA, and unexpected by the experimenters. As for real life, I would speculate pretty confidently (uh-oh...) that we can't know, because we're still short on dino sequence, and a fossil is a snapshot, not a video. (One of the beautiful things about biology is that certain speculations like this that are logically foolproof are often wrong) Wnt (talk) 23:27, 17 June 2013 (UTC)
Related question
If dinosaurs fit between crocodilians and birds in a taxonomic sense, then why would you repair their fragmented DNA with frog DNA? Why not use bird or reptile DNA? 39.214.54.44 (talk) 04:41, 18 June 2013 (UTC)
- Good question! A crocodile's DNA would be a particularly good match in this case -- MUCH better than frog DNA! But I guess they needed some kind of plausible explanation for the dinosaurs breeding despite being all of the same sex (a similar kind of handwaving used for the movie "Godzilla", as far as I remember...) 24.23.196.85 (talk) 06:19, 18 June 2013 (UTC)
- Pardon my ignorance, but if missing DNA portions are filled with that of another species, how do you still get a dinosaur, as opposed to some other, exotic creature? ←Baseball Bugs What's up, Doc? carrots→ 13:49, 18 June 2013 (UTC)
- The premise what that the majority of the DNA was intact, and the frog DNA was only used to patch up the little bits that were missing. Closely related species will share very similar genes (think of the humans/chimps share 95% of their genome claims), so the hope was that any differences would be minor enough that there was still a viable dinosaur embryo. Technically, the DNA would be different than the actual dinosaur it was based on, but it would be close enough that there isn't a point in calling it something new. Of course, frogs aren't that close to dinosaurs at all, but that was part of the hand-wavy "science" that allowed the dinosaurs to breed in the end. Some highly-conserved genes do stay functionally identical across huge gaps. For example, a human HOX gene for a limb can be used in a fruit fly to cause the fly to grow a fruit fly limb. 209.131.76.183 (talk) 14:40, 18 June 2013 (UTC)
- Pardon my ignorance, but if missing DNA portions are filled with that of another species, how do you still get a dinosaur, as opposed to some other, exotic creature? ←Baseball Bugs What's up, Doc? carrots→ 13:49, 18 June 2013 (UTC)
- The reason is likely to be that Jurassic Park was published in 1990 (and probably written over a couple of years before that). Solid evidence that birds are dinosaurs didn't really start to appear until the early 1990's - and wasn't widely known and accepted until the mid-1990's. So Crighton (who went to medical school - not to learn archeology) was unlikely to be aware of this new understanding as he wrote. Frogs are certainly an odd choice as a source of DNA patches - why not lizards or some other reptile? My best guess is that he wanted to use the "changing gender" thing as a plot point and the claim to have introduced frog DNA was his way to make that seem more plausible. In the end, it's fiction - and there doesn't have to be an answer to the "WHY?" question. SteveBaker (talk) 15:33, 18 June 2013 (UTC)
- Obviously, Crighton was aware of the understanding that dinosaurs were closely related to birds, as his characters discuss it a several points during the story. 182.1.217.41 (talk) 23:56, 18 June 2013 (UTC)
Why do some algae make PUFA (poly unsaturated fatty acids)?
Some species of algae make a large amount of PUFA. This leads me to ask the question of, why? What are the functions of PUFA for single celled organisms? As I get it, one of the reasons is to maintain membrane fluidity under a variety of temperature and salinity conditions. But, why do different algal species make chains of varying lengths? For instance, some make C20:5 others C22:6. What's the added advantage of an extra double bond? Especially considering that these organisms tend to inhabit the same ecological space (thus enviromental effects are the same). If anyone has any ideas, or can refer me to papers published on the subject, I'd be eternaly grateful. 137.224.252.10 (talk) 14:53, 17 June 2013 (UTC)
- As I've commented recently, it is hard to say "why" evolution does something. But I should point out that every organism has a cell membrane, made up of phosopholipids, typically 16-20 carbons long according to the article (which sounds right). Even in humans, which you would think would do more to regulate such things internally, polyunsaturated fatty acids incorporate into membranes and change their mechanical properties concerning "rafts" of lipid-modified proteins, cholesterol etc. [14] If you can name the species you have in mind it would be easier to try to research further about the specific system, but tracking exactly why two extra carbons are used by one rather than the other can't be easy, and might even be chance. Wnt (talk) 17:01, 17 June 2013 (UTC)
The article Race (biology) is kind of incomplete. I still want to know how do biologists put into words the difference between humans who belong to what's socially considered different races. I understand that the socially relevant definition might be biologically inaccurate, but how do you express that? OsmanRF34 (talk) 17:04, 17 June 2013 (UTC)
- Existing human races aren't different enough for scientists to consider them subspecies, which would lead to terms like Homo sapiens causcasicus. Individual traits which are considered racially salient include the cephalic index, objective descriptions of human skin color, eye color, and human hair. There are other traits like Steatopygia and the epicanthic fold, blood types, limb length, tooth shape, lactose tolerance, and many other things that can vary along with what is perceived as race. μηδείς (talk) 17:38, 17 June 2013 (UTC)
- The problem is that many of these indicators vary more within a "race" than between races. That is the source of the statement that "race has no biological basis in humans". --Stephan Schulz (talk)
- Right, your quote is a good answer to Osman's final question. It's not that the social notion of race is biologically inaccurate, it's that it is biologically unfounded, or perhaps ill-defined. SemanticMantis (talk) 18:36, 17 June 2013 (UTC)
- That's a rather facile and unhelpful, but typical denial. Something like "naive social notions of race are rarely rigorously defined or helpful biologically" would be much more accurate. The desire to remove the term race from all scientific discussion is a political one, not a scientific one. μηδείς (talk) 19:01, 17 June 2013 (UTC)
- I agree with μηδείς. Unless you believe that skin color varies more within a race (defining by a society) than between races, you have to admit that there is something biological there, and that some societies consider these differences to group people. It's not all chaos when it comes to the social identity. OsmanRF34 (talk) 22:44, 17 June 2013 (UTC)
- The problem even with this trait is you are referring to, skin colour, is that if you go solely by it you will often be highly mislead. Someome with dark skin could easily be more closely related to someone with light skin. This shouldn't exactly be a surprise. A chimpanzee (either species) is far more closely related to a a human than a New World monkey even though both the monkey and chimpanzee have a lot more fur. And Opossums are quite distantly related to primates despite some having shared features like opposable thumbs and a prehensile tail. Nil Einne (talk) 00:17, 18 June 2013 (UTC)
- To be fair to Stephan, if you define the traditional three old-world races, then compare the nominally Caucasian Saami people and Tamil people, you are going to find a far greater difference in skin color than you will betweeen any various populations of Sub-Saharan blacks. But that is taking a very specific and limited definition of race as if it were the only way that four-letter word beginning with arr could be used. Obviously it is quite reasonable to talk about the racial differences between Madagascans and Tanganyikans, or between Ainu and Japanese, as opposed to the linguistic or religious differences between such peoples. Arguing that the word has no use, or that there are no scientific correlates for some of the word's various senses, is simple sophistry. μηδείς (talk) 01:16, 18 June 2013 (UTC)
- Biologists usually talk about differences in terms of ancestor groups and geographical regions. For example a paper might refer to people of sub-Saharan African descent, or people of Polynesian descent, etc. Looie496 (talk) 18:39, 17 June 2013 (UTC)
- There are biological terms for groups of a species which vary by less than those of two subspecies. There's a population, a breed (as in cats or dogs), and a color phase, to start. Those seem closer to what we call "race", in humans. Of course, it's complicated because there's so much interbreeding between historically distinct populations in humans. So, we're all "mixed breeds", if you want to put it in those terms. StuRat (talk) 19:46, 17 June 2013 (UTC)
- Presumably the races arose due to lengthy isolations of groups from each other in prehistoric times. With that isolation having been significantly compromised in relatively recent times, and with more and more cross-race children being produced, the notion of race is getting increasingly murkier. We've got a ways to go, though. ←Baseball Bugs What's up, Doc? carrots→ 23:42, 17 June 2013 (UTC)
- See Haplogroup. The way people have been classifying race is sort of like a dog pound that gets lots of French poodles and chihuahuas. Something is either a French poodle, a chihuahua, or "sort of like one or the other". Or, of course, it could be Spanish-speaking, in which case it is opted out of the classification scheme entirely. ;) Though what's sort of clear is that by and large, genetic variation beyond a certain point is called "black". Wnt (talk) 20:03, 17 June 2013 (UTC)
- Spanish-speaking chihuahuas? I didn't know they really existed! 24.23.196.85 (talk) 20:15, 17 June 2013 (UTC)
- Were you expecting it to speak Norwegian, perhaps, hmmm? Bielle (talk) 01:22, 18 June 2013 (UTC)
Conservation of momentum in time dimension
Is momentum something that applies and is conserved in all dimensions? So if I push something back in time, I can be accelerated through time? Or is that nonsense? --92.19.68.88 (talk) 23:20, 17 June 2013 (UTC)
- First of all, there's no way to "push something back in time" -- this would in itself violate the laws of physics. 24.23.196.85 (talk) 23:37, 17 June 2013 (UTC)
- I don't understand. Could a mass A travelling through time at a greater rate than another mass B, collide elastically with mass B and transfer some of its energy, increasing the rate of passage through time of B? Or do those laws apply specifically to three dimensions of which time is not one? --92.19.68.88 (talk) 23:48, 17 June 2013 (UTC)
- You can interpet a particle moving back in time as its anti-particle moving forward in time. So, pushing an electron to move back in time is equivalent to that electron getting annihilated by a positron that it collides with. Two photons will be created, you do obviously have conservation of energy and momentum in the conventional sense. Count Iblis (talk) 23:54, 17 June 2013 (UTC)
- I don't understand. Could a mass A travelling through time at a greater rate than another mass B, collide elastically with mass B and transfer some of its energy, increasing the rate of passage through time of B? Or do those laws apply specifically to three dimensions of which time is not one? --92.19.68.88 (talk) 23:48, 17 June 2013 (UTC)
- If the OP would be so kind as to define acceleration "through time..." we might possibly answer the question. Acceleration is the rate of change of the change of position per unit of time. (In other words, the second derivative of position with respect to time). Is the question supposing to ask about the rate of change in the change in time per unit time? That quantity makes very little physical sense, whether it is expressed in plain English or expressed mathematically. Things don't travel through time; they change with respect to time. In other words, the latter part of the OP's question is nonsense. Nimur (talk) 00:18, 18 June 2013 (UTC)
- Are we moving along a time dimension like a cart moves along a track in, say, x dimension? Or is that nonsense again? 92.19.68.88 (talk) 00:48, 18 June 2013 (UTC)
- Obviously we are not moving along time in a cart! So the direct answer to your question is "NO!" But your question can be answered with a little bit more substance, albeit with a lot of abstraction - if you're willing to think a little bit abstractly.
- In physics, we use very precise terminology. This helps us avoid logical errors or semantic ambiguity.
- Matter has position. We say something has moved when its position changes: displacement. Let's say we have an object with a measurable position. In classical physics, we define this type of object to be a particle - just a thing with a position. If we measure a starting position (on the x-axis, for example), and a final position, we have a displacement - let's call it Δx for a displacement along the x-axis. If we know when we took the starting measurement, and when we took the final measurement, we also have a change in time: let's call it Δt. The average velocity is therefore, by definition, Δx/Δt. We can talk about the particle moving on the x axis during the experiment time. We can say that the object was at a position x1 at time t1. Equivalently, we can use time as a generalized coordinate and describe a position ( x1, t1) - but this is just a different way of describing the same event.
- When we describe motion in this way, we are conducting the science of kinematics - the pure mathematical relationships of motion. When we attribute these motions to observable physical law, we are progressing to the science of dynamics - the study of physical motion.
- In kinematics, we can easily consider time as just another coordinate, but when we study dynamics - in any of its forms, including the modern style relativistic mechanics, we find equations of motion where the dependence on time is not symmetric with respect to the dependence on position. One very trivial example of this is a simple case of energy conservation. We find that in many cases, potential energy and kinetic energy are interchangeable, but the dynamics of the real world show us that the dependence on position is a first-order effect; and the dependence on time is a second-order effect. We call this coupled first- and second- order time-space relationship Hooke's law and we use it to describe simple harmonic oscillation, one of the simplest and most fundamental physical descriptions of motion. (Some people use this to model the behavior of a "spring").
- So: we can use mathematical abstraction to describe displacement in space, and displacement in time, using the same language; but when we study even the most simple cases, we quickly find that time- and space- do not behave in the same way. Dynamical systems almost always exhibit different properties - rates of change, stability, and so on - with respect to time and with respect to position. Nimur (talk) 01:12, 18 June 2013 (UTC)
- Nimur's very excellent and easy to understand explanation is the inherent reasoning behind Minkowski space, which is the four-dimensional space created to do the geometric calculations behind special relativity. The four dimensions in Minkowski space are not identical. Three are "space-like" dimensions, which behave like space, and one is a "time-like" dimension, which behaves as time does. Space and time are all different dimensions, but they do not behave the same way, and cannot be treated equivalently, so one's "motion in time" is not equivalent to one's "motion in space" and one cannot discuss the two concepts as though they were interchangeable. --Jayron32 12:12, 18 June 2013 (UTC)
- Are we moving along a time dimension like a cart moves along a track in, say, x dimension? Or is that nonsense again? 92.19.68.88 (talk) 00:48, 18 June 2013 (UTC)
- Yipes. The OP has asked a reasonable question, but the answers confuse and miss the point. An object does not have a position in space or time without specifying a slice through spacetime (e.g. "now"): it has a world-line through spacetime, and acceleration is a change in angle of this worldline: a curve in spacetime. Objects do not travel through spacetime. Energy–momentum is conserved in all four dimensions. — Quondum 01:50, 18 June 2013 (UTC)
- Last week, the term "now" referred to last week; today it refers to today. Presumably if that change were to happen more quickly, it would be an example of the kind of thing the OP is talking about. Card Zero (talk) 01:53, 18 June 2013 (UTC)
- Isn't that a bit like saying if a metre were longer, things'd be further apart? If by "that change" and "quickly" you are referring to some concept of movement through time in terms of elapsed time, you're confusing things. By "now" I simply meant a given instant in time as perceived by some observer. — Quondum 02:20, 18 June 2013 (UTC)
- Last week, the term "now" referred to last week; today it refers to today. Presumably if that change were to happen more quickly, it would be an example of the kind of thing the OP is talking about. Card Zero (talk) 01:53, 18 June 2013 (UTC)
- This is a sort of have-your-cake-and-eat-it-too question. There is no meaning in the idea of changing your rate of travel through time. You always go through time at a rate of exactly one second per second. Looie496 (talk) 05:08, 18 June 2013 (UTC)
- unless you speed up. --Jayron32 12:12, 18 June 2013 (UTC)
- ... but it's your observation of someone else's time that appears to be "speeded up". Your own time remains always one second per second. Dbfirs 15:34, 18 June 2013 (UTC)
- unless you speed up. --Jayron32 12:12, 18 June 2013 (UTC)
- According to Noether's theorem the consevration of (linear) momentum arises from (or is, at least, intimately connected to) the invariance of physical laws under arbitrary translations in space. The equivalent conservation law that arises from invariance under translations in time is conservation of energy. Gandalf61 (talk) 13:09, 18 June 2013 (UTC)
June 18
Homozygote-to-heterozygote assortative mating
Are there any known examples of heterozygous genetic traits subject to assortative mating, but for which homozygotes prefer heterozygous mates and vice-versa? (This would be equivalent to the same allele coding a disassortative recessive trait and an assortative dominant trait. The reason I ask is that if autism-spectrum disorders were partly the result of a partially dominant trait, then people "square enough for the peg and round enough for the hole" would more often become partners and parents of those that needed them, as I have anecdotal evidence to suggest.) NeonMerlin 03:25, 18 June 2013 (UTC)
- I have no answer here, only the observation that the same thought had occurred to me. I think the 'square enough/round enough' phenomenon you're describing is what's known as the 'broader autism phenotype'. If anyone can supply some genetics insight here, I'd be fascinated. Thanks! AlexTiefling (talk) 11:37, 18 June 2013 (UTC)
- That article mentions disassortative mating, which I think is what you want (heterozygous mating with homozygous preferentially and v/v) - it's a keyword that will pull up things like [15]. You might also be curious about [16] (MHC disassortative mating?). Note that our article gives the opposite speculation - assortative mating - about autism. Wnt (talk) 21:57, 18 June 2013 (UTC)
Audio Source Signatures and Transforming
Given samples of two sources/instruments/voices performing/speaking the same piece in identical fashion is it possible to workout a transform between the two so that given new samples of one, we could transform it into a decent approx. of the other? For example, given a violin and an acoustic guitar playing various scales at various rates, could we generate an algorithm to map violin pieces to guitar pieces? What about for voices and speaking (This is my primary interest, but the situation is interesting in general)? Finally, what about less specific cases, for example: changing a male voice into a "female" voice (not a specific one, just removing male characteristics and adding feminine ones); or, with music, changing a string instrument to a brass version of it? I'd be interested in any information pertaining to this, including info on what characterizes one source from another (and the science/mathematics of it). Thank you for any help:-)Phoenixia1177 (talk) 04:52, 18 June 2013 (UTC)
- To give you an entry into the literature, what you are asking for in technical terminology is a digital filter that transforms a recording of a violin into something that sounds like a guitar, or a recording of a male voice into something that sounds like a female voice. All of these things can be done, but some are simple and others required a very sophisticated filter. The main thing that gives a sound source its character is its power spectrum, but there are other important things too -- for example a guitar is plucked while a violin is usually played with a bow, and it is very difficult to transform a plucked sound into a bowed sound. Looie496 (talk) 15:06, 18 June 2013 (UTC)
- This is definitely not easy stuff. There is software out there (eg this one) that can extract the pitch and duration of notes from simple music. (This is generally called "music-to-MIDI" because MIDI is the lingua franca of music synthesis) - and once you have that, you can relatively easily produce synthesized music played on any other instrument for which good synthesis exists. But if you have complicated polyphony or lots of different instruments playing different parts, then extracting the note information is much harder - perhaps impossible. Some software can do this in realtime - so that you can play music on a guitar and have it sound like a pipe organ or a xylophone...but, again, it's quite limited. Most good software takes longer to do the transcription than the music takes to play - so you can only use it on recordings.
- There are "voice changers" out there (just google "voice changer" and you'll see a bazillion of them) - but they mostly just shift the pitch of the voice up and down or add distortion of one kind or another. They don't change other "characteristics" that might identify the gender of the speaker.
- There is software out there that can make synthetic voices from sound samples of real people. I heard a very convincing demo of Winston Churchill reading some modern text that was created by dumping all of his recorded speeches into some analysis software - deducing the characteristics of his voice and then using that to synthesize his new speech. But software like this is quite new and specialized - it would probably require you paying a company who specializes in this kind of thing to do that work for you. I don't think the process is entirely automatic either.
- Thank you both for the answers:-) In general, I have an interest in this subject, but I do have an oddly specific need for a part of it. Supposing that I, a man, recorded my voice speaking with a female rhythm, vocabulary choices, inflection, etc. what would I need to do to it, mathematically, to make it passably feminine? I'm not wanting to do anything nefarious, but am making an fps for my own interest and use and don't feel like hunting down voice actors. Obviously, what I'm proposing isn't going to end up with a great sound for female characters, but it sounds fun and a neat side project to learn something from. (I've acted both male and female roles extensively, and written them, so I can imitate how a female speaks outside of actual physical properties.) On this elaboration, additionally, would it be feasible to modify a given masculine voice to sound like a different male speaker more easily? Phoenixia1177 (talk) 05:57, 19 June 2013 (UTC)
- With regard to voice transformation, some work was done by NASA in the 1960's with a view to using analogue methods of bandwidth compression, which were never used. I remember they published an interesting paper, the essentials of which are: 1) the human voice comprises 3 frequency bands, low, originating in the chest & contributing to the quality of the voice; midband originating in the throat, contributing mainly to the the speech evelope and to the identity of the speeker, and high, originating in the mouth, contributing the intellence (ie conveyed meaning) of the speech. To convert male speech into female sounding speech, you need to suppress the mids and substitue new mids at higher pitch and with less harmonic structure. For best illusion, you need to shift the lows up in pitch a bit. You need to leave the highs alone, or you'll get a Donald Duck voice with poor diction. None of this is easy. I cannot find the NASA paper now, but this may give you some clues for your own search.
- If you have (or a friend has) good electronics construction skills, you may find it easier to try the 1940's VODER approach - using a key or finger controlled electronic voice. See http://www.davidszondy.com/future/robot/voder.htm. Scroll down until you find the video clip. Do not be discouraged by the sound quality - the machine was built by a chap interested in encription and novelty, not high quality sound. Operators needed to build up considerable skill to operate this machine. However, about 30 years ago, myself and some other workers revisited the VODER idea for helping children who had lost their voice due to cancer and for who a throat box artifical larynch is not a option. The usual approach was to give them a pocket computer with a text-to-speech program, but the sound is so unnatural that people tended to ignore it. Or a device with some pre-recorded emergency messages ("please, where is the toilet?", "please phone my mum on 456-98765") which also tended to get ignored. And conversations were impossible due to the delay in typing. The idea was that they could take everywhere a pocket size VODER machine, which might be possible with 1980's electronics instead of 1940's electronics. We found that producing a tolerable child voice to match the child was not difficult, but we coudn't do an adult (male or female) voice, as to do that you need a full size hi-fi loudspeaker. We found that using the key arrangement of an English stenography/court reporter machine (which is a bit like a multi-key computer mouse, with 5 keys positioned to naturally fall under each thumb and finger tip. Different combinations of key pressed simultanously code for each steno "sound") and the right choice in key coding with pressure coded volume, made it easy to learn. A bright 8-year old could learn to make useful speech in a few hours, sounding much better than computer text-to-speech software, and could easily do it fast enough for a natural conversation. With the steno keyblock in the right hand, and a pitch control in the left, they could do a pretty good job at singing! Our project was not very successful however. Batteries then were not good enough, and we couldn't make it cheap enough. — Preceding unsigned comment added by 124.182.180.102 (talk) 07:01, 19 June 2013 (UTC)
- Thank you both for the answers:-) In general, I have an interest in this subject, but I do have an oddly specific need for a part of it. Supposing that I, a man, recorded my voice speaking with a female rhythm, vocabulary choices, inflection, etc. what would I need to do to it, mathematically, to make it passably feminine? I'm not wanting to do anything nefarious, but am making an fps for my own interest and use and don't feel like hunting down voice actors. Obviously, what I'm proposing isn't going to end up with a great sound for female characters, but it sounds fun and a neat side project to learn something from. (I've acted both male and female roles extensively, and written them, so I can imitate how a female speaks outside of actual physical properties.) On this elaboration, additionally, would it be feasible to modify a given masculine voice to sound like a different male speaker more easily? Phoenixia1177 (talk) 05:57, 19 June 2013 (UTC)
my 2001 bmw 740i is rattling
what do i do? i can't figure it out and ain't got no money. it rattles upon slow acceleration and is a light vibrator noise coming from my right rear wheel. — Preceding unsigned comment added by 108.212.70.237 (talk) 05:38, 18 June 2013 (UTC)
- I'm not a mechanic, but my personal opinion is that your wheels need to be balanced. Lucky for you, this can be done pretty easily and inexpensively at most tire shops. (BTW, if you "ain't got no money", perhaps you might consider selling your luxury BMW and buying a more affordable car to make ends meet?) 24.23.196.85 (talk) 06:13, 18 June 2013 (UTC)
- Hmmm.. A 2001 740i could have been purchased for as low as $5000 if in poor condition and with no maintenance logs, even though it is a top line luxury vehicle. Sometimes young guys buy these before realising that a car costs a lot more to own than the purchase price, so the question could be genuine. Check the driveline: Check each universal joint. These rwd cars have a split prop shaft like trucks and some GM cars. Check the prop shaft carrier/bearing underneath the center of the car. When the resient mounting fails you get just the symptoms you describe. If the carrier and bearing are ok and the universal joints are ok, check things like engine mounts and suspension components. Note that if engine is misfiring one cylinder this can combine with other problems to cause rattles during mild acceleration. Also note that if te car has been correctly serviced by properly certified mechanics since new, all these faults are just about impossible. — Preceding unsigned comment added by 124.182.43.219 (talk) 10:20, 18 June 2013 (UTC)
- I agree with the above suggestion. If you don't think you can do the checks yourself, look for an underbody shop that will provide a free inspection. They'll lift it up, look over things, and show you exactly what has gone wrong and what needs to be replaced. They'll provide you with an estimate, which I would expect to be $75-$250 in labor (assuming you're in the US) on top of the normal cost of the parts, depending on the exact problem. From there you can decide whether it is worth it to handle the repair on your own or not. 209.131.76.183 (talk) 14:30, 18 June 2013 (UTC)
- It is unfortunate that you don't have the money to get it looked at by a professional. Wheel vibration has to be taken very seriously because the causes can be anything from something as simple as a balance weight fallen off of the rim - all the way up to a problem with wheel bearings or a broken axle that can literally cause the wheel to fall off at freeway speeds! With a car as old as this one, wheel bearing issues are quite likely - and if the car was for sale at a suspiciously cheap price, that could easily be the reason why!
- It often takes an expert to figure out which of those things it is. At the very least, I'd want to get it put up on a lift so that the wheel and it's associated suspension parts can be examined properly. When you know how serious the problem is, you can judge whether you can fix it yourself, or whether you can afford to get someone to fix it for you - or maybe it's a problem you can just decide to live with (very unlikely in the case of wheel vibration!).
- If you absolutely must do something yourself:
- I suppose it could be something as simple as a loose lug nut or a bad tire (look for suspicious bulges or other signs that it's not perfectly round and smooth)...those are things you can easily check - but it's relatively unlikely.
- You could jack up that corner of the car (make sure it's in gear or in park and with the parking brake on!) until the wheel is just off of the ground by a half inch or so - then try to wobble the wheel with your hands - both side-to-side and up-and-down. If you don't know what to expect (and I guess you don't), try the same thing with the wheel that's not causing vibration. Does they feel the same? If you can feel a definite difference between them then you probably have a wheel bearing problem - which is quite serious and potentially dangerous. If that's the problem then you're in a position where "If you have to ask the question - you're not qualified to fix it" - and this is going to cost you serious money to get it fixed. BMW's are amongst the more expensive cars to get work done on.
- Going to a cheap tire place and asking them to balance the wheel might help - and shouldn't cost an arm and a leg.
- Failing that, try to find a garage who will assess the problem for no charge - some places will do that. That would at least tell you whether this is something potentially life-threatening - or just an annoyance that should be fixed to avoid premature tire wear or something.
- General "rattles" are only to be expected in a car of this age - and they may well be exacerbated by your wheel wobble - so get that seen to first. A rattle can be anything from a loose dashboard panel to a bent exhaust heat baffle to a broken motor mount! There is no way we can diagnose it for you without a lot more information.
- I have to say: If you want a cheap car - then don't buy a cheap luxury car - those things are hard to maintain, the parts are expensive - and if you need professional help, remember that mechanics who specialize in luxury cars charge prices that only luxury car owners can afford!
- If you have visions of becoming a "shade tree mechanic" and doing the work yourself then you need to start with something older, smaller and simpler!
- SteveBaker (talk) 14:49, 18 June 2013 (UTC)
- This is a rear wheel drive car with 4-wheel independent suspension, so a broken axle is most unlikely - the symptoms would be quite a bit more dramatic (think: extreme grinding noises; unable to move). However, a fauly wheel bearing is a possible cause of the rear wheel vibration only, especially if the car has high mileage and a history of poor maintenance (faults like this always come back to poor maintenance, which in turn is indicated if the purchase price was low). I agree with the rest of Steve's comments. The corrollary to them is that taking luxury cars to average mechanics can cost you even more than the expensive specialist mechanics. Simply because the average mechanic sees GM cars, Fords, Toyotas, etc all the time. Experience with them gives them the ability to diagnose faults straight away without having to puzzle them out with logical thinking. And they can get on the phone to their parts supplier (if they don't already have the parts) right away and call out a list off the top of their head. For car they hardly ever see they'll have to a) obtain/download the relavent parts manual (a story in itself these days) b) study it, c) work out what parts they need, d) find out where to get them and order and d) when they parts arrive, realise they need something else. And YOU pay for the time. The practical advantage of luxury cars is minimal these days anyway. All the major brands all have good design and good safety. The benefit of buying high end luxury cars is being able to say "look at me - I am a big success - I can afford it" - which does not apply if you bought an old cheapie. Back in the 1960's makes like BMW, Mercedes had clear advantages over GM and Ford, with their all-independent suspension, proper auto aircon, durability, and safety, but the gap has closed a lot now. Now you pay for the name. — Preceding unsigned comment added by 124.182.180.153 (talk) 23:18, 18 June 2013 (UTC)
Possible improvements in Wikipedia entry on Milankovitch Cycles
Introduction:
James Hansen's book, "Storms of My Grandchildren" make it clear that our understanding of Ice Ages, which has come about from a combination of Milankovitch Theory with much hard work by geologists on coring and complex analyses of those cores, is at the centre of our understanding of what is likely to happen to us as Global Warming picks up pace.
Unfortunately, even though I am a climate physicist of 40 years experience, engaged on writing a popular book on this subject, I cannot yet say I understand Milankovitch theory as described in this article (or any other popular books on the subject) well enough to write a simple but authoritative account of it for my readers.
I am not blaming the authors of the article at all for this -- it is genuinely a brute of a subject for anyone to get one's head around, and I have learned a lot from what they say.
My question:
Could the Reference Desk kindly get me into email contact with the authors of the article? In particular, with whoever provided the box, found on the right side of the first page of this article? Whoever the person is who came up with this box, it's clearly got nearly all of what I need to understand. I would like to email back and forth with him/her to explain what I am trying to do, and to work out some wording that satisfies both me and that person as a description of the physical things that govern the summer warming of icecaps. What's already there is very good. However, my purpose is to explain the observational history of the past few centuries that led to these astrophysical results, and this is the area where I most need the advice of the authors of the article. (Ivars Peterson's "Newton's Clock: Chaos in the Solar System", Freeman and Co 1993, unfortunately just misses giving what I need). 06:45, 18 June 2013 (UTC)~ — Preceding unsigned comment added by Neergtrish (talk • contribs)
- Hi. Firstly, it's probably worth pointing out that almost all Wikipedia articles are collaborative efforts, so it's unlikely that there is a single 'author' of the article, which seems to be what you're looking for. Nevertheless, if you want to find out who has contributed to the article, simply click 'View History' at the top of the article and you will be able to see all the changes made throughout its history.
- As for the image in the top right, this was added on 3rd June 2009 by Incredio. If you wish to contact them, the best way would be through their talk page. Some editors allow others to email them through Wikipedia's EmailUser, but Incredio has not enabled this, so on-Wiki communication is the only way.
- Alternatively, since this is the reference desk, we may be able to help. If you post the questions you have about the article, maybe we can find some sources to aid your understanding. - Cucumber Mike (talk) 07:55, 18 June 2013 (UTC)
- The basic problem here is that this just isn't how Wikipedia works. It's a collaborative environment - the idea is that any discussions you may wish to have about the content of the article should be publicly available to anyone who wishes to be involved in editing the article - either now, or in years to come. That discussion forms a valuable record of why particular editing choices were made. Hence private email communication between editors about article content is generally considered inappropriate - even in cases where the user allows you to communicate with them in private. By far the best thing for you to do here is to raise your concerns on the "Talk" page of the article itself - generally your input will be seen as interesting and valuable - so long as it is constructive and on-topic. Beware though that most of the time people here have little respect for your personal qualifications (after all On the Internet, nobody knows you're a dog) and you'll need to make your points by linking to reference information such as books and scientific papers that back up whatever point you're trying to make.
- Another part of Wikipedia culture that's important here is encapsulated in the Be Bold philosophy. It's perfectly OK (indeed, encouraged) for you to dive in and fix the article yourself. If there is some problem with what you write, it may get reverted - or improved in some way by another editor - and that should be the starting point for a discussion thread on the corresponding talk page. It's more efficient to make a change to the article and only to discuss it if people disagree than it is to debate each change before it goes into the article.
- If you absolutely must have private email communication, you could try enabling that for your own account and posting a request to the editors' talk page requesting that (s)he contact you via email. However, don't be surprised if the don't. Most editors (myself included) would prefer to keep communications about article content on the relevant talk page.
- Wikipedia is a very different way of working than you may be used to - but it's surprisingly productive - and the outcomes are usually good. SteveBaker (talk) 14:07, 18 June 2013 (UTC)
- The article (for convenience) is Milankovitch cycles, and I've added a thumbnail of the image in question here. Note that the creator, Incredio, has not edited Wikipedia since November 2012, and therefore is probably not paying attention to his or her talk page (but you never know). Looie496 (talk) 14:51, 18 June 2013 (UTC)
- Yeah - but there are plenty of other people working on the article - and a vibrant, active talk page. If our OP raises his issues there, I'm quite sure that they'll be properly discussed and any problems swiftly dealt with or explained. SteveBaker (talk) 23:58, 18 June 2013 (UTC)
What's this turtle doing?
These two photos were taken just a few days ago in Vermont. I'm guessing that it's a common snapping turtle. It was a bit aggravated by me being as close as I was and I didn't want to bother it too much. What was it likely doing? Would the other two, for lack of a better word, divots in the soil next to the road in the foreground of the second image have been dug out by the turtle? Was she(?) laying eggs? There wasn't a large body of water near by. She was scratching at the dirt when I found her. Thanks, Dismas|(talk) 07:11, 18 June 2013 (UTC)
- Yes, laying eggs I think. They live is small streams, don't they ? So you might not know there's one nearby. Since turtles don't exactly travel long distances on land, I'd expect the stream it came from to be quite close (maybe even a drainage culvert). They don't seem to understand the danger of roads, either, unfortunately. StuRat (talk) 07:31, 18 June 2013 (UTC)
- Snapping turtles prefer standing water, not flowing streams. They like warmer elevated areas for nesting, that roadside would have met both those requirements. μηδείς (talk) 16:54, 18 June 2013 (UTC)
Can neutrinos undergo gravitational lensing?
Thanks!76.218.104.120 (talk) 11:28, 18 June 2013 (UTC)
- Yes. Like all elementary particles, neutrinos have mass/energy - therefore their paths are affected by gravity, and therefore they undergo gravitational lensing. Gandalf61 (talk) 11:36, 18 June 2013 (UTC)
- More specifically, high-energy neutrinos (the only ones that we can detect directly) will follow paths that are essentially indistinguishable from the paths of photons in terms of gravitational lensing. — Quondum 13:58, 18 June 2013 (UTC)
Complex space & Quantum mechanics
I don't have a background in particle physics, but I have been doing some reading. I recently picked up on some ideas brought up with Imaginary time and the complex plane. So is it possible to project an imaginary axis on a 3D, maybe as (x,y,z,i), n-dimensional Euclidean space? I could only assume this logic could be used to explain occurrences such as particle-wave duality. In a similar way this little diagram (http://commons.wikimedia.org/wiki/File:Dualite.jpg) shows how what we may see as 2 separate figures may in fact be the same because we fail to see a superfluous axis of space. Bugboy52.4 ¦ =-= 13:51, 18 June 2013 (UTC)
- I assume you mean the coordinates (x,y,z,it), since i is not a variable. If that's the case than yes, there's value in that approach as a way to better understand Minkowski space, but that is not related to the concept of particle-wave duality. Dauto (talk) 15:40, 18 June 2013 (UTC)
- If you're thinking about complex numbers, three spatial dimensions, and time, the classic object building off of those is the quaternions. I'm not aware of applications to quantum mechanics, but they have been used for many types of theoretical physics, and you might enjoy reading up on them anyway.
| “ | Time is said to have only one dimension, and space to have three dimensions. […] The mathematical quaternion partakes of both these elements; in technical language it may be said to be "time plus space", or "space plus time": and in this sense it has, or at least involves a reference to, four dimensions. And how the One of Time, of Space the Three, Might in the Chain of Symbols girdled be. | ” |
- What are the extra j and k axis in quaternions and its application in physics? this is fascinating, Id like to learn more. Oh and I was just considering the possibility since particles can appear and reappear or have a superposition then maybe we are only visualizing these particles from the perspective of our space because they are moving through an extra dimention. Bugboy52.4 ¦ =-= 23:05, 18 June 2013 (UTC)
- The j and k are extra imaginary components - that is j^2 = k^2 = -1. Note that quaternions do not obey commutativity of product: ij = k, ji = -k for instance. Your ideas about particle-wave duality are off the mark. It has nothing to do with extra dimensions. Particle-wave duality really is simply a symptom of the inadequacy of the concepts of particles and waves to begin with. Dauto (talk) 03:47, 19 June 2013 (UTC)
- I noticed that as well, how a product was able to have 2 seperate qoutients. Is there anywhere I can read more indept about this cerriculurly? 99.38.224.22 (talk) 04:36, 19 June 2013 (UTC)
Siphoning gasoline - which cars to choose?
I'm just watching something postapocalyptic and they need to siphon gasoline from abandoned cars. How would they know which contain the right type? Are there just two (diesel and unleaded)? Would it still be good after 20 years sat in the tank? — Preceding unsigned comment added by 89.241.228.217 (talk) 15:35, 18 June 2013 (UTC)
- This is harder than it looks, because the nature of fuel can vary between countries and even between regions. (Of all of these the most comical, and I would think lucrative for the right people, is Opal (fuel); but I think it still is subject to stability issues despite the lower volatility) See Gasoline#Stability to get started. Wnt (talk) 16:15, 18 June 2013 (UTC)
- Not hard at all - every car parts store sells siphons - and locking gas caps to prevent theft from your own car. And how many times have you had to alter your car when you went on vacation? Rmhermen (talk) 17:29, 18 June 2013 (UTC)
- The neck of the gas tank is a different diameter for gasoline and diesel - in an effort to prevent people from accidentally grabbing the wrong hose at the gas station and filling their car with the wrong stuff...so it's easy to tell which is which if you know what you're looking for. Also, many cars now have stickers inside the gas cap telling you what octane range you need - which would also be a clue.
- However, after 20 years, gasoline (and probably diesel too) is going to be almost useless for running a car on. The lighter fractions of the complex chemical brew will evaporate off and leave only the heavier fractions. As gasoline gets more than a year old - it gets harder and harder to start you car using it, and eventually, it flat out doesn't work. SteveBaker (talk) 19:25, 18 June 2013 (UTC)
- Well, the "Opal" I mentioned has fewer volatiles - however, I think that the main problem isn't loss of volatiles, but reaction with oxygen to cause gummy deposits. I would allow for the possibility that ingenious postapocalyptic people can modify engines to be more robust (or find ancient relics or modern eco-prototypes that can choke down anything) but not be able to reach in and turn the key on a car they find beside the road, even with that battery they were carrying around in their backpack for such occasions. But I don't know these things well - it would be nice to hear an actual mechanic chime in. Wnt (talk) 20:56, 18 June 2013 (UTC)
- There are details about what happens to "old" gasoline here Gasoline#Stability - suffice to say that 20 year old gasoline is going to be fairly useless as a fuel for cars. Incidentally - I checked up on diesel - and the problem is pretty much the same. This BP document says that diesel fuel is not "useable" after 6 to 12 months depending on temperature.
- Truly, after just a few years, the gasoline in car tanks would be very poor for running cars...after 20 years, there's no chance. SteveBaker (talk) 23:53, 18 June 2013 (UTC)
- Well, the "Opal" I mentioned has fewer volatiles - however, I think that the main problem isn't loss of volatiles, but reaction with oxygen to cause gummy deposits. I would allow for the possibility that ingenious postapocalyptic people can modify engines to be more robust (or find ancient relics or modern eco-prototypes that can choke down anything) but not be able to reach in and turn the key on a car they find beside the road, even with that battery they were carrying around in their backpack for such occasions. But I don't know these things well - it would be nice to hear an actual mechanic chime in. Wnt (talk) 20:56, 18 June 2013 (UTC)
All the above makes some assumptions. Internal combustion engines all around the world have used all sorts of horrible fuels, so long as you have air fuel and spark a mechanic will eventually get an SI engine to run, and a diesel engine is in theory not much less flexible. Greglocock (talk) 01:52, 19 June 2013 (UTC)
Generic medications
I am a physician and today at work a dispute arose between our pharmacist and myself. Some of my patients complain that the medications they take often change their appearance in color and shape. They claim that some of the generic variants are in fact ineffective and they refuse to take them. I believe that there might be a scientific explanation why this happens. Different generic manufacturers probably use different fillers (clays) and in some cases the active chemical (medication) gets bound to them and passes the GI tract thus decreasing bio availability. Our pharmacist contends that all generics are tightly regulated by the FDA and such an event is not possible.
The question is: are the fillers regulated by the FDA?
Thanks, - Alex — Preceding unsigned comment added by 168.178.74.53 (talk) 16:27, 18 June 2013 (UTC)
- They are in Canada. I doubt the US would be different. I have found, personally, with one specific drug, that the original and one of the generics work for me, but not another of the generics. My pharmacist says that this is not uncommon on an individual basis. Bielle (talk) 16:37, 18 June 2013 (UTC)
- You should take a look at Generic drug#Quality standards. Ironically, it includes a statement that, "A physician survey in the US found only 17% of prescribing physicians correctly identified the USFDA's standards for bioequivalency of generic drugs". Looie496 (talk) 16:48, 18 June 2013 (UTC)
- (Let me note that we are flirting dangerously close here to Wikipedia's prohibition against giving medical advice -- the fact that the questioner identifies as a physician isn't really relevant.) Looie496 (talk) 16:52, 18 June 2013 (UTC)
- No, we are not. And yes, it isn't. --Stephan Schulz (talk) 17:54, 18 June 2013 (UTC)
- Kainaw's criterion is not being breached. We can certainly answer this question. SteveBaker (talk) 19:14, 18 June 2013 (UTC)
- No, we are not. And yes, it isn't. --Stephan Schulz (talk) 17:54, 18 June 2013 (UTC)
- (Let me note that we are flirting dangerously close here to Wikipedia's prohibition against giving medical advice -- the fact that the questioner identifies as a physician isn't really relevant.) Looie496 (talk) 16:52, 18 June 2013 (UTC)

- I believe that it's well known that the packaging of generics can make a difference. Our article generic drug says "A generic drug must contain the same active ingredients as the original formulation. According to the U.S. Food and Drug Administration (FDA), generic drugs are identical or within an acceptable bioequivalent range to the brand-name counterpart with respect to pharmacokinetic and pharmacodynamic properties. By extension, therefore, generics are considered (by the FDA) identical in dose, strength, route of administration, safety, efficacy, and intended use." - which suggests that (in the USA at least) your patient's fears are unfounded. However, the article goes on to say "The FDA's use of the word "identical" is very much a legal interpretation, and is not literal." - so these generics are "legally identical" but maybe not actually identical.
- Later in that article, under Generic_drug#Efficacy - it describes a study done in 2009 on oxybutynin that showed that the generic version was less effective - and the image there shows a clear difference in the rate at which bupropion is released between the generic and the original version of the drug. Our Bioequivalence article has more to say on the matter.
- So, yes, there are probably some small differences. The question is whether these differences are sufficient to be truly noticeable by your patients. Clearly the FDA don't think they should be.
- We should also suspect that a nocebo effect is taking place here - because the patients disbelieve that the generic is as good ("How can it possibly be as good? It's cheaper!") they are getting a reverse-placebo effect that is making them genuinely sicker for reasons unrelated to the content of the drug itself. They simply lost confidence in it and the nocebo effect did the rest.
- An alternative hypothesis for what you're seeing is that (randomly) some patients get better after a while on the treatment and some get worse - regardless of whether they are taking the generic or the original drug. The ones who get better don't say anything - and the ones who didn't switch to the generic don't attribute their change to the treatment because it didn't change. But the patients who (by chance) get worse right around the time they switched to the generic version will always attribute the change to the first thing that comes to mind - which is that the generic drug doesn't work as well. Since you're only hearing from the ones who have something to complain about - you're going to see a rather severe "confirmation bias" effect going on here.
- In terms of what you can do about it - that's a tougher matter. Having an authority figure (such as a doctor) boldly assert that the generic is utterly identical and just as good as the original might well have an effect - but we're into the realms of psychology and "bedside manner" rather than hard science. Worse still, if you yourself now have doubts over the generic drug - then that might well be subconsciously passed onto the patients, which would be a clear case where the nocebo effect could happen.
I am the original poster. Thanks to everyone who responded. I am not sure I am doing this post correctly, I've never before posted answers. One of the later contributors extrapolated on the difference between generic and brand names. This is not my situation at all. We are talking about a perceptible difference between two generics. Today's case in one of many in my practice. In the environment I work in everything might be suspect (I am a prison psychiatrist. I see up to 30 patients a day). This case however stands out. The man said that he had taken citalopram for 7 years and it always worked for him. Two weeks ago he was given citalopram from a different maker and now he is complaining that it is not working, he is getting depressed.
About 15 or 20 years ago I read an article about an attempt to compare the bio availability of brand name Tegretol with various generic carbamazepines. Surprisingly some of the generics appeared to have substantially better bio availability than the brand name medication. That article led me to believe that the fillers are responsible for the difference and that they are not regulated.
Thanks. - Alex — Preceding unsigned comment added by 168.178.74.53 (talk) 20:17, 18 June 2013 (UTC)
- One important consideration is that even though generics need to meet the same standards as all other drugs, they are in fact much less closely monitored. Also, because the profit margins are significant smaller for generic drugs than for branded drugs that not yet off patent, there is a very real and very significant pressure to cut manufacturing and distribution costs. That's a situation that is simply ripe for abuse. See for example the massive criminal fraud that pervaded generic manufacturer Ranbaxy: http://features.blogs.fortune.cnn.com/2013/05/15/ranbaxy-fraud-lipitor/ DrMatthias (talk) 20:28, 18 June 2013 (UTC)
- There's also the possibility that the generics are outright counterfeits. You might want to have the patient return to the original med while you have the new generic tested, and also monitor any other patients who were switched to the new med. I personally am highly suspicious of any change to prescribed meds ("If it ain't broke, don't fix it").
- As for differences in the binder, my dad had an interesting case where he was using a pill cutter on the name brand to split it into two, but then the generic equivalent just shattered into sharp shards when he tried to use it there. StuRat (talk) 20:38, 18 June 2013 (UTC)
- The differences between generic and branded is likely to be similar to that between one generic and another. They certainly do differ in the binders and other non-active ingredients that they use - but (in theory) that's not supposed to make any difference because the FDA requires them not to. My previous comments about nocebo and observer bias still apply. It would probably be unethical - but I'd be strongly tempted to get a batch of the generic that your patient did fine on and dunk them into some food coloring to make them look like something else. If he continues to claim that it's not working then you may suspect the nocebo/observer-bias effect. Sadly, that doesn't help you to do anything about it. SteveBaker (talk) 23:42, 18 June 2013 (UTC)
Generating electricity by evaporating water
Theoretically, if the relative humidity is r and the ambient temperature is T, then given N molecules of water, you can extract approximately an amount of W = -N k T Log(r) of work by letting the water evaporate (this is assuming the ideal gas law for water vapor, I leave this as an exercise for the Ref Deskers). This means that at a relative humidity of 60% at 20 C, one liter of water could theoretically generate about 69 kJ of energy.
The question is if we can generate electricity using a process that involves evaporating water. One can think of the following process. If we have two cups of water and we add salt to one cup, then one can generate electricity using electrodes one of which can absorb sodium ions, the other electrode can absorb chlorine ions. When the electrodes are saturated one puts them in the cup of water without salt, electricity will then run in the other direction as the ions from the electrodes dissolve into the water. This process exploits the difference in salt concentration of the two cups of water and it has been tested in practice. So, we can just use this process until the salt concentrations are equal. Then we wait until all the water has evaporated away, we then get the salt back and we can start the same process again. But this seems to me a cumbersome process that doesn't generate a lot of power. Count Iblis (talk) 17:01, 18 June 2013 (UTC)

- I don't see how evaporation contributes to the energy generation there. Looie496 (talk) 17:42, 18 June 2013 (UTC)
- Yup - and given that the world is neither short of salty water nor fresh(ish) water, why bother with the evaporation at all? Just swap the water over at the appropriate stage... AndyTheGrump (talk) 17:46, 18 June 2013 (UTC)
- See osmotic power. You could certainly increase the concentration of some salt via evaporation and then use the resulting gradient to provide power. It's probably not very effective, though. --Stephan Schulz (talk) 17:58, 18 June 2013 (UTC)
- Yup - and given that the world is neither short of salty water nor fresh(ish) water, why bother with the evaporation at all? Just swap the water over at the appropriate stage... AndyTheGrump (talk) 17:46, 18 June 2013 (UTC)
- There are of course other ways that evaporation of water can provide power - hydroelectricity is in most cases ultimately driven by the process... AndyTheGrump (talk) 18:09, 18 June 2013 (UTC)
- One form of Ocean Thermal Energy Conversion involves warm surface water evaporating under reduced pressure, the vapor then driving a huge low-pressure turbine (essentially an enclosed windmill) and finally condensing on contact with cold deep-sea water. (Personally, I'm not so sure this would work at all -- and even if it does, it would be EXTREMELY inefficient because of the low temperature difference.) 24.23.196.85 (talk) 01:23, 19 June 2013 (UTC)
Merkel
How much does Angela Merkel weight in atomic mass units? --Wissbegieriges (talk) 18:16, 18 June 2013 (UTC)
- Well, Ms Merkel's weight does not seem to be published anywhere I could find. This says that an average 58 year old caucasian woman weighs 70kg - and that the 25/75 percentile span is +/- 10kg. She doesn't appear to be excessively large or skinny - so that's a reasonable range. An atomic mass unit is 1.6x10-27 so just divide one by the other and you'll have your answer - somewhere between 36,000,000,000,000,000,000,000,000,000 and 48,000,000,000,000,000,000,000,000,000. SteveBaker (talk) 18:44, 18 June 2013 (UTC)
Problems with the definition of the newton in relativity
If the newton is the force required to accelerate a 1 kg object at 1 m/(s^2), then that seems to be only accurate classically, because F=ma is not valid relativistically. In my opinion the Newton should be defined as the force that will cause a 1 kg m/s change in an object's momentum if exerted over the course of a single second. But do we actually gain by using a relativistic definition of the newton?--Jasper Deng (talk) 19:27, 18 June 2013 (UTC)
- Forces need to be treated with a Lorentz transform when considering relativistic reference frames. If you do this correctly, "all the math works out." See Lorentz invariance with respect to momentum and force. Nimur (talk) 21:17, 18 June 2013 (UTC)
- But the problem is, we have:
- (if my differentiation is correct)
- which means, a constant force does not lead to a uniform acceleration, so 1 N does not exactly produce an acceleration of 1 m/s^2 for a 1 kg object.--Jasper Deng (talk) 21:39, 18 June 2013 (UTC)
- Note relativistic mass. In fact, this equation can be derived from the assumption that momentum remains conserved while mass increases, if I understand correctly. Wnt (talk) 22:02, 18 June 2013 (UTC)
- Usually, when we refer to the mass of an object, we refer to its rest mass, I'm pretty sure.--Jasper Deng (talk) 23:40, 18 June 2013 (UTC)
- When we refer to mass and we know we are worried about relativistic reference frames, we don't leave the interpretation up to chance! Specify what you mean. For example, the article I linked expressly defines inertial mass and invariant mass. The article defines and uses a different mathematical symbol for each value. Things get way too confusing if everyone is interpreting "mass" to mean different things! Nimur (talk) 00:19, 19 June 2013 (UTC)
- I never really grokked what Lorentz transforms were for-- so thanks OP for the question and Nimur for the answer! SemanticMantis (talk) 02:23, 19 June 2013 (UTC)
- The definition of the Newton doesn't specify which kind of mass is referred to.--Jasper Deng (talk) 05:39, 19 June 2013 (UTC)
- Oh, is that so? Perhaps you aren't looking hard enough: there's a whole club full of unit nerds who get together and standardize this sort of definition - accounting for all sorts of theoretical and practical specifics. The more pedantically one nitpicks at such things, the more pedantic the responses have to become. Nimur (talk) 06:04, 19 June 2013 (UTC)
- When we refer to mass and we know we are worried about relativistic reference frames, we don't leave the interpretation up to chance! Specify what you mean. For example, the article I linked expressly defines inertial mass and invariant mass. The article defines and uses a different mathematical symbol for each value. Things get way too confusing if everyone is interpreting "mass" to mean different things! Nimur (talk) 00:19, 19 June 2013 (UTC)
- Usually, when we refer to the mass of an object, we refer to its rest mass, I'm pretty sure.--Jasper Deng (talk) 23:40, 18 June 2013 (UTC)
- Note relativistic mass. In fact, this equation can be derived from the assumption that momentum remains conserved while mass increases, if I understand correctly. Wnt (talk) 22:02, 18 June 2013 (UTC)
- None of that is necessary. The Newton is defined as equivalent to 1 kg m / s^2. That definition works in relativety just as well as in classic physics. Dauto (talk) 03:32, 19 June 2013 (UTC)
- Not really. For example, torque is measured in N m, which is equivalent to the joule, but is clearly a different kind of quantity.--Jasper Deng (talk) 05:37, 19 June 2013 (UTC)
% fat-free
I'm wondering about how (under US regulations) a product can claim to be 97% fat-free when it's 80% fat. Any ideas? — kwami (talk) 23:04, 18 June 2013 (UTC)
- On the face of it, that seems impossible - so I'm guessing there is something missing in your description. What exactly is the product and what are the other claimed ingredients? SteveBaker (talk) 23:20, 18 June 2013 (UTC)
- Is that like those "low fat oils?" Either they have replaced it for soemthing else, or I don't know. OsmanRF34 (talk) 23:50, 18 June 2013 (UTC)
- Varying definitions of "fat". HiLo48 (talk) 00:http://images.google.com/11, 19 June 2013 (UTC)
- OK, it could have low saturated fat, or low monounsaturated fat, or low cis fat or whatever. But I doubt the marketing people are not aware that they are playing with the ignorance of the people who associate fat = bad, so low fat = good. OsmanRF34 (talk) 01:20, 19 June 2013 (UTC)
- This page says "When a label claims to be 95 percent free, it is not saying—contrary to popular belief—that only 5 percent of the calories come from fat. The legal definition of 95 percent fat free is 95 percent of the weight of the product doesn’t contain fat". ( spam filtered 3fatchicks.com/does-95-percent-fat-free-mean-that-only-5-percent-of-the-calories-come-from-fat/ ). Perhaps not the most reliable source, but I think they're on to something, regarding the labels being regulated by weight, not calorie content. When I googled for this, I also found this link from the US FDA[www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm064911.htm], but it doesn't load for me. SemanticMantis (talk) 02:34, 19 June 2013 (UTC)
- I typically see this with luncheon meats. I doubt weight alone would account for it, as I don't think sliced meat contains that much water. Even 98%-fat-free chicken soup is only 35% fat.[17] Weight would account for this meat,[18] which is 97% fat-free and 19% fat, but I've come across meats with similar claims that are 70 or 80% fat by calories. I can't figure that out. — kwami (talk) 05:12, 19 June 2013 (UTC)
Cleaning the shower stall.
Recently moved into a house with a glassed-in shower stall - which has a whitish film coating the surface that looks like hard water deposits or soap residue or something. So far, we've tried to clean it with:
- Calcium Lime Rust aka "CLR" - (now Phosphate-free)
- CLR-brand shower cleaner
- Scrubbing Bubbles
- Vinegar with Baking soda
- Bar Keepers Friend
- Hard work with various scrubbers and cloths.
Result so far...nothing, zip, nada. Shower still has deposits on glass. I was quite surprised that CLR didn't get it clean - but maybe the new phosphate-free formulation isn't effective.
Any suggestions? OK...any PRACTICAL suggestions?! :-)
SteveBaker (talk) 23:35, 18 June 2013 (UTC)
- I would try Acetic acid or Citric acid in reasonable concentrations (say 25% of Acetic acid). Adding baking soda is counterproductive, as it will neutralize the acid. --Stephan Schulz (talk) 23:48, 18 June 2013 (UTC)
- OK - I double checked with my g/f and she has tried vinegar by itself...same problem. But according to Vinegar, the white vinegar we used is only 8% acetic acid - so maybe need to get some of the real thing. We're planning to try some neat lemon juice. SteveBaker (talk) 00:16, 19 June 2013 (UTC)
- Be careful with the "real thing", if by that you mean glacial acetic acid. I understand that it's fairly nasty stuff. My dad said just the odor would knock you off your feet. --Trovatore (talk) 00:19, 19 June 2013 (UTC)
- Over here, 25% Acedic acid is sold in 1/2l and 1l bottles, as "Essigessence" (essence of vinegar). It's primarily marketed as a food product (dilute 5-1 with wine, juice, or, boringly, water, to make various vinegars), but also for cleaning. --Stephan Schulz (talk) 06:47, 19 June 2013 (UTC)
- Is it possible to reduce white vinegar (reduce in the cooking sense, not chemical)? Does it form an azeotrope, or can you obtain glacial without burning it? Plasmic Physics (talk) 00:22, 19 June 2013 (UTC)
- Be careful with the "real thing", if by that you mean glacial acetic acid. I understand that it's fairly nasty stuff. My dad said just the odor would knock you off your feet. --Trovatore (talk) 00:19, 19 June 2013 (UTC)
- OK - I double checked with my g/f and she has tried vinegar by itself...same problem. But according to Vinegar, the white vinegar we used is only 8% acetic acid - so maybe need to get some of the real thing. We're planning to try some neat lemon juice. SteveBaker (talk) 00:16, 19 June 2013 (UTC)
- If it corrodes metal should you reduce it in a Teflon coated kitchen vessel, under good ventilation or outside? Plasmic Physics (talk) 00:28, 19 June 2013 (UTC)
- I don't remember off the top of my head, but the WP data page on acetic acid doesn't show any azeotropes on the distillation curve -- so yes, if you heat the stuff hot enough (116 C or more) you can get glacial acid. WARNING -- DO NOT try this at home without BOTH a fume hood AND a gas mask, because the smell would knock you out cold! (I know from experience -- I've accidentally inhaled just a tiny whiff of glacial acetic acid vapor, and it almost burned my nose right off!) Also, whatever you do, DO NOT concentrate acetic acid in a metal container -- it will undergo a single displacement reaction, forming the corresponding metal acetate (i.e. burning a hole in the metal) and releasing hydrogen gas (which could cause an explosion if you're using an open flame as your heat source). And no, a Teflon-coated saucepan WILL NOT do -- the Teflon degrades over time with high temperatures, and can also easily become scratched during normal use, exposing the metal underneath. Use a glass or plastic container instead. 24.23.196.85 (talk) 01:55, 19 June 2013 (UTC)
- Except for being highly toxic and a disposal issue (i.e. you don't want it in the water supply), chromic acid will clean the white off of rice. It's great at getting sparkling glass, though it is nasty stuff. IIRC, you make it with a 50/50 mixture of concentrated sulfuric acid with a saturated solution of sodium dichromate. But again, it's so nasty, unless you have a means of using it in a well-ventilated space, avoid all skin contact, have proper breathing protection, and are scrupulously careful to properly dispose of every drop you use, it's probably not practical. But if chromic acid doesn't get it clean, it isn't meant to be clean. --Jayron32 01:16, 19 June 2013 (UTC)
- Yes, maybe Steve Baker is scrubbing like mad a frosted glass. OsmanRF34 (talk) 01:22, 19 June 2013 (UTC)
- Maybe not even deliberately frosted. Glass can become etched, and if it is etched, it can be as scrupulously clean and look milky or cloudy. --Jayron32 01:59, 19 June 2013 (UTC)
- Yes, maybe Steve Baker is scrubbing like mad a frosted glass. OsmanRF34 (talk) 01:22, 19 June 2013 (UTC)
June 19
The psychology of flirting between men and women
Can anyone help me learn the basic instinct behind the actions men and women reflexively take when trying to attract each other? Out of all the junk Google spews at me whenever I try to research the subject I got one good link from one site that took me to another website that said, for example, that women try to make themselves appear smaller when flirting with a man and men try to make themselves appear bigger when flirting with a woman. This sounds interesting, like animal behavior. I'd like to learn the "Why" behind these primal? instincts within us ("Women try to make themselves appear smaller BECAUSE thousands of years ago...") as well as see a list of actions that men and women take when flirting with each other. I don't care about trivial modern stuff like "Women should wear red!" and "Men, don't wear too much cologne!" I'm trying to explore the basic or primal instincts in our brains that's left over from ancient times. — Preceding unsigned comment added by 174.65.51.113 (talk) 00:39, 19 June 2013 (UTC)
- Have you tried Google Scholar instead of regular Google? It does a good job of seperating the wheat from the chaff. Select the "More" option at the top, then select "Even more" from that menu, and page down, there's a "Scholar" option on the next page. That's usually a good way to seek actual research and scholarly papers on topics. --Jayron32 01:11, 19 June 2013 (UTC)
- The "Women should wear red!" thing is not trivial modern stuff. Dauto (talk) 03:25, 19 June 2013 (UTC)
- Flirting is like foreplay. ←Baseball Bugs What's up, Doc? carrots→ 03:39, 19 June 2013 (UTC)
if a neutrino goes on a bullseye towards a nucleus will it usually pass right thru?
Or will the weak force interaction that I've read about probably occur? 2. Same questions for a neutrino on bullseye toward and into a lone proton. Will it usually just go past those 3 little quarks inside the proton like Road Runner or will it more likely interact? Thanks76.218.104.120 (talk) 05:16, 19 June 2013 (UTC)
- Straight through most of the time. Some relevant detail in neutrino and neutrino detector. Dragons flight (talk) 05:33, 19 June 2013 (UTC)
Searching for Jimmy Hoffa...
(For those of you not familiar with the case, Jimmy Hoffa was the head of the Teamsters Union who apparently was murdered by the Mafia in Detroit and buried in an unknown location, and they try to find him by digging up some new place every few years.)
My question is, in the latest excavation, they brought in a cadaver dog to sniff him out. Now, the remains of somebody dead for decades can't stink of decomp any more, right (at least in normal circumstances) ? So would the dogs do any good ? StuRat (talk) 06:29, 19 June 2013 (UTC)
- According to [19] some dogs can still detect dry bones 30 years after death. Dragons flight (talk) 06:54, 19 June 2013 (UTC)

