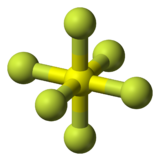
Fluorine
 Liquid fluorine (F2 at extremely low temperature) | |||||||||||||||||||||
| Fluorine | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | |||||||||||||||||||||
| Allotropes | alpha, beta (see Allotropes of fluorine) | ||||||||||||||||||||
| Appearance | gas: very pale yellow liquid: bright yellow solid: alpha is opaque, beta is transparent | ||||||||||||||||||||
| Standard atomic weight Ar°(F) | |||||||||||||||||||||
| Fluorine in the periodic table | |||||||||||||||||||||
| |||||||||||||||||||||
| Atomic number (Z) | 9 | ||||||||||||||||||||
| Group | group 17 (halogens) | ||||||||||||||||||||
| Period | period 2 | ||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||
| Electron configuration | [He] 2s2 2p5[3] | ||||||||||||||||||||
| Electrons per shell | 2, 7 | ||||||||||||||||||||
| Physical properties | |||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||
| Melting point | (F2) 53.48 K (−219.67 °C, −363.41 °F)[4] | ||||||||||||||||||||
| Boiling point | (F2) 85.03 K (−188.11 °C, −306.60 °F)[4] | ||||||||||||||||||||
| Density (at STP) | 1.696 g/L[5] | ||||||||||||||||||||
| when liquid (at b.p.) | 1.505 g/cm3[6] | ||||||||||||||||||||
| Triple point | 53.48 K, .252 kPa[7] | ||||||||||||||||||||
| Critical point | 144.41 K, 5.1724 MPa[4] | ||||||||||||||||||||
| Heat of vaporization | 6.51 kJ/mol[5] | ||||||||||||||||||||
| Molar heat capacity | Cp: 31 J/(mol·K)[6] (at 21.1 °C) Cv: 23 J/(mol·K)[6] (at 21.1 °C) | ||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||
| Oxidation states | −1, 0[8] (oxidizes oxygen) | ||||||||||||||||||||
| Electronegativity | Pauling scale: 3.98[3] | ||||||||||||||||||||
| Ionization energies | |||||||||||||||||||||
| Covalent radius | 64 pm[10] | ||||||||||||||||||||
| Van der Waals radius | 135 pm[11] | ||||||||||||||||||||
| Other properties | |||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||
| Crystal structure | cubic | ||||||||||||||||||||
| Thermal conductivity | 0.02591 W/(m⋅K)[12] | ||||||||||||||||||||
| Magnetic ordering | diamagnetic (−1.2×10−4)[13][14] | ||||||||||||||||||||
| CAS Number | 7782-41-4[3] | ||||||||||||||||||||
| History | |||||||||||||||||||||
| Naming | after the mineral fluorite, itself named after Latin fluo (to flow, in smelting) | ||||||||||||||||||||
| Discovery | André-Marie Ampère (1810) | ||||||||||||||||||||
| First isolation | Henri Moissan[3] (June 26, 1886) | ||||||||||||||||||||
| Named by | |||||||||||||||||||||
| Isotopes of fluorine | |||||||||||||||||||||
| |||||||||||||||||||||
Fluorine is the chemical element with symbol F and atomic number 9. It is the lightest halogen and has a single stable isotope, fluorine‑19. At standard pressure and temperature, the element is a pale yellow gas composed of diatomic molecules, F2. Fluorine is the most electronegative element and also the most reactive, requiring great care in handling. Compounds of fluorine are called fluorides.
In stars, fluorine is rare—for a light element—because it is consumed in fusion reactions. Within Earth's crust, fluorine is the thirteenth‑most abundant element. The most important mineral of fluorine, fluorite, was first formally described in 1529. The mineral's name derives from the Latin verb fluo, meaning "flow", because fluorite was added to metal ores to lower their melting points. Suggested as a chemical element in 1811, the dangerous element injured many experimenters who tried to isolate it. In 1886, French chemist Henri Moissan succeeded. His method of electrolysis remains the industrial production method for fluorine gas. The largest use of elemental fluorine, uranium enrichment, began during the Manhattan Project.
Global fluorochemical sales are over US$15 billion per year. Because of the difficulty in making elemental fluorine, 99% of commercially used fluorine is never converted to the free element. About half of all mined fluorite is used directly in steel-making. The other half is converted to hydrofluoric acid, a precursor to other chemicals. The most important synthetic inorganic fluoride is cryolite, a commodity that is critical for aluminium refining. Organic fluorides have very high chemical and thermal stability, which enables significant commercial applications. The largest use is in refrigerant gases; even though traditional CFCs are now mostly banned, the replacement molecules still contain fluorine. The predominant fluoropolymer is Teflon, which is used in electrical insulation and cookware.
While a few plants and bacteria synthesize organofluorine poisons, fluorine has no metabolic role in mammals. The fluoride ion, when directly applied to teeth, reduces decay. For this reason, it is used in toothpaste and water fluoridation. A growing fraction of modern pharmaceuticals contain fluorine; Lipitor and Prozac are prominent examples.
Characteristics
Isotopes
The nucleus of a fluorine atom contains nine protons. One stable isotope occurs naturally: fluorine-19, which contains ten neutrons.[16] This makes the element both monoisotopic and mononuclidic, characteristics that enable its use in uranium enrichment and in nuclear magnetic resonance.
Seventeen radioisotopes have been synthesized: mass numbers 14–18 and 20–31.[17] Fluorine-18 is the most stable, with a half-life of 109.77 minutes. The lightest fluorine isotopes, those with mass numbers of 14–16, decay via electron capture. Flourine-17 and -18 undergo beta plus decay (emission of a positron). All isotopes heavier than fluorine-19 decay by beta minus mode (emission of an electron); some also decay by neutron emission.[17]
Atomic structure
The neutral fluorine atom has nine electrons, one fewer than neon, arranged in the electronic configuration [He] 2s2 2p5.[18] Fluorine's outer electrons are relatively separate from each other. They hence do not shield each other from the nucleus and therefore experience a high effective nuclear charge. Fluorine has a small covalent radius, around 60 picometers, which is similar to oxygen and neon.[note 1][19][20]

Fluorine is reluctant to ionize. Instead, it exhibits a very strong preference for one more electron to achieve the extremely stable neon‑like electron configuration.[18] Fluorine is the most electronegative element.[21] It'sionization energy (energy required to remove an electron to form F+) is higher than that of any element except neon or helium.[22] It's electron affinity (energy released by adding an electron to form F–) is higher than that of any element except chlorine.[23]
Molecular structure
Fluorine forms diatomic molecules, F2. The difluorine bond is relatively weak, with a bond energy much less than that in Cl2 or Br2. It is similar to the easily cleaved oxygen–oxygen bonds of peroxides or nitrogen–nitrogen bonds of hydrazines.[24][25]
While an individual fluorine atom has one unpaired electron, molecular fluorine has all the electrons paired. This makes it diamagnetic (slightly repelled by magnets). In contrast, neighboring element oxygen, with two unpaired electrons per molecule, is paramagnetic (attracted to magnets).[13][26]
Phases
Fluorine is gaseous at room temperature.[27] Though sometimes cited as yellow-green, pure fluorine is actually a very pale yellow.[28] The gas has a "pungent" characteristic odor that is noticeable in concentrations as low as 20 ppb.[29] Fluorine condenses to a bright yellow liquid at −188 °C (−307 °F),[30] which is near the condensation temperatures of oxygen and nitrogen.
Fluorine solidifies at −220 °C (−363 °F)[30] into a cubic structure called beta-fluorine. This phase is transparent and soft with significant disorder of the molecules. With further cooling to −228 °C (−378 °F), fluorine undergoes a solid–solid phase transition into a monoclinic structure called alpha-fluorine. This phase is opaque and hard with close-packed, shingled, layers of molecules. The solid state phase change releases more energy than the melting point transition and can be violent, shattering samples and blowing out sample holder windows. In general, fluorine's solid state phases are more similar to oxygen's than to its fellow halogens'.[31][32]

|

|
| Low-temperature fluorine phases | Alpha-fluorine crystal structure |
Chemical reactivity
| External videos | |
|---|---|
Reactions with elemental fluorine are often sudden or explosive. Many substances that are generally regarded as unreactive—such as powdered steel, glass fragments, and asbestos fibers—are readily consumed by cold fluorine gas. Wood and even water burn with flames when subjected to a jet of fluorine, without the need for a spark.[27][33]
Reactions of elemental fluorine with metals require different conditions that depend on the metal. Many—such as aluminium, iron, and copper—must be powdered to overcome the protective metal fluoride layers formed by passivation.[25] The alkali metals, such as sodium, react with a bang. The alkaline earth metals, such as calcium, react not quite as dramatically. The noble metals gold, platinum, palladium, rhodium, and ruthenium react least readily, requiring pure fluorine gas at 300–450 °C (575–850 °F).[34]
Fluorine reacts explosively with hydrogen in a manner similar to that of alkali metals.[35] The other halogens react readily with fluorine gas[36] as does the heavy noble gas radon.[37] The lighter noble gases xenon and krypton can be made to react with fluorine under special conditions, while argon will undergo chemical trasformations only with hydrogen fluoride.[38] Oxygen and fluorine gases do not normally react, but they can be combined with electric discharge at low concentrations.[citation needed] Nitrogen, with its very stable triple bonds, requires electric discharge and very high temperatures to react with fluorine.[39]
Carbon, in graphite or diamond form, is impervious to fluorine gas at room temperature. Above 400 deg C, graphite reacts with fluorine to make a fluorinated solid, "carbon monofluoride". At even higher temperatures, gaseous fluorocarbons start to be produced; the reaction can become explosive.[40] Carbon dioxide and carbon monoxide gases react with fluorine gas at room temperature or a little above. The reactions make mixtures of products, are heat-generating, and are difficult to control.[41][42] Organic chemicals, such as paraffins, react strongly when exposed to fluorine.[43] Even fully halogenated organic molecules, such as the normally incombustible solvent carbon tetrachloride, can explode.[44]
The other solid nonmetals or metalloids (boron, silicon, arsenic, sulfur, germanium, phosphorus, selenium, tellurium) burn with a flame in room temperature fluorine.[45] Hydrogen sulfur and sulfur dioxide react readily with fluorine gas; the latter can be explosive. Sulfuric acid reacts much more sluggishly.[45]
Origin and occurrence
In the universe
| Atomic number |
Element | Relative amount |
|---|---|---|
| 6 | Carbon | 4,800 |
| 7 | Nitrogen | 1,500 |
| 8 | Oxygen | 8,800 |
| 9 | Fluorine | 1 |
| 10 | Neon | 1,400 |
| 11 | Sodium | 24 |
| 12 | Magnesium | 430 |
From the perspective of cosmology, fluorine is relatively rare with 400 ppb in the universe. Within stars, any fluorine that is created is rapidly eliminated through nuclear fusion: either with hydrogen to form oxygen and helium, or with helium to make neon and hydrogen. The presence of fluorine at all—outside of temporary existence in stars—is somewhat of a mystery because of the need to escape these fluorine-destroying reactions.[47][48]
Three theoretical solutions to the mystery exist. In type II supernovae, atoms of neon could be hit by neutrinos during the explosion and converted to fluorine. In Wolf-Rayet stars (blue stars over 40 times heavier than the Sun), a strong solar wind could blow the fluorine out of the star before hydrogen or helium can destroy it. In asymptotic giant branch (a type of red giant) stars, pulses of fusion reactions could allow convection to lift fluorine out of the inner star. Only the red giant hypothesis has supporting evidence from observations.[47][48]
In space, fluorine commonly combines with hydrogen to form hydrogen fluoride. (This compound has been suggested as a tracer to enable tracking reservoirs of hydrogen in the universe.)[49] In addition to HF, monatomic fluorine has been observed in the interstellar medium.[50][51] Fluorine cations have been seen in planetary nebulae and in stars, including our Sun.[52]
On Earth
Fluorine is the thirteenth most common element in Earth's crust, comprising between 600 and 700 ppm of the crust by mass. Because of its reactivity, it is usually found as fluoridated compounds. Three minerals exist that are industrially relevant sources: fluorite, fluorapatite, and cryolite.[53][54]
| Major fluorine-containing minerals | ||

|

|

|
| Fluorite | Fluorapatite | Cryolite |
- Fluorite (CaF2), also called fluorspar, is the main source of commercial fluorine. Fluorite is a colorful mineral associated with hydrothermal deposits. It is common and found worldwide. China supplies more than half of the world's demand; Mexico is the second-largest producer. The United States produced most of the world's fluorite in the early 20th century, but its last mine, in Illinois, shut down in 1995.[54][55][56][57][58]
- Fluorapatite (Ca5(PO4)3F) is mined along with other apatites for its phosphate content and is used mostly for production of fertilizers. Most of the Earth's fluorine is bound in this mineral, but because the percentage within the mineral is low (3.5%), the fluorine is discarded as waste. Only in the United States is there significant recovery. There, the hexafluorosilicates produced as byproducts are used to supply water fluoridation.[54]
- Cryolite (Na3AlF6) is the least abundant of the three major fluorine-containing minerals, but is a concentrated source of fluorine. It was formerly used directly in aluminium production. However, the main commercial mine, on the west coast of Greenland, closed in 1987.[54]
Minor occurrences
Several other minerals, such as the gemstone topaz, contain fluoride. Fluoride is not significant in seawater or brines, unlike the other halides, because the alkaline earth fluorides precipitate out of water.[54] Commercially insignificant quantities of organofluorines have been observed in volcanic eruptions and in geothermal springs. Their ultimate origin (from biological sources or geological formation) is unclear.[59]
The possibility of small amounts of gaseous fluorine within crystals has been debated for many years. One form of fluorite, antozonite, has a smell suggestive of fluorine when crushed. The mineral also has a dark black color, perhaps from free calcium (not bonded to fluoride). In 2012, a study reported detection of trace quantities (0.04% by weight) of diatomic fluorine in antozonite. It was suggested that radiation from small amounts of uranium within the crystals had caused the free fluorine defects.[60]
History
Early discoveries

The word "fluorine" derives from the Latin stem of the main source mineral, fluorite, which was first mentioned in 1529 by Georgius Agricola, who described it as a flux—an additive that helps lower the melt temperature during smelting.[61][62] Agricola, the "father of minerology", invented several hundred new terms in his Latin works describing 16th century industry. For fluorite rocks (schöne Flüsse in the German of the time), he created the Latin noun fluorés, from fluo (flow). The name for the mineral later evolved to fluorspar (still commonly used)[63] and then to fluorite.[58][64]
Hydrofluoric acid was known as a glass-etching agent from the 1720s, perhaps as early as 1670.[note 2] Andreas Sigismund Marggraf made the first scientific report on its preparation in 1764 when he heated fluorite with sulfuric acid; the resulting solution corroded its glass container.[67][68] Swedish chemist Carl Wilhelm Scheele repeated this reaction in 1771,[68][69] recognizing the product as an acid, which he called "fluss-spats-syran" (fluor-spar-acid).
In 1810, French physicist André-Marie Ampère suggested that the acid was a compound of hydrogen with an unknown element, analogous to chlorine.[70] Fluorite was then shown to be mostly composed of calcium fluoride.[66] Sir Humphry Davy originally suggested the name fluorine, taking the root from the name of "fluoric acid" and the -ine suffix, similarly to other halogens. This name, with modifications, came to most European languages, although Greek, Russian, and some others (following Ampère's suggestion) use the name ftor or derivatives, from the Greek φθόριος (phthorios), meaning "destructive".[71][72] The New Latin name (fluorum) gave the element its current symbol, F, although the symbol Fl was used in early papers.[73][note 3]
Isolation of the element
Progress in isolating the element was slowed by the exceptional dangers of generating fluorine: several 19th century experimenters, the "fluorine martyrs", were killed or blinded.[note 4] Initial attempts to isolate the element were also hindered by material difficulties: the extreme corrosiveness and reactivity of hydrogen fluoride (and of fluorine gas) as well as problems getting a suitable conducting liquid for electrolysis.[66][75]
Edmond Frémy thought that passing electric current through pure hydrofluoric acid (dry HF) might work. Previously, hydrogen fluoride was only available in a water solution. Frémy therefore devised a method for producing dry hydrogen fluoride by acidifying potassium bifluoride (KHF2). Unfortunately, pure hydrogen fluoride did not pass an electric current.[66][75][76]
French chemist Henri Moissan, formerly one of Frémy's students, continued the search. After trying many different approaches, he combined potassium bifluoride and dry hydrogen fluoride. The mixture conducted electricity. Moissan also constructed especially corrosion-resistant equipment: containers crafted from a mixture of platinum and iridium (more chemically resistant than pure platinum) with fluorite stoppers.[77][75]
After 74 years of effort by many chemists, on 26 June 1886, Moissan isolated elemental fluorine.[76][78] In 1906, two months before his death, Moissan received the Nobel Prize in chemistry.[79][80][81] The citation:[75][note 5]
...in recognition of the great services rendered by him in his investigation and isolation of the element fluorine...The whole world has admired the great experimental skill with which you have studied that savage beast among the elements.

|

|
| Moissan's cell, 1887 publication | Henri Moissan, Nobel Prize photo |
Later developments
During the 1930s and 1940s, the DuPont company commercialized organofluorine compounds at large scales. Following trials of chlorofluorcarbons as refrigerants by General Motors, DuPont developed large-scale production of Freon-12 in 1930. It proved to be a marketplace hit, rapidly replacing earlier, more toxic, refrigerants and growing the overall market for kitchen refrigerators. In 1938, Teflon was discovered by accident by a recently hired DuPont Ph.D., Roy J. Plunkett. While working with a cylinder of tetrafluoroethylene, he was unable to release the gas although the weight had not changed. Scraping down the container, he found white flakes of a polymer new to the world. Tests showed the substance was more resistant to corrosion and had better high temperature stability than any other plastic. By 1941, a crash program was making significant quantities of Teflon.[68][82][83][84]
Large-scale productions of elemental fluorine began during World War II. Germany used high-temperature electrolysis to produce tons of chlorine trifluoride, a compound planned to be used as an incendiary.[85] The Manhattan project in the United States produced even more fluorine for use in uranium separation. Gaseous uranium hexafluoride was used to separate uranium-235, an important nuclear explosive, from the heavier uranium-238. Because uranium hexafluoride releases small quantities of corrosive fluorine, the separation plants were built with special materials. All pipes were coated with nickel; joints and flexible parts were fabricated from Teflon.[82]
In the 1970s and 1980s, concerns developed over the role chlorofluorocarbons play in damaging the ozone layer. By 1996, almost all nations had banned chlorofluorocarbon refrigerants, and commercial production ceased. Fluorine continued to play a role in refrigeration though: hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) were commercialized as replacement refrigerants.[86][87]
Industry and applications
An estimate pegged the fluorochemicals global market size at over $15 billion in 2011 and predicted 5% growth to 2018, when the market would exceed $20 billion.[88] A different report said that, in 2016, global fluorochemicals production would be almost $20 billion on 3.5 million metric tons per year volume.[89]
The main source of fluorine, fluorite mining, was estimated in 2003 to be a $550 million industry, extracting 4.5 million tons per year.[68] Mined fluorite is concentrated by flotation separation into two main grades, with about equal production of each. Metspar (60–85% purity) is used almost exclusively for iron smelting. Acidspar (97%+ purity) is primarily converted to hydrofluoric acid (by reaction with sulfuric acid). The resultant HF is mostly used to produce organofluorides and synthetic cryolite.[68][90][55]

Inorganic fluorides
About 3 kg (6.5 lb) of metspar grade fluorite are added to make each metric ton of steel. The fluoride ions from CaF2 lower the melt's temperature and viscosity, making it runnier. Metspar is similarly used to produce cast iron and for other iron alloys.[55][91]
Acidspar grade fluorite is added to ceramics, enamels, glass fibers, clouded glass, cement, and the outer coating of welding rods.[55] Most acidspar is used to make hydrofluoric acid, a chemical intermediate for most fluorine-containing compounds. Significant direct uses of HF include pickling (cleaning) steel, etching glass, and cracking alkanes in the petrochemical industry.[55]

One third of HF (one sixth of mined fluorine) is used to make synthetic cryolite (sodium hexafluoroaluminate) and aluminium trifluoride. These compounds are used in the electrolysis of aluminium by the Hall–Héroult process. About 23 kg (51 lb) are required for every metric ton of aluminium.[55] Although small amounts are lost over time through various side reaction, the fluorides are not reactants in the smelting process, but fluxes that lower the temperature of the melt.[92]
Fluorosilicates are the next most significant inorganic fluorides formed from HF. Sodium fluorosilicate is used for water fluoridation, as an intermediate for synthetic cryolite and silicon tetrafluoride, and for treatment of effluents in laundries.[93]
Other inorganic fluorides made in large quantities include cobalt difluoride (for organofluorine synthesis), nickel difluoride (electronics), lithium fluoride (a flux), sodium fluoride (water fluoridation), potassium fluoride (flux), ammonium fluoride (various uses), and magnesium fluoride (antireflection optical coatings).[55] Sodium and potassium bifluorides are significant to the chemical industry.[94][95]
Fluorocarbons
Making organic fluorides is the main use for hydrofluoric acid, consuming over 40% of it (over 20% of all mined fluorite). Within organofluorides, refrigerant gases are still the dominant segment, consuming about 80% of HF. Fluoropolymers are less than one quarter the size of refrigerant gases in terms of fluorine usage but are growing faster.[55][96] Fluorosurfactants (materials like Scotchgard, used in durable water repellents) are a small segment in mass but are significant economically—over $1 billion yearly revenue.[97]
Refrigerant gases
Traditionally chlorofluorocarbons (CFCs) were the predominant class of fluorinated organic chemical. CFCs are identified by a system of numbering (the R-number system) that explains the amount of fluorine, chlorine, carbon and hydrogen in the molecules.[98] The DuPont brand Freon has been colloquially used for CFCs and similar halogenated molecules; brand-neutral terminology uses "R" ("refrigerant") as the prefix. Prominent CFCs included R-11 (trichlorofluoromethane), R-12 (dichlorodifluoromethane), and R-114 (1,2-dichlorotetrafluoroethane).[55]
Production of CFCs grew strongly through the 1980s, primarily for refrigeration and air conditioning but also for propellants and solvents. Since the end use of these materials is now banned in most countries, this industry has shrunk dramatically. By the early 21st century, production of CFCs was less than 10% of the mid-1980s peak.[55]
Hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) now serve as replacements for CFC refrigerants; few were commercially manufactured before 1990. Currently more than 90% of fluorine used for organics goes into these two classes, in about equal amounts. Prominent HCFCs include R-22 (chlorodifluoromethane) and R-141b (1,1-dichloro-1-fluoroethane). The main HFC is R-134a (1,1,1,2-tetrafluoroethane).[55]
Fluoropolymers
The 2011 global fluoropolymer market was estimated at slightly under $6 billion revenue and predicted to grow 6.5% per year until 2016.[99] As of about 2006–2007, fluoropolymer volume was estimated at over 180,000 metric tons per year. The corresponding revenue estimate was over $3.5 billion.[100]
Polytetrafluoroethylene (PTFE) is 60–80% of the world's fluoropolymer production on a weight basis.[100] The DuPont brand Teflon is sometimes used generically for the substance.[101] The largest application for PTFE is in electrical insulation. It is an excellent dielectric. It is also used extensively in the chemical process industry where corrosion resistance is needed: in coating pipes, in tubing, and gaskets. Another major use is architectural fabric (PTFE-coated fiberglass cloth used for stadium roofs and such). The major consumer application is non-stick cookware.[101]
| Major PTFE applications | ||

|

|

|
| PTFE dielectric separating core and outer metal in a specialty coaxial cable | First Teflon branded frying pan, 1961 | The interior of the Tokyo Dome. The roof is PTFE-coated fiberglass and air-supported.[102] |
When stretched with a jerk, a PTFE film makes a fine-pored membrane: expanded PTFE (ePTFE). The term "Gore-tex" is sometimes used generically for this material, but that is a specific brand name. ePTFE is used in rainwear, protective apparel, and liquids and gas filters. PTFE can also be formed into fibers which are used in pump packing seals and bag house filters for industries with corrosive exhausts.[101]
Other fluoropolymers tend to have similar properties to PTFE—high chemical resistance and good dielectric properties—which leads to use in the chemical process industry and electrical insulation. They are easier to work with (to form into complex shapes), but are more expensive than PTFE and have lower thermal stability. Films from two different fluoropolymers serve as glass-replacements in solar cells. Fluorinated ethylene propylene (FEP) is the second most produced fluoropolymer.[101][103][104]
Fluorinated ionomers (polymers that include charged fragments) are expensive, chemically resistant materials used as membranes in electrochemical cells. Nafion, developed in the 1960s, was the first example and remains the most prominent material in the class. The initial application was as a fuel cell material in spacecraft. Since then, the material has been transforming the 55 million tons per year chloralkali industry; it is replacing hazardous mercury-based cells with membrane cells. Recently, the fuel cell application has reemerged with efforts to get proton exchange membrane (PEM) fuel cells into automobiles.[105][106][107]
Fluoroelastomers are rubber-like substances that are composed of crosslinked mixtures of fluoropolymers. Viton is a prominent example. Chemical-resistant O-rings are the primary application.[101]
Fluorine gas
For countries with available data, about 17,000 metric tons of fluorine are produced per year. Fluorine is relatively inexpensive, costing about $5–8 per kilogram ($2–4 per pound) when sold as uranium hexafluoride or sulfur hexafluoride. Because of difficulties in storage and handling, the price of fluorine gas is much higher. Processes demanding large amounts of fluorine gas generally vertically integrate and produce the gas onsite.[108]

The largest application for elemental fluorine (up to 7,000 metric tons per year) is the preparation of uranium hexafluoride, which is used in the production of nuclear fuels. To obtain the compound, uranium dioxide is first treated with hydrofluoric acid, to produce uranium tetrafluoride. This compound is then further fluorinated by direct exposure to fluorine gas to make the hexafluoride.[108] Fluorine's monoisotopic natural occurrence makes it useful in uranium enrichment because uranium hexafluoride molecules will differ in mass only because of mass differences between uranium-235 and uranium-238. These mass differences are used to concentrate uranium-235 by diffusion or centrifugation.[55][108]
The second largest application for fluorine gas (about 6,000 metric tons per year) is in sulfur hexafluoride, which is used as a dielectric medium in high voltage transformers and circuit breakers. SF6 gas has a much higher dielectric strength than air and is extremely chemically inert. Switchgear using SF6 has no hazardous polychlorinated biphenyls (PCBs), in contrast to traditional oil-filled devices.[109]
Several compounds made from elemental fluorine serve the electronics industry. Rhenium and tungsten hexafluorides are used for chemical vapor deposition of thin metal films onto semiconductors. Tetrafluoromethane is used for plasma etching in semiconductor and flat panel display manufacturing.[110][111][112] Nitrogen trifluoride is used for cleaning equipment at display manufacturing plants.[55]
Some organic fluorides are prepared from elemental fluorine rather than from HF. However, because direct fluorination is usually too hard to control, intermediate strength fluorinators are made from fluorine gas. The halogen fluorides ClF3, BrF3, and IF5 provide gentler fluorination, with a series of strengths, and are easier to handle. Sulfur tetrafluoride is used for making fluorinated pharmaceuticals.[55]
Production of fluorine gas

Electrolytic synthesis
Today, elemental fluorine is produced using Moissan's process of electrolyzing potassium fluoride/hydrogen fluoride mixtures, but with an apparatus made of different materials: the steel container acts as the cathode, attracting H+ ions and releasing hydrogen gas; a carbon block (similar to that used in aluminum production) acts as the anode, attracting F- ions and releasing fluorine gas. The voltage is 8–12 volts.[55][113]
When combined, potassium fluoride and hydrogen fluoride spontaneously produce potassium bifluoride (KHF2), which increases the conductivity of the solution. A mixture with the approximate composition KF•2HF melts at 70 °C (158 °F) and is electrolyzed at 70–130 °C (160–265 °F). Because HF alone cannot be electrolyzed, the presence of some KF is critical, even though it is not consumed in the cell and remains in solution.[68][114][115]
Chemical routes
In 1986, when preparing for a conference to celebrate the 100th anniversary of the discovery of fluorine, Karl Christe discovered a purely chemical preparation of fluorine gas. However, he stated in his work that the basics were known 50 years before the actual reaction.[116] The main idea is that some metal fluoride anions do not have a neutral counterpart (or those are very unstable) and their acidifying would result in chemical oxidation, rather than formation of the expected molecules. Christe lists the following reactions as a possible way:[117]
- 2 KMnO4 + 2 KF + 10 HF + 3 H2O2 → 2 K2MnF6 + 8 H2O + 3 O2↑
- 2 K2MnF6 + 4 SbF5 → 4 KSbF6 + 2 MnF3 + F2↑
Handling
Pure fluorine gas may be stored in steel cylinders, where the inside surface is passivated, as long as the temperature is kept below 200 °C (400 °F). Above that temperature, nickel is required.[68][114] Regulator valves are made of nickel. Fluorine piping is generally made of nickel or Monel (nickel-copper alloy).[118] Care must be taken to passivate all surfaces frequently and to exclude any water or greases. In the laboratory, fluorine gas may be used in glass tubing provided the pressure is low and moisture is excluded,[118] but some sources recommend systems made of nickel, Monel, and PTFE.[119]

Biological aspects
Natural biochemistry

Fluoride is not considered an essential mineral element for mammals or humans.[121] Small amounts of fluoride may be beneficial for bone strength, but fluoride deficiency is an issue only in the formulation of artificial diets.[122]
Biologically synthesized organofluorines have been found in microorganisms and plants,[59] but not in animals.[123] The most common example is fluoroacetate, with an active poison molecule identical to commercial "1080". It is used as a defense against herbivores by at least 40 green plants in Australia, Brazil, and Africa.[124] Other biologically synthesized organofluorines include ω-fluoro fatty acids, fluoroacetone, and 2-fluorocitrate.[123]
The enzyme adenosyl-fluoride synthase, which makes the carbon–fluorine bond, was isolated in bacteria in 2002. The discovery was touted as possibly leading to biological routes for organofluorine synthesis.[125]
Dental care
Since the mid-20th century, it has been understood (from population studies) that fluoride reduces tooth decay, somehow. Initially, researchers hypothesized that fluoride helped by converting tooth enamel from the mineral hydroxyapatite to the more acid-resistant mineral fluorapatite. However, recent studies showed no difference in the frequency of caries (cavities) amongst teeth that were pre-fluoridated to different degrees. Current thinking is that fluoride prevent cavities primarily by helping teeth that are in the very early stages of tooth decay to regrow tooth enamel. In any case, it is only the fluoride that is directly present in the mouth (topical treatment) that prevents cavities. Fluoride ions that are swallowed do not benefit the teeth.[126]
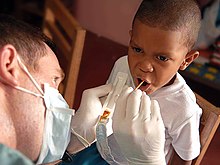
Water fluoridation is the controlled addition of fluoride to a public water supply to reduce tooth decay.[127] Its use began in the 1940s, following studies of children in a region where water was naturally fluoridated. It is now used for about two thirds of the U.S. population on public water systems[128] and for about 6% of people worldwide.[129] Although the best available evidence shows no association with adverse effects other than dental fluorosis, most of which is mild,[130] water fluoridation has been contentious for ethical, safety, and efficacy reasons.[129] Opposition to water fluoridation exists despite its support by public health organizations.[131] The benefits of water fluoridation have lessened recently—presumably because of the availability of fluoride in other forms—but are still measurable, particularly for low income groups.[132] Reviews of the scholarly literature in 2000 and 2007 showed significant reduction of cavities in children associated with water fluoridatation.[133]
Toothpaste may contain fluorine in the form of sodium fluoride, tin difluoride, or (most commonly) sodium monofluorophosphate. The first fluoride toothpaste was introduced in 1955 in the United States. Now, almost all toothpaste in developed countries is fluoridated.[132] Fluoride may also be applied to teeth in gels, foams, or varnishes. It is also contained in prescription and non-prescription mouthwashes.[134]
Pharmaceuticals
About 20% of modern pharmaceuticals contain fluorine, including commercially significant drugs in many different pharmaceutical classes.[135] One of these, the cholesterol-reducer atorvastatin (Lipitor), was the number one money-making drug for nearly a decade. The branded asthma medication Serevent (Advair), a top-ten revenue drug as of the mid-2000s, also contains a fluorinated molecule: fluticasone.[136][137]
Even a single atom of fluorine, added to a drug molecule, can greatly change its chemical properties and thus how it interacts with the body. For instance, because of the considerable stability of the carbon-fluorine bond, many drugs are fluorinated to delay their metabolism and elimination by the body. This allows longer times between doses.[138] Also, adding fluorine to organics increases their lipophilicity (ability to dissolve in fats) because the carbon–fluorine bond is even more hydrophobic than the carbon–hydrogen bond. This effect often increases a drug's bioavailability because of increased cell membrane penetration.[137]
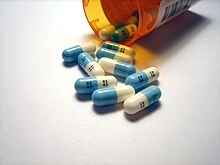
Many modern antidepressants are fluorinated molecules that selectively limit the body's binding of serotonin (low serotonin availability in brain cells is a cause of depression). Prior to the 1980s, traditional antidepressants, such as the tricyclics, altered not only serotonin uptake but also affected several other neurotransmitters. This non-selective interaction caused many side effects. One of the first drugs to alter only serotonin uptake—and be free of most side effects of previous drugs—was fluorine-containing Prozac (fluoxetine). It became the best-selling antidepressant and prompted the popular book Listening to Prozac. Some other selective serotonin reuptake inhibitor (SSRI) antidepressants that are fluorinated are Celexa (and its isomer Lexapro), Luvox, and Paxil.[139][140]
Quinolones are artificial compounds that are broad-spectrum antimicrobials. Most of the recent quinolone drugs (and those in current common use) are fluorinated to make the drugs more powerful. Prominent examples of such fluoroquinolones include ciprofloxacin (Cipro) and levofloxacin (Levaquin). The latter was the highest selling U.S. antibiotic in 2010.[141][142][143][144]
Fluorine also finds use in many steroidal drugs.[145] Fludrocortisone (Florinef) is a synthetic mineralocorticoid (a compound used to retain sodium and water and thus raise blood pressure).[146] Dexamethasone (Decadron) and triamcinolone (Kenalog) are potent synthetic glucocorticoids (anti-inflammatories).[146]
Several inhaled anesthetics, including the most common ones, are heavily fluorinated. The first fluorinated anesthetic, halothane, proved to be much safer (neither explosive nor flammable) and longer-lasting than those previously used. Modern fluorinated anesthetics are longer-lasting still and almost insoluble in blood, which accelerates the awakening. Examples include sevoflurane, desflurane, enflurane, and isoflurane, all fluorinated ethers.[147][148]
Agrichemicals and poisons

An estimated 30% of agrichemical compounds contain fluorine.[149] Most of them are poisons, but a few stimulate growth instead.
Sodium monofluoroacetate, the same acid as in vinegar but with one hydrogen changed out for fluorine, is a powerful poison. It was first synthesized in the late 19th century and was recognized as an insecticide in the early 20th century. Later, it became widely used to control mammalian pests (e.g. rats). The name "1080" refers to the catalogue number of the poison, which became its brand name. The compound is now banned in the European community and the United States[note 6] but is still used in Australia and some other countries. Fluoroacetate deprives cells of energy by replacing acetate in the Krebs cycle, halting a key part of cell metabolism.[124][150] Several insecticides contain sodium fluoride, which is much less toxic than fluoroacetate.[151]
Trifluralin was once a very important weedkiller; for example, in 1998 over a half of U.S. cotton field area was coated with the chemical.[152] However, its suspected carcinogenic properties caused some Northern European countries to ban it in 1993.[153] Although currently banned throughout the whole European Union, this decision has recently been contested.[154]
The currently used agrichemicals utilize another tactic: instead of directly poisoning organisms by interfering with metabolism, they transform the metabolic processes so that the organism itself produces poisonous compounds. For example, insects fed 29-fluorostigmasterol (a fluorinated steroid) produce fluoroacetates. Because vertebrates do not break down the fluorosteroid, the insecticide is more safe than 1080. If a fluorine is transferred to a body cell, it blocks metabolism at the position occupied.[155]
Use of fluorine agrichemicals may increase if the cost of synthesis decreases and if green chemistry accentuates the differences between these agrichemicals and other less-environmentally-friendly alternatives.[156]
Scanning

Compounds containing fluorine-18, a radioactive isotope that emits positrons, are often used in positron emission tomography (PET) scanning, because the isotope's half-life of about 110 minutes is long by positron-emitter standards. One such radiopharmaceutical is 2-deoxy-2-(18F)fluoro-D-glucose (generically referred to as fludeoxyglucose), commonly abbreviated as 18F-FDG, or simply FDG.[157] In PET imaging, FDG can be used for assessing glucose metabolism in the brain and for imaging cancer tumors. After injection into the blood, FDG is taken up by "FDG-avid" tissues with a high need for glucose, such as the brain and most types of malignant tumors.[158] Tomography, often assisted by a computer to form a PET/CT (CT stands for "computer tomography") machine, can then be used to diagnose or monitor treatment of cancers; especially Hodgkin's lymphoma, lung cancer, and breast cancer.[159]
Natural fluorine is monoisotopic, consisting solely of fluorine-19. Fluorine compounds are highly amenable to nuclear magnetic resonance (NMR), because fluorine-19 has a nuclear spin of ½, a high nuclear magnetic moment, and a high magnetogyric ratio. Fluorine compounds typically have a fast NMR relaxation, which enables the use of fast averaging to obtain a signal-to-noise ratio similar to hydrogen-1 NMR spectra.[160] Fluorine-19 is commonly used in NMR study of metabolism, protein structures and conformational changes.[161] In addition, inert fluorinated gases have the potential to be a cheap and efficient tool for imaging lung ventilation.[162]
Hazards
Fluorine gas
Elemental fluorine is highly toxic. Above a concentration of 25 ppm, fluorine causes significant irritation while attacking the eyes, respiratory tract, lungs, liver and kidneys. At a concentration of 100 ppm, human eyes and noses are seriously damaged.[163]

Hydrofluoric acid
Hydrogen fluoride is a toxic gas. Its water solution, hydrofluoric acid, is a contact poison and must be handled with extreme care, far beyond that accorded to other mineral acids. From 1984-1994, at least 9 U.S. workers died from accidents with HF.

Owing to its lesser chemical dissociation in water (remaining a neutral molecule), hydrogen fluoride penetrates tissue more quickly than typical acids. Poisoning can occur readily through the skin or eyes or when inhaled or swallowed. Once in the blood, hydrogen fluoride reacts with calcium and magnesium, resulting in electrolyte imbalance (potentially hypocalcemia). The consequent cardiac arrhythmia may be fatal.[165] Formation of insoluble calcium fluoride also causes strong pain.[166] Burns with areas larger than 160 cm2 (25 in2), about the size of a man's hand, can cause serious systemic toxicity.[167]
Symptoms of exposure to hydrofluoric acid may not be immediately evident, with 8-hour delay for 50% HF and up to 24-hour for lower concentrations. Hydrogen fluoride interferes with nerve function, meaning that burns may not initially be painful. Accidental exposures can go unnoticed, delaying treatment and increasing injury.
If the burn has been initially noticed, then HF should be washed off with a forceful stream of water for ten to fifteen minutes, to prevent its further penetration into the body. Clothing used by the person burned may also exhibit danger.[168] Hydrofluoric acid exposure is often treated with calcium gluconate, a source of Ca2+ that binds with the fluoride ions. Skin burns can be treated with a water wash and 2.5% calcium gluconate gel[169][170] or special rinsing solutions.[171]
Because HF is absorbed, medical treatment is necessary. Calcium gluconate may be injected or administered by IV. Use of calcium chloride is contra-indicated and may lead to severe complications. Sometimes surgical excision of tissue or amputation may be required.[167][172]
Fluoride ion

Soluble fluorides are moderately toxic. For sodium fluoride, the lethal dose for adults is 5–10 g, which is equivalent to 32–64 mg of elemental fluoride per kilogram of body weight.[173] The dose that may lead to adverse health effects is about one fifth the lethal dose.[174] Chronic excess fluoride consumption can lead to skeletal fluorosis, a disease of the bones that affects millions in Asia and Africa.[174][175]
The fluoride ion is readily absorbed by the stomach and intestines. Ingested fluoride forms hydrofluoric acid in the stomach. In this form, fluoride crosses cell membranes and then binds with calcium and interferes with various enzymes.
Fluoride is excreted through urine. Fluoride exposure limits are based on urine testing which has determined the human body's capacity for ridding itself of fluoride.[174][176]
Historically, most cases of fluoride poisoning have been caused by accidental ingestion of insecticides containing inorganic fluoride,[177] Currently, most calls to poison control centers for possible fluoride poisoning come from the ingestion of fluoride-containing toothpaste.[174] Malfunction of water fluoridation equipment has occurred several times, including an Alaskan incident which sickened nearly 300 people and killed one.[178]
Environmental concerns
Atmosphere
Because they deplete the ozone layer, Chlorofluorocarbons (CFCs) and bromofluorocarbons (BFCs) have been strictly regulated via a series of international agreements called the Montreal Protocol. It is the chlorine and bromine from these molecules that cause harm, not fluorine. Because of the inherent stability of these fully halogenated molecules (which makes them so nonflammable and useful), they are able to reach the upper reaches of the atmosphere before decomposing. At those altitudes, they release chlorine and bromine which then attack the ozone molecules.[179] Predictions are that generations will be required, even after the CFC ban, for these molecules to leave the atmosphere and for the ozone layer to recover. Early indications are that the CFC ban is working—ozone depletion has stopped and recovery is underway.[180][181]

Hydrochlorofluorocarbons (HCFCs) are current replacements for CFCs, with about one tenth the ozone damaging potential (ODP).[182] They were originally scheduled for elimination by 2030 in developed nations (2040 in undeveloped). In 2003, the U.S. Environmental Protection Agency prohibited production of one HCFC and capped the production of the two others.[183] In 2007, a new treaty was signed by almost all nations to move HCFC phaseout up to 2020 because HFCs, which have no chlorine and thus zero ODP, are available.[184]
Fluorocarbon gases of all sorts (CFCs, HFCs, etc.) are greenhouse gases about 4,000 to 10,000 times as potent as carbon dioxide. Sulfur hexafluoride exhibits an even stronger effect, about 20,000 times the global warming potential of carbon dioxide.[185][186]
Biopersistance
Because of the strength of the carbon–fluorine bond, organofluorines endure in the environment. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), used in waterproofing sprays, are persistent global contaminants. Trace quantities of these substances have been detected worldwide, from polar bears in the Arctic to the global human population. PFOA has been detected in breast milk and the blood of newborns. One study indicates that PFOS levels in wildlife are starting to go down because of reducing production.[187][188]
In the body, PFOA binds to a protein, serum albumin. PFOA's tissue distribution in humans is unknown, but studies in rats suggest it is likely to be present primarily in the liver, kidney, and blood. PFOA is not metabolized by the body, but is excreted by the kidneys.[187][188]
The potential health effects of PFOA are unclear. Unlike chlorinated hydrocarbons, PFOA is not lipophilic (stored in fat), nor is it genotoxic (damaging genes). While both PFOA and PFOS cause cancer in high quantities in animals, studies on exposed humans have not been able to prove an impact at current exposures. Bottlenose dolphins have some of the highest PFOS concentrations of any wildlife studied; one study suggests an impact on their immune systems.[187][188]
Because biological systems do not metabolize fluorinated molecules easily, fluorinated pharmaceuticals (often antibiotics and antidepressants) are among the major fluorinated organics found in treated city sewage and wastewater.[189] Fluorine-containing agrichemicals are measurable in farmland runoff and nearby rivers.[190]
Compounds
Fluorine's common oxidation state is −1.[note 7] With other atoms, fluorine forms either ionic bonds or polar covalent bonds. Covalent bonds involving fluorine atoms are almost always single bonds.[note 8][193] Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding.[194] Fluorine has a rich chemistry including compounds formed with hydrogen, metals, main group nonmetals, and even noble gases, as well as a diverse set of organic compounds.[note 9][195]
Hydrogen fluoride
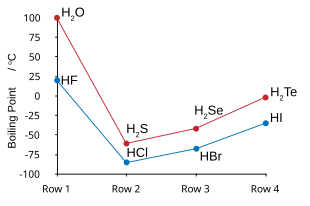
Fluorine combines with hydrogen to make a compound called hydrogen fluoride (HF) or, in the context of water solutions, hydrofluoric acid. HF molecules cluster together weakly via hydrogen bonds. Because of this, hydrogen fluoride behaves more like water than like HCl.[196][197][198] Hydrogen fluoride boils at a much higher temperature than the heavier hydrogen halides. HF is also fully miscible with water (dissolves in any proportion), unlike HCl, HBr, or HI.[199]
In water solution, hydrogen fluoride is a weak acid, having lower dissociation constant compared to the other hydrohalic acids.[200][note 10] Hydrofluoric acid is non-ideal: instead of having a constant acid dissociation constant (PKa), HF's inherent acidity increases at higher concentrations through a phenomenon called homoconjugation.[202][203] Although hydrofluoric acid is weak, it is very corrosive, even attacking glass.[202]
Metal fluorides
Metal fluorides share some similarities with other metal halides but are more ionic, often more like oxides in their bonding and crystal structures.[204] However, there is a trend of the binary metal fluorides becoming more covalently-bonded and volatile as the amount of fluorination increases. For example, sodium monofluoride boils at 1704 °C (3099 °F),[205] while rhenium heptafluoride has a boiling point below water's.[206] Similar to fluorine itself, the higher metal fluorides are very reactive. Platinum hexafluoride was the first compound to oxidize molecular oxygen[207] and xenon.[208] The solubility of fluorides varies greatly but tends to decrease with more fluorine.[209]
The alkali metals form monofluorides that, like the alkali metal chlorides, are very ionic and soluble. They have the same atomic arrangement—the rock salt crystal structure—as sodium chloride.[210][94] Other metal monofluorides show clear difference versus their chlorides. For example, thallium[211] and silver[73] monofluorides are soluble, while the chlorides are not.
The difluorides of the alkaline earths are also very ionic, but are generally very insoluble. In contrast, the alkali earth dichlorides are fairly soluble.[73] Beryllium difluoride differs from those of the other alkaline earths: the bonding has some covalent character, the structures are similar to SiO2 (quartz), and the compound is water soluble.[212]
Many metals form trifluorides, such as iron, bismuth, the rare earths, some actinides, and the metals in the aluminium and scandium columns of the periodic table.[citation needed] Gold forms a trifluoride that has a polymer structure, with helical chains.[213] In contrast, gold trichloride is molecular.[213]
The tetrafluorides show a mixture of ionic and covalent bonding. Zirconium[214] and hafnium,[215] along with several actinides,[216] form tetrafluorides with an ionic structure based on 8-coordinate metal cations.[217] Melting points are around 1000 °C (1850 °F).[note 11] On the other hand, the tetrafluorides of titanium,[220], vanadium,[221] niobium[222] are polymeric. The first melts at 284 °C (543 °F);[223] the latter two decompose at around 350 °C (650 °F).[224]
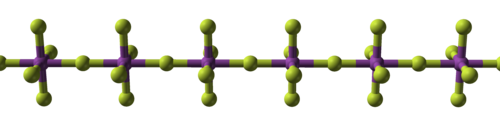
The pentafluorides are even more covalently bonded, forming low dimensionality polymers or oligomeric molecules.[225] Bismuth and uranium pentafluorides share the same structure: straight chains of connected octahedra.[226][227][228] Gold's pentafluoride is a dimer (doubled molecule), Au2F10.[225] Niobium and tantalum pentafluorides are tetrameric molecules (fourfold clusters).[225] The former melts at 80 °C (176 °F),[229] the latter at 95 °C (203 °F).[230]
There are thirteen metal hexafluorides.[note 12] All are octahedral molecules.[231] At room temperature tungsten hexafluoride is a gas.[219] Molybdenum hexafluoride and rhenium hexafluoride are liquids.[232] The rest are volatile solids.[citation needed]
The only definite metal heptafluoride, rhenium heptafluoride, is a low-melting molecular solid. Its structure is pentagonal bipyramidal.[204][note 13]
| Progression of structure type with metal charge in the metal fluorides | ||

|
|

|
| Sodium fluoride, ionic | Bismuth pentafluoride, polymeric | Rhenium heptafluoride, molecular |
Nonmetal fluorides
The binary fluorides of the main group nonmetals and metalloids are generally volatile, covalently bonded molecules. The fluorides of those elements in the second row of the period table (carbon, nitrogen, and oxygen) follow the octet rule. Boron is an exception—its fluoride has an incomplete octet (less bonds than normal). Nonmetals from the third row of the period table and below form fluorides which are hypervalent (more bonds than normal).[234] The discussion is organized by periodic table column.
Boron trifluoride is a planar molecule. It is a weak Lewis acid and readily accepts a Lewis base, forming adducts (combinations). The lone pair of a Lewis base, such as ammonia, allows the boron to complete its octet. With another fluoride ion (F− is a Lewis base), BF3 reacts to form the relatively unreactive BF−
4 anion.[235]
Silicon tetrafluoride and germanium tetrafluoride adopt a tetrahedral structure[236] and are Lewis acids.[237][238] Germanium forms a difluoride, less stable than its tetrafluoride.[239]
The pnictogens (nitrogen's column) form trifluorides that are weak Lewis bases and are more reactive as the pnictogen becomes heavier.[240] In comparison, the pnictogen pentafluorides are much more reactive,[241][242] with antimony pentafluoride being the strongest Lewis acid of all charge-neutral compounds.[242] PF5 and AsF5 are trigonal bipramidal in all phases, as is gaseous SbF5. Liquid and solid SbF5 have more complicated structures with octahedral antimony and bridging fluorines.[226] Nitrogen is different than other pnictogens as it forms a trifluoride that is stable against hydrolysis and is not a Lewis base.[240] Also, nitrogen pentafluoride does not exist.[243]
The chalcogens (oxygen's column) form a variety of fluorides. Unstable difluorides are known for oxygen (the only compound where oxygen is at oxidation state +2) as well as sulfur and selenium.[citation needed] Sulfur and selenium tetrafluorides are molecular while TeF4 is a polymer. The tetrafluorides are thermally unstable and hydrolyze.[244] The hexafluorides are the result of direct fluorination of sulfur, selenium, and tellurium, while other hexahalides of the elements do not even exist. SF6 is extremely inert, while SeF6 and TeF6 show increasingly higher reactivity.[244]
The well-characterized heavier halogens (chlorine, bromine, and iodine) all form mono-, tri-, and pentafluorides: XF, XF3, and XF5. Of the neutral +7 species, only iodine heptafluoride is known.[245] The corresponding cations, ClF+
6 and BrF+
6, are known and are extremely strong oxidizers.[246] For the radioactive astatine only the non-volatile astatine monofluoride has been studied,[247] but its existence is debated.[248] Many of the halogen fluorides are powerful fluorinators (sources of fluorine atoms). ClF3 readily fluorinates asbestos and refractory oxides, and industrial use requires precautions similar to those for fluorine gas.[249][250]
| Notable nonmetal fluorides | ||||

|

|
|

| |
| Boron trifluoride (BF3) | Silicon tetrafluoride (SiF4) | Antimony pentafluoride (SbF5) | Sulfur hexafluoride (SF6) | Chlorine trifluoride (ClF3) |
Noble gas compounds

The noble gases are generally non-reactive because they have complete electronic shells. Until the 1960s, no chemical bond with a noble gas was known. In 1962, Neil Bartlett reported the first chemical compound of xenon, xenon hexafluoroplatinate.[251] Later in 1962, xenon was reported to react directly with fluorine to form the di- and tetrafluorides. Since then, xenon hexafluoride, various oxyfluorides, and their derivatives have been prepared.[252][253]
Krypton, xenon's lighter homolog, also forms difluoride and a few more complicated fluorine-containing compounds.[254] The possibility of a tetrafluoride[255] or a hexafluoride has been debated.[256]
Radon, xenon's heavier homolog has been shown to readily react with fluorine to form a solid compound. It is generally thought to be radon difluoride, but the exact formula is not known.[247] If radon were not so radioactive and difficult to collect, its chemistry might be at least as extensive as xenon's.[257]
The lightest noble gases do not form stable binary fluorides. Argon, however, reacts in extreme conditions with hydrogen fluoride to form argon fluorohydride.[258] Helium and neon do not form any stable chemical compounds at all, but helium fluorohydride has been observed and it is unstable in gas phase, but it may be stable under enormous pressure.[259] Neon is considered less reactive than helium and is not expected to form a stable compound capable of synthesis.[260]
Organic compounds
The carbon–fluorine chemical bond is the strongest bond in organic chemistry.[261] This C–F bond strength, along with the low polarizability of molecules containing C-F, makes organofluorines very stable.[262] Fluorinated organics have similar sizes to corresponding unfluorinated molecules because of the small van der Waals radius of fluorine.[262]
The range of organofluorine compounds is diverse, reflecting the inherent complexity of organic chemistry. A vast number of small molecules can exist with varying amounts of fluorine substitution, as well as many polymers. Research in particular areas is driven by the commercial value of applications.[82]
Small molecules
Partially fluorinated alkanes are hydrofluorocarbons (HFCs). Monofluoroalkanes (alkanes with one hydrogen replaced with fluorine) have properties similar to unfluorinated alkanes. They are soluble in many nonpolar solvents and have some chemical and thermal instability. As more fluorines are substituted for hydrogens, the properties change. Solubility in hydrocarbons decreases and stability increases. Also, melting and boiling points decrease, while density goes up.[263]
When all hydrogens are replaced with fluorines to make perfluorocarbons (the "per" means maximum), a great difference is revealed. Such compounds are extremely stable, and only sodium in liquid ammonia attacks them at standard conditions. They are also very insoluble, with few organic solvents capable of dissolving them.[263]

|

|
| Perfluorodecalin (trans isomer shown) is a liquid at room temperature. It boils at 142 °C (288 °F).[264] Its hydrocarbon analog, decalin, has a higher boiling point: 187 °C (369 °F).[265] | Perfluorocarbon density demonstration: upper layer is water with blue dye; lower layer is perfluoroheptane. The goldfish cannot swim down. The crab floats at the boundary. (Animals were rescued after the photo.) |
Perfluorinated compounds, hydrocarbons that are fully fluorinated except for one functional group, have a combination of properties.[266] They exhibit many perfluorocarbon properties (e.g. inertness, stability, non-wetting by water and oils, slipperiness).[267] However, the functional group is available for reactions or may make the molecule behave as a surfactant.[citation needed] If a perfluorinated compound has a fluorinated tail but also a few non-fluorinated carbons (typically two) near the functional group, it is called a fluorotelomer. Industrially, such compounds are treated as perfluorinated.[267]
Fluorinating an organic acid raises its acidity. For example, acetic acid and its mono-, di-, and trifluoroacetic derivatives show a trend of lowering pKa: 4.74, 2.66, 1.24, 0.23 (thus increasing acidity).[268] This happens because of fluorine's inductive effect (it stabilizes anions by spreading negative charge, allowing the hydrogen to be released).[269] Similarly, the acidity is greatly increased for other perfluorocarboxyl acids, as well as the amines (which are not acids but become less basic if fluorinated).[262] The perfluoroalkanesulfonic acids are also very notable for their acidity. Trifluoromethanesulfonic acid, is comparable to strong mineral acids.[270][270]
Polymers

As with small molecules, replacing hydrogen with fluorine in a polymer increases chemical stability and reduces flammability. Melting points are typically much higher than in the corresponding hydrocarbon polymers.[271]
The simplest fluoroplastic is polytetrafluoroethylene (PTFE, DuPont brand Teflon), which is a simple linear chain polymer with the repeating structural unit: –CF2–. It has no hydrogens and is the perfluoro analog of polyethylene (structural unit: –CH2–). PTFE has high chemical and thermal stability, as expected for a perfluorocarbon, much stronger than polyethylene. However, its very high melting point makes it difficult to fashion into parts.[272]
Various PTFE derivatives have lower maximum usage temperatures but have the benefit of being more melt-processable. FEP (fluorinated ethylene propylene is structurally similar to PTFE but has some fluorines replaced with the –CF3 groups). PFA (perfluoroalkoxy has some fluorines replaced with –OCF3).[272]
There are other fluoroplastics that are not perfluorinated (contain some C-H). Polyvinylidene fluoride (PVDF, structural unit: –CF2CH2–), is an analog of PTFE with half the fluorines. PVF (polyvinyl fluoride, structural unit: –CH2CHF–) contains one one-fourth the fluorines of PTFE. Despite this, it still has many properties of more fluorinated compounds.[273]
Nafion is a structurally complicated polymer. It has a PTFE-like backbone, but also contains side chains of perfluoro ether that end in sulfonic acid (–SO2OH) groups. Because of the polar side chains, it is very hydroscopic. With the addition of water and cations such as Na+, Nafion can be made into an ionic conductor.[274][275]
Notes
- ^ Exact comparison of the sizes of fluorine, oxygen and neon atoms is not possible because of conflicting estimates from different sources.
- ^ See the differing accounts of Partington[65] and Weeks.[66]
- ^ Since 2012, the symbol Fl has been used for flerovium (element 114), a man-made transuranic element.[74]
- ^ The injured included Davy, Joseph Louis Gay-Lussac, Louis Jacques Thénard, and Irish chemists Thomas and George Knox. Belgian chemist Paulin Louyet and French chemist Jerome Nickles died. Moissan also experienced HF poisoning.[66][75]
- ^ Moissan's Nobel also honored his invention of the electric arc furnace
- ^ A minor allowed use is in collars of sheep and cattle to kill predators like coyotes.
- ^ It differs from this value in elemental fluorine, where the atoms are bonded to each other and thus at oxidation state 0. The very unstable anions F2- and F3- with intermediate oxidation states exist at very low temperatues, decomposing at around 40 K.[191] Also, the F4+ cation and a few related species have been predicted to be stable.[192]
- ^ Boron monofluoride and nitrogen monofluoride are metastable compounds with higher than single bonded fluorine. Boron monofluoride is stable as a ligand in some metal complexes.
- ^ In this article, metalloids are lumped with the definite main group nonmetals because the fluoride chemistry is similar. The noble gases are treated separately. Hydrogen is discussed in the Hydrogen fluoride section; carbon in the Organic compounds section. P-block period 7 elements have not been studied and thus are not included. This is illustrated by the image to the right: the dark gray elements are metals, the green ones are nonmetals, the light blue ones are the noble gases, the purple one is hydrogen, the yellow one is carbon, and the light gray elements have unknown properties.
- ^ For more detail, see explanation by Jim Clark.[201]
- ^ ZrF4 melts at 932 °C (1764 °F).[218] HfF4 sublimes at 968 °C.[citation needed] UF4 melts at 1036 °C (1897 °F).[219]
- ^ IrF6, MoF6, OsF6, NpF6, PoF6, PuF6, PtF6, ReF6, RhF6, RuF6, TcF6, UF6, and WF6
- ^ In 1966, osmium heptafluoride was reported from daunting synthesis conditions (400 atmospheres of fluorine gas at 600 °C). The product was not structurally characterized. In 2006, the synthesis was re-attempted but only OsF6 was observed.[233]
Citations
- ^ "Standard Atomic Weights: Fluorine". CIAAW. 2021.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c d Jaccaud et al. 2000, p. 381.
- ^ a b c Haynes 2011, p. 4.121.
- ^ a b Jaccaud et al. 2000, p. 382.
- ^ a b c Compressed Gas Association 1999, p. 365.
- ^ "Triple Point | The Elements Handbook at KnowledgeDoor". KnowledgeDoor.
- ^ Himmel, D.; Riedel, S. (2007). "After 20 Years, Theoretical Evidence That 'AuF7' Is Actually AuF5·F2". Inorganic Chemistry. 46 (13). 5338–5342. doi:10.1021/ic700431s.
- ^ Dean 1999, p. 4.6.
- ^ Dean 1999, p. 4.35.
- ^ Matsui 2006, p. 257.
- ^ Yaws & Braker 2001, p. 385.
- ^ a b Mackay, Mackay & Henderson 2002, p. 72.
- ^ Cheng et al. 1999.
- ^ Chisté & Bé 2011.
- ^ National Nuclear Data Center. "NuDat 2.1 database – Fluorine-19". Brookhaven National Laboratory. Retrieved 31 September 2005.
{{cite web}}: Check date values in:|accessdate=(help) - ^ a b National Nuclear Data Center. "NuDat 2.1 database". Brookhaven National Laboratory. Retrieved 31 September 2005.
{{cite web}}: Check date values in:|accessdate=(help) - ^ a b Jaccaud et al. 2005, p. 1.
- ^ Cordero, Beatriz; Gómez, Verónica; Platero-Prats, Ana E.; Revés, Marc; Echeverría, Jorge; Cremades, Eduard; Barragán, Flavia; Alvarez, Santiago (2008). "Covalent radii revisited". Dalton Translations (21): 2832–2838. doi:10.1039/b801115j.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1002/chem.200901472, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1002/chem.200901472instead. - ^ Moore, John W.; Stanitski, Conrad L.; Jurs, Peter C. (2010). Principles of chemistry: The molecular Science. Cengage Learning. p. 156. ISBN 978-0-495-39079-4. Retrieved 7 May 2011.
- ^ Dean 1999, p. 564.
- ^ Lide 2004, pp. 10-137–10-138.
- ^ Macomber, Roger S. (1996). Organic chemistry. Vol. 1. University Science Books. p. 230. ISBN 0-935702-90-3. Retrieved 26 July 2011.
- ^ a b Greenwood & Earnshaw 1998, p. 804.
- ^ Cheng, H.; Fowler, D. E.; Henderson, P. B.; Hobbs, J. P.; Pascaloni, M. R. (1999). "On the magnetic susceptibility of fluorine". Journal of Physical Chemistry A. 103 (15): 2861–2866. doi:10.1021/jp9844720.
- ^ a b Jaccaud et al. 2005, p. 2.
- ^ Burdon, J.; Emson, B.; Edwards, A. J. (1987). "Is fluorine really yellow?". Journal of Fluorine Chemistry. 34 (3–4): 471–474. doi:10.1016/S0022-1139(00)85188-X.
- ^ Lide 2004, p. 4-12.
- ^ a b Dean 1999, p. 523.
- ^ Cite error: The named reference
phaseswas invoked but never defined (see the help page). - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1063/1.1711946, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1063/1.1711946instead. - ^ Nelson, Eugene W. (1947). "'Bad man' of the elements". Popular Mechanics. 88 (2): 106–108, 260.
- ^ Lidin, Molochko & Andreeva 2000, pp. 442–455.
- ^ Greenwood & Earnshaw 1998, p. 844.
- ^ Jaccaud et al. 2005, p. 3.
- ^ Pitzer, Kenneth S. (1975). "Fluorides of radon and element 118". Journal of the Chemical Society: Chemical communications (18): 760b–761. doi:10.1039/C3975000760B.
- ^ Khriachtchev, Leonid; Pettersson, Mika; Runeberg, Nino; Lundell, Jan; Räsänen, Markku (2000). "A stable argon compound". Nature. 406 (6798): 874–876. doi:10.1038/35022551. PMID 10972285. Retrieved 29 April 2011.
- ^ Lidin, Molochko & Andreeva 2000, p. 252.
- ^ http://pubs.acs.org/doi/abs/10.1021/j100892a049?journalCode=jpchax
- ^ http://www.sciencedirect.com/science/article/pii/S0022113906002983
- ^ http://www.academia.edu/2508809/Direct_fluorination_of_carbon_monoxide_in_microreactors
- ^ Lagow, James; The reactions of elemental fluorine; a new approach to fluorine chemistry (Ph.D. thesis); 1970
- ^ http://www.cdc.gov/niosh/docs/81-123/pdfs/0107-rev.pdf
- ^ a b JW Mellor; A comprehensive treatise on theoretical and inorganic chemistry (1927); pp 9-15
- ^ Cameron, A. G. W. (1973). "Abundance of the elements in the Solar System" (PDF). Space Science Review. 15: 121–146. Bibcode:1973SSRv...15..121C. doi:10.1007/BF00172440.
- ^ a b Croswell, Ken (2003). "Fluorine: An element–ary mystery". Sky and Telescope. Retrieved 3 May 2011.
- ^ a b Renda, A.; Fenner, Y.; Gibson, B.K.; Karakas, A.I.; Lattanzio, J.C.; Campbell, S.; Chieffi, A.; Cunha, K.; Smith, V.V. (2004). "On the origin of fluorine in the Milky Way" (PDF). Monthly Notices of the Royal Astronomical Society. 354 (2): 575–580. arXiv:astro-ph/0410580. Bibcode:2004MNRAS.354..575R. doi:10.1111/j.1365-2966.2004.08215.x.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Neufeld, David; Bergin, Edwin; Gerin, Maryvonne (2010). "Tracing the Milky Way's hidden reservoirs of gas". European Space Agency.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Snow, T. P., Jr.; York, D. G. (1981). "The detection of interstellar fluorine in the line of sight toward Delta Scorpii". Astrophysical Journal. 247: L39. Bibcode:1981ApJ...247L..39S. doi:10.1086/183585.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Snow, Theodore, P. (2007). "The abundance of interstellar fluorine and its implications". The Astrophysical Journal. 655 (1): 285–298. arXiv:astro-ph/0611066. Bibcode:2007ApJ...655..285S. doi:10.1086/510187.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Zhang, Y.; Liu, X.-W. (2005). "Fluorine abundances in planetary nebulae". The Astrophysical Journal. 631 (1): L61–L63. arXiv:astro-ph/0508339. Bibcode:2005ApJ...631L..61Z. doi:10.1086/497113.
- ^ Jaccaud et al. 2005, p. 4.
- ^ a b c d e Greenwood & Earnshaw 1998, p. 795.
- ^ a b c d e f g h i j k l m n o Villalba, Gara; Ayres, Robert U.; Schroder, Hans (2008). "Accounting for fluorine: production, use, and loss". Journal of Industrial Ecology. 11: 85–101. doi:10.1162/jiec.2007.1075.
- ^ Kelly, T.D. "Historical fluorspar statistics" (PDF). United States Geological Service. Retrieved 25 January 2012.
{{cite web}}: Cite has empty unknown parameter:|1=(help) - ^ Lusty, P. A. J. (2008). "The need for indigenous fluorspar production in England". British Geological Survey. Retrieved 25 January 2012.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Norwood, Charles J. (1907). "Fluorspar and its Occurrence". Kentucky geological survey Bulletin 9: Fluorspar deposits of Kentucky. Globe Printing Company. p. 52.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Gribble, Gordon W. (2002). "Naturally occurring organofluorines". The Handbook of Environmental Chemistry. The Handbook of Environmental Chemistry. 3N: 121–136. doi:10.1007/10721878_5. ISBN 3-540-42064-9.
- ^ Template:De icon Schmedt, Jörn; Mangst, Martin; Kraus, Florian (2012). "Elementares Fluor F2 in der Natur – In-situ-Nachweis und Quantifizierung durch NMR-Spektroskopie". Angewandte Chemie. 124 (31): 7968–7971. doi:10.1002/ange.201203515.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Greenwood & Earnshaw 1998, p. 790.
- ^ Senning, Alexander (2007). Elsevier's dictionary of chemoetymology: The whies and whences of chemical nomenclature and terminology. Elsevier. p. 149. ISBN 978-0-444-52239-9. Retrieved 7 May 2011.
- ^ Greenwood & Earnshaw 1998, p. 109.
- ^ Agricola, Georgius (1912). De Re Metallica. trans. Hoover, Herbert Clark; Hoover, Henry Lou. pp. preface, 380–381 (3, 416–417 in linked viewer).
- ^ Partington, J. R. (1923). "The early history of hydrofluoric acid". Memoirs and Proceedings of the Manchester Literary and Philosophical Society. 67 (6): 73–87.
- ^ a b c d e Weeks, Mary Elvira (1932). "The discovery of the elements. XVII. The halogen family". Journal of Chemical Education. 9 (11): 1915–1939. Bibcode:1932JChEd...9.1915W. doi:10.1021/ed009p1915.
- ^ Template:Fr icon Marggraf, Andreas Sigismun (1770). "Observation concernant une volatilisation remarquable d'une partie de l'espece de pierre, à laquelle on donne les noms de flosse, flüsse, flus-spaht, et aussi celui d'hesperos; laquelle volatilisation a été effectuée au moyen des acides". Mémoires de l'Académie royale des sciences et belles-lettres: 3–11.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ a b c d e f g Kirsch, Peer (2004). "Fluorine". Modern fluoroorganic chemistry: Synthesis, reactivity, applications. pp. 3–10. ISBN 978-3-527-30691-6. Retrieved 7 May 2011.
- ^ Template:Sv icon Scheele, Carl Wilhelm (1771). "Undersŏkning om fluss-spat och dess syra". Kungliga Svenska Vetenskapsademiens Handlingar (Proceedings of the Royal Swedish Academy of Science). 32: 129–138.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ Template:Fr icon Ampere, André-Marie (1816). "Suite d'une classification naturelle pour les corps simples". Annales de chimie et de physique. 2: 1–5. Retrieved 7 May 2011.
- ^ "09 Fluorine". elements.vanderkrogt.net. Retrieved 24 January 2012.
- ^ φθόριος. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project
- ^ a b c Storer, Frank Humphreys (1864). First outlines of a dictionary of solubilities of chemical substances. Cambridge University Press. pp. 278–280. ISBN 978-1-176-62256-2.
- ^ "Element 114 is named flerovium and element 116 is named livermorium". IUPAC. 30 May 2012. Retrieved 1 June 2012.
- ^ a b c d e "Fluorine, an obsession with a tragic past"; Toon, Richard; Education in Chemistry, September 2011, 148-151; http://www.rsc.org/images/EiC_Sept2011_fluorine_tcm18-207109.pdf
- ^ a b Asimov, Isaac (1966). The noble gases. Basic Books. p. 162. ISBN 978-0-465-05129-8.
- ^ Greenwood & Earnshaw 1998, pp. 789–791.
- ^ Template:Fr icon Moissan, Henry (1886). "Action d'un courant électrique sur l'acide fluorhydrique anhydre". Comptes rendus hebdomadaires des séances de l'Académie des sciences. 102: 1543–1544. Retrieved 7 May 2011.
- ^ "The Nobel Prize in chemistry 1906". nobelprize.org. Retrieved 7 July 2009.
- ^ Greenwood & Earnshaw 1998, p. 791.
- ^ James, Laylin K. (1993). Nobel laureates in chemistry, 1901–1992. Chemical Heritage Foundation. p. 35. ISBN 978-0-8412-2690-6.
- ^ a b c Okazoe, Takashi (2009). "Overview on the history of organofluorine chemistry from the viewpoint of material industry" (PDF). Proceedings of the Japan Academy, Series B. 85 (8): 276–289. Bibcode:2009PJAB...85..276O. doi:10.2183/pjab.85.276.
- ^ Hounshell & Smith 1988, pp. 156–157.
- ^ "Freon". dupont.com. Retrieved 10 November 2012.
- ^ Meyer, Eugene (1977). Chemistry of hazardous materials (1st ed.). p. 111. ISBN 9780131292390.
{{cite book}}:|journal=ignored (help) - ^ U.S. Environmental Protection Agency (2008). "Ozone depletion glossary". Retrieved 3 September 2008.
- ^ U.S. Environmental Protection Agency (2006). "Brief questions and answers on ozone depletion | Ozone layer protection". Retrieved 8 November 2011.
- ^ Transparency Market Research (2013). "Fluorochemicals market is expected to reach USD 21.5 billion globally by 2018: Transparency Market Research". Transparency Market Research Blog. Retrieved 3 June 2013.
- ^ "World Fluorochemicals Market, Freedonia". PR Newswire. 2012. Retrieved 25 May 2013.
- ^ Kogel; Trivedi, Nikhil C.; Barker, James M. (2006). Industrial minerals & rocks: Commodities, markets, and uses. Society for Mining, Metallurgy, and Exploration (U.S.). pp. 461–473. ISBN 978-0-87335-233-8.
- ^ Miller, M. Michael (2003). "Fluorspar". U.S. Geological Survey Minerals Yearbook (PDF). U.S. Geological Survey. pp. 27.1–27.12.
- ^ Energetics, Inc. (1997). Energy and Environmental Profile of the U.S. Aluminum Industry (PDF) (Report). pp. 41, 50.
- ^ Aigueperse et al. 2005, p. 33.
- ^ a b Aigueperse et al. 2005, pp. 25–27.
- ^ Willey, Ronald R. (2007). Practical equipment, materials, and processes for optical thin films. Willey Optical. p. 113. ISBN 9780615143972.
- ^ "Global fluorochemicals Market to exceed 2.6 million tons by 2015, according to a new report by Global Industry Analysts, Inc". Global Industry Analysts (via PRWeb). 2010. Retrieved 26 January 2012.
- ^ Renner, R. (2006). "The long and the short of perfluorinated replacements". Environmental Science & Technology. 40 (1): 12–3. Bibcode:2006EnST...40...12R. doi:10.1021/es062612a. PMID 16433328.
- ^ "Understanding the refrigerant "R" nomenclature". DuPoint. 2013. Retrieved 14 June 2013.
- ^ MarketsandMarkets (2013). "Fluoropolymers market is poised to grow at a CAGR of 6.5% & to reach $9,446.0 Million by 2016 - New report by MarketsandMarkets".
- ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1134/S1070363209030335, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1134/S1070363209030335instead. - ^ a b c d e Martin, John Wilson (2007). Concise encyclopedia of the structure of materials. Elsevier. pp. 187–194. ISBN 978-0-08-045127-5.
- ^ Nakagawa, Ulara (2011). "15 sights that make Tokyo so fascinating". CNN. Retrieved 31 December 2011.
- ^ Bhiwankar, Nikhil (2011). "Weathering the storm: Fluoropolymer films protect solar modules and ensure performance". altenergymag.com. Retrieved 31 December 2011.
- ^ DeBergalis, Michael (2004). "Fluoropolymer films in the photovoltaic industry" (PDF). Journal of Fluorine Chemistry. 125 (8): 1255–1257. doi:10.1016/j.jfluchem.2004.05.013.
- ^ Grot, Walter (2011). Fluorinated ionomers. Elsevier. pp. 1–10. ISBN 978-1-4377-4457-6.
- ^ Ramkumar, Jayshreee (2012). "Nafion Persulphonate Membrane: Unique Properties and Various Applications". In Banerjee, S (ed.). Functional materials: Preparation, processing and applications. Elsevier. pp. 549–578. ISBN 978-0-12-385142-0.
- ^ Burney, H.S. (1999). "Past, Present, and Future of the Chlor-Alkali Industry". Chlor-alkali and chlorate technology: R.B. MacMullin memorial symposium. The Electrochemical Society. pp. 105–126. ISBN 1-56677-244-3.
- ^ a b c Jaccaud et al. 2005, p. 12.
- ^ Aigueperse et al. 2005, p. 35.
- ^ Jaccaud et al. 2005, pp. 11–12.
- ^ El-Kareh, Badih (1994). "Fluorine –Based Plasmas". Fundamentals of semiconductor processing technology. p. 317. ISBN 978-0-7923-9534-8. Retrieved 7 May 2011.
- ^ Arana, Leonel R.; de Mas, Nuria; Schmidt, Aleksander J.; Franz, Martin A.; Jensen, Schmidt F.; Jensen, Klaus F. (2007). "Isotropic etching of silicon in fluorine gas for MEMS micromachining". Journal Micromechanical Microenergy. 17 (2): 384. Bibcode:2007JMiMi..17..384A. doi:10.1088/0960-1317/17/2/026.
- ^ Jaccaud et al. 2005, p. 6.
- ^ a b Jaccaud et al. 2005, pp. 4–5.
- ^ Greenwood & Earnshaw 1998, p. 796–797.
- ^ Kirsch, Peer (2004). Modern fluoroorganic chemistry: Synthesis, reactivity, applications. John Wiley & Sons. p. 7. ISBN 3-527-30691-9.
- ^ Christe, K. (1986). "Chemical synthesis of elemental fluorine". Inorganic Chemistry. 25 (21): 3721–3724. doi:10.1021/ic00241a001.
- ^ a b Jaccaud et al. 2005, pp. 10–11.
- ^ Shriver, Duward; Atkins, Peter (2010). Solutions manual for inorganic chemistry. Macmillan. p. 427. ISBN 978-1-4292-5255-3.
- ^ Lehrstuhl für Molekül- und Koordinationschemie (2013). "Fluorine Laboratory". University of Freiburg. Retrieved 28 May 2013.
- ^ Olivares, M.; Uauy, R. (2004). Essential nutrients in drinking water (Draft) (PDF) (Report). WHO. Retrieved 30 December 2008.
- ^ Nielsen, Forrest H. (2009). "Micronutrients in parenteral nutrition: Boron, silicon, and fluoride". Gastroenterology. 137 (5 Suppl): S55–S60. doi:10.1053/j.gastro.2009.07.072. PMID 19874950.
- ^ a b Murphy, C.; Schaffrath, C.; O'Hagan, D. (2003). "Fluorinated natural products: The biosynthesis of fluoroacetate and 4-fluorothreonine in Streptomyces cattleya". Chemosphere. 52 (2): 455–461. doi:10.1016/S0045-6535(03)00191-7. PMID 12738270.
- ^ a b Proudfoot, A. T.; Bradberry, S. M.; Vale, J. A. (2006). "Sodium fluoroacetate poisoning". Toxicological Reviews. 25 (4): 213–219. doi:10.2165/00139709-200625040-00002. PMID 17288493. Cite error: The named reference "Proudfoot" was defined multiple times with different content (see the help page).
- ^ O'Hagan, D.; Schaffrath, C.; Cobb, S. L.; Hamilton, J. T.; Murphy, C. D. (2002). "Biochemistry: Biosynthesis of an organofluorine molecule". Nature. 416 (6878): 279. Bibcode:2002Natur.416..279O. doi:10.1038/416279a. PMID 11907567.
- ^ Pizzo G.; Piscopo, M. R.; Pizzo, I.; Giuliana, G. (2007). "Community water fluoridation and caries prevention: a critical review" (PDF). Clinical Oral Investigation. 11 (3): 189–193. doi:10.1007/s00784-007-0111-6. PMID 17333303.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Centers for Disease Control and Prevention (2001). "Recommendations for using fluoride to prevent and control dental caries in the United States". MMWR Recommendations and Reports. 50 (RR–14): 1–42. PMID 11521913.
- ^ Ripa, L. W. (1993). "A half-century of community water fluoridation in the United States: review and commentary" (PDF). Journal of Public Health Dentistry. 53 (1): 17–44. doi:10.1111/j.1752-7325.1993.tb02666.x. PMID 8474047. Archived from the original (PDF) on 4 March 2009.
- ^ a b Cheng, K. K.; Chalmers, I.; Sheldon, T. A. (2007). "Adding fluoride to water supplies" (PDF). BMJ. 335 (7622): 699–702. doi:10.1136/bmj.39318.562951.BE. PMC 2001050. PMID 17916854.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marya, C. M. (2011). A textbook of public health dentistry. JP Medical Limited. p. 343. ISBN 9789350252161.
- ^ Armfield, J. M. (2007). "When public action undermines public health: A critical examination of antifluoridationist literature". Australia and New Zealand Health Policy. 4 (1): 25. doi:10.1186/1743-8462-4-25. PMC 2222595. PMID 18067684.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Fejerskov, Ole (2008). Dental caries: The disease and its clinical management. John Wiley & Sons. p. 518. ISBN 978-1-4051-3889-5.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: extra punctuation (link) - ^ a b National Health and Medical Research Council (Australia) (2007). "A systematic review of the efficacy and safety of fluoridation" (PDF). Retrieved 24 February 2009. Summary: Yeung, C. A. (2008). "A systematic review of the efficacy and safety of fluoridation". Evidence-Based Dentistry. 9 (2): 39–43. doi:10.1038/sj.ebd.6400578. PMID 18584000.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help) - ^ Cracher, Connie Myers (2009). "Current concepts in preventive dentistry" (PDF). dentalcare.com. p. 12. Retrieved 20 January 2012.
- ^ Emsley, John (2011). Nature's building blocks: An A–Z guide to the elements (2nd ed.). Oxford University Press. p. 178. ISBN 978-0-19-960563-7.
- ^ "Lipitor becomes world's top-selling drug". Crain's New York Business. 28 December 2011.
- ^ a b Swinson, Joel (2005). "Fluorine – A vital element in the medicine chest" (PDF). PharmaChem. Pharmaceutical Chemistry: 26–27. Retrieved 26 August 2010.
- ^ Hagmann, W.K. (2008). "The many roles for fluorine in medicinal chemistry". Journal of Medicinal Chemistry. 51 (15): 4359–69.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Mitchell, E. Siobhan; Triggle, D. J. (2004). Antidepressants. Infobase Publishing. pp. 37–39. ISBN 978-1-4381-0192-7.
- ^ Preskorn, Sheldon H. (1996). "2 - Rational Drug Discovery and SSRIs". Clinical Pharmacology of SSRI's (1st ed.). Professional Communications, Inc. ISBN 1-884735-08-8.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1186/1471-2334-11-187, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1186/1471-2334-11-187instead. - ^ Brody, Jane E. (2012). "Popular antibiotics may carry serious side effects". The New York Times. Retrieved 3 June 2013.
- ^ Nelson, J. M.; Chiller, T. M.; Powers, J. H.; Angulo, F. J. (2007). "Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: A public health success story" (PDF). Clinical Infectious Diseases. 44 (7): 977–980. doi:10.1086/512369. PMID 17342653.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ King, Dana E.; Malone, Robb; Lilley, Sandra H. (2000). "New classification and update on the quinolone antibiotics". American Family Physican. 61 (9): 2741–2748.
- ^ Goulding, Nicolas J.; Flower, Rod J. (2001). Glucocorticoids. Springer. p. 40. ISBN 9783764360597.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Raj, P. Prithvi; Erdine, Serdar (2012). Pain-relieving procedures: The illustrated guide. John Wiley & Sons. p. 58. ISBN 9781118300459.
- ^ Filler, R.; Saha, R. (2009). "Fluorine in medicinal chemistry: A century of progress and a 60-year retrospective of selected highlights" (PDF). Future Medicinal Chemistry. 1 (5): 777–791. doi:10.4155/fmc.09.65. PMID 21426080.
- ^ Bégué, Jean-Pierre; Bonnet-Delpon, Daniele (2008). Bioorganic and Medicinal Chemistry of Fluorine. John Wiley & Sons. pp. 335–336. ISBN 9780470281871.
- ^ "Fluorine's treasure trove". ICIS news. 2 October 2006. Retrieved 20 February 2011.
- ^ Eisler, Ronald (1995). Biological report 27: Sodium monofluoroacetate (1080) Hazards to fish, wildlife and invertebrates: A synoptic review (PDF) (Report). Patuxent Environmental Science Center (U.S. National Biological Service). Retrieved 5 June 2011.
- ^ "Class I ozone-depleting substances". Sodium fluoride – pesticidal uses. Scorecard. Retrieved 20 February 2011.
- ^ "Fact sheet: Trifluralin". Pesticides News. 52: 20–21. 2001.
- ^ European Commission (2007). Trifluralin (PDF) (Report).
- ^ Case T-475/07, Dow AgroSciences Ltd vs. European Commission (2011). The General Court of European Union (Third Camber).
- ^ Barnette, William E. (1995). "Physical Organic Aspects of Fluorinated Argichemicals". Fluorine in agriculture. Smithers Rapra Publishing. pp. 1–19. ISBN 9781859570333.
- ^ Theodoridis, George (2006). Fluorine and the Environment: Agrochemicals, Archaeology, Green Chemistry & Water. Elseiver. pp. 121–176. ISBN 9780444526724.
- ^ Schmitz, A.; Kälicke, T.; Willkomm, P.; Grünwald, F.; Kandyba, J.; Schmitt, O. (2000). "Use of fluorine-18 fluoro-2-deoxy-D-glucose positron emission tomography in assessing the process of tuberculous spondylitis" (PDF). Journal of spinal disorders. 13 (6): 541–544. doi:10.1097/00002517-200012000-00016. PMID 11132989.
- ^ Bustamante, Ernesto; Pedersen, Peter L. (1977). "High aerobic glycolysis of rat hepatoma cells in culture: Role of mitochondrial hexokinase". Proceedings of the National Academy of Sciences. 74 (9): 3735–3739. Bibcode:1977PNAS...74.3735B. doi:10.1073/pnas.74.9.3735. PMC 431708. PMID 198801.
- ^ Hayat, M. A. (2007). Cancer imaging: Lung and breast carcinomas. Academic Press. p. 41. ISBN 9780123742124.
- ^ Nelson, J. H. (2003). Nuclear magnetic resonance spectroscopy. Prentice Hall. pp. 129–139. ISBN 0-13-033451-0.
- ^ Danielson, Mark A.; Falke, Joseph J. (1996). "Use of 19F NMR to probe protein structure and conformational changes". Annual Review of Biophysics and Biomolecular Structure. 25: 163–195. doi:10.1146/annurev.bb.25.060196.001115. PMC 2899692. PMID 8800468.
- ^ Kuethe, Dean O.; Caprihan, Arvind; Fukushima, Eiichi; Waggoner, R. Allen (2005). "Imaging lungs using inert fluorinated gases". Magnetic Resonance in Medicine. 39 (1): 85–88. doi:10.1002/mrm.1910390114. PMID 9438441.
- ^ Keplinger, M. L.; Suissa, L. W. (1968). "Toxicity of fluorine short-term inhalation". American Industrial Hygiene Association Journal. 29 (1): 10–18. doi:10.1080/00028896809342975. PMID 5667185.
- ^ "UN/NA 1045 (United Nations/North America fluorine data sheet)". National Oceanic and Atmospheric Administration. Retrieved 17 June 2011.
- ^ Blodgett, David W.; Suruda, Anthony J.; Crouch, Barbara Insley (2001). "Fatal unintentional occupational poisonings by hydrofluoric acid in the U.S" (PDF). American Journal of Industrial Medicine. 40 (2): 215–220. doi:10.1002/ajim.1090. PMID 11494350.
- ^ Goldfrank's manual of toxicologic emergencies. McGraw-Hill Professional. 2007. p. 1333. ISBN 978-0-07-144310-4. Retrieved 3 May 2011.
- ^ a b "Recommended medical treatment for hydrofluoric acid exposure" (PDF). Honeywell Specialty Materials. pp. 2–12. Retrieved 6 May 2009.
- ^ Sullivan, John Burke; Krieger, Gary R. (2001). Clinical environmental health and toxic exposures. Lippincott Williams & Wilkins. pp. 458–459. ISBN 9780683080278.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ El Saadi, M. S.; Hall, A. H.; Hall, P. K.; Riggs, B. S.; Augenstein, W. L.; Rumack, B. H. (1989). "Hydrofluoric acid dermal exposure". Veterinary and human toxicology. 31 (3): 243–47. PMID 2741315.
- ^ Roblin, Isabelle; Urban, Martine; Flicoteau, Domitille; Martin, Chantel; Pradeau, Dominique (2006). "Topical treatment of experimental hydrofluoric acid skin burns by 2.5% calcium gluconate". Journal of Burn Care & Research. 27 (6): 889–894. doi:10.1097/01.BCR.0000245767.54278.09. PMID 17091088.
- ^ Hultén, Peter; Höjer, J.; Ludwigs, U.; Janson, A. (2004). "Hexafluorine vs. standard decontamination to reduce systemic toxicity after dermal exposure to hydrofluoric acid". Journal of Toxicology – Clinical Toxicology. 42 (4): 355–361. doi:10.1081/CLT-120039541. PMID 15461243.
- ^ Zorich, Robert (1991). Handbook of Quality Integrated Circuit Manufacturing. Academic Press. pp. 182–183. ISBN 9780323140553.
- ^ Liteplo, R.; Gomes R.; Howe, P.; Malcolm, H. (2002). Environmental health criteria 227 (Fluoride). United Nations Environment Programme; International Labour Organization; World Health Organization. p. 100. ISBN 92-4-157227-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Nochimson, Geofrey (2011). "Fluoride toxicity". emedicine. Retrieved 19 May 2011.
- ^ Reddy, D. R. (2009). "Neurology of endemic skeletal fluorosis". Neurology India. 57 (1): 7–12. doi:10.4103/0028-3886.48793. PMID 19305069.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Baez, Ramon J.; Baez, Martha X.; Marthaler, Thomas M. (2000). "Urinary fluoride excretion by children 4–6 years old in a south Texas community". Revista Panamericana de Salud Pública. 7 (4): 242–248. doi:10.1590/S1020-49892000000400005.
- ^ Augenstein, W. L.; Spoerke, David G.; Kulig, Ken W. (1991). "Fluoride ingestion in children: a review of 87 cases". Pediatrics. 88 (5): 907–912. PMID 1945630.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Gessner, Bradford D.; Beller, Michael; Middaugh, John P.; Whitford, Gary M. (1994). "Acute fluoride poisoning from a public water system". New England Journal of Medicine. 330 (2): 95–99. doi:10.1056/NEJM199401133300203. PMID 8259189.
- ^ Aucamp, Pieter J.; Björn, Lars Olof. "Questions and answers about the environmental effects of the ozone layer depletion and climate change: 2010 update" (PDF). United Nations Environmental Programme. pp. 4–6, 41, 46–47. Retrieved 6 January 2012.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/news.2011.293, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/news.2011.293instead. - ^ Barry, Patrick L.; Phillips, Tony (26 May 2006). "Good news and a puzzle". NASA. Retrieved 6 January 2012.
- ^ "Class I Ozone-depleting Substances". U.S. Environmental Protection Agency. Retrieved 6 January 2012.
- ^ "Phaseout of HCFCs (Class II Ozone-Depleting Substances)". U.S. Environmental Protection Agency. 2012. Retrieved 15 August 2012.
- ^ McCoy, Michael (2007). "Nations speed up HCFC elimination". Chemical & Engineering News. 85 (40): 11. doi:10.1021/cen-v085n023.p011a. Retrieved 25 December 2012.
- ^ "Greenhouse gases: Some definitions". gdrc.org. Retrieved 20 February 2011.
- ^ Intergovernmental Panel on Climate Change. "Changes in Atmospheric Constituents and in Radiative Forcing.". Climate change 2007: The physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate (PDF). Cambridge University. p. 212. ISBN 978-0-521-70596-7.
- ^ a b c Steenland, Kyle; Fletcher, Tony; Savitz, David A. (2010). "Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA)". Environmental Health Perspectives. 118 (8): 1100–8. doi:10.1289/ehp.0901827. PMC 2920088. PMID 20423814. Retrieved 3 May 2011.
- ^ a b c Betts, Kellyn S. (2007). "Perfluoroalkyl acids: What is the evidence telling us?". Environmental Health Perspectives. 115 (5): A250–A256. doi:10.1289/ehp.115-a250. PMC 1867999. PMID 17520044. Retrieved 5 September 2011.
- ^ Lietz, A. C.; Meyer, Michael T. (2006). Evaluation of emerging contaminants of concern at the south district wastewater treatment plant based on seasonal sampling events, Miami-Dade Country, Florida, 2004 (PDF) (Report). U.S. Geological Survey Scientific Investigations. pp. 7–8. Retrieved 6 June 2011.
{{cite report}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1039/c0em00373e, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1039/c0em00373einstead. - ^ Wiberg, Wiberg & Holleman 2001, p. 422.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1039/C1RA00804H, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1039/C1RA00804Hinstead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11792193, please use {{cite journal}} with
|pmid=11792193instead. - ^ Smart, Bruce E.; Tatlow, J. C. (1994). Organofluorine chemistry: Principles and commercial applications. Springer. p. 515. ISBN 0306446103.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.ccr.2008.07.014, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.ccr.2008.07.014instead. - ^ Pauling, Linus A. (1960). The nature of the chemical bond and the structure of molecules and crystals: An introduction to modern structural chemistry. Cornell University Press. pp. 454–464. ISBN 978-0-8014-0333-0.
- ^ Atkins, Peter; Jones, Loretta (2008). Chemical principles: The quest for insight. W. H. Freeman & Co. pp. 184–185. ISBN 978-1-4292-0965-6.
- ^ Emsley, John (1981). "The hidden strength of hydrogen". New Scientist. 91 (1264): 291–292. Retrieved 25 December 2012.
- ^ Greenwood & Earnshaw 1998, pp. 812–816.
- ^ Wiberg, Wiberg & Holleman 2001, p. 425.
- ^ Clark, Jim (2002). "The acidity of the hydrogen halides". Retrieved 4 September 2011.
- ^ a b Chambers, C.; Holliday, A. K. (1975). Modern inorganic chemistry (An intermediate text) (PDF). The Butterworth Group. pp. 328–329.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Hannan, Henry J. (2010). Course in chemistry for IIT-JEE 2011. Tata McGraw Hill Education Private Limited. pp. 15–22. ISBN 9780070703360.
- ^ a b Greenwood & Earnshaw 1998, p. 819.
- ^ Lide 2004, p. 4-84.
- ^ Lide 2004, p. 4-79, 4-91.
- ^ Bartlett, Neil; Lohmann, D. H. (1962). "Dioxygenyl hexafluoroplatinate (V), O2+[PtF6]−". Proceedings of the Chemical Society (3). Chemical Society: 115. doi:10.1039/PS9620000097.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bartlett, Neil (1962). "Xenon hexafluoroplatinate (V) Xe+[PtF6]−". Proceedings of the Chemical Society (6). Chemical Society: 218. doi:10.1039/PS9620000197.
- ^ Oxtoby, David W.; Gillis, H. Pat; Campion, Alan (2012). Principle of modern chemistry. Cengage learning. p. 693. ISBN 9780840049315.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Arai, Toshihiro (1999). Mesoscopic materials and clusters: Their physical and chemical properties. Springer. p. 267. ISBN 9783540648840.
- ^ Remy, Heinrich (1956). Treatise on inorganic chemistry: Introduction and main groups of the periodic table. Elsevier Publishing Company. p. 383.
- ^ Walsh, Kenneth A (1 August 2009). Beryllium chemistry and processing. ASM International. pp. 99–102, 118–119. ISBN 978-0-87170-721-5.
- ^ a b http://pubs.rsc.org/en/content/articlelanding/1967/J1/j19670000478
- ^ Brown, Paul L.; Mompean, Federico J.; Perrone, Jane; Illemassène, Myriam (2005). Chemical Thermodynamics of Zirconium. Gulf Professional Publishing. p. 144. ISBN 0-444-51803-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Perry, D. L. (2011). Handbook of Inorganic Compounds (2nd ed.). CRC Press. p. 193. ISBN 9781439814611.
{{cite book}}:|access-date=requires|url=(help) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1063/1.467963, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1063/1.467963instead. - ^ Lide 2004, pp. 4–60, 4–76, 4–92, 4–96.
- ^ Lide 2004, p. 4-96.
- ^ a b Lide 2004, p. 4-92.
- ^ Greenwood & Earnshaw 1998, p. 964.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1002/anie.199004061, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1002/anie.199004061instead. - ^ Greenwood & Earnshaw 1998, p. 990.
- ^ Lide 2004, p. 4-91.
- ^ Lide 2004, p. 4-72, 4-93.
- ^ a b c Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1002/1521-3773(20011001)40:19<3690::AID-ANIE3690>3.0.CO;2-5, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1002/1521-3773(20011001)40:19<3690::AID-ANIE3690>3.0.CO;2-5instead. - ^ a b Greenwood & Earnshaw 1998, pp. 561–563.
- ^ Emeléus & Sharpe 1983, pp. 256–277.
- ^ Mackay, Mackay & Henderson 2002, pp. 243–244.
- ^ Lide 2004, p. 4-72.
- ^ Lide 2004, p. 4-88.
- ^ Drews, T.; Supeł, J.; Hagenbach, A.; Seppelt, K. (2006). "Solid state molecular structures of transition metal hexafluorides". Inorganic Chemistry. 45 (9): 3782–3788. doi:10.1021/ic052029f. PMID 16634614.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lide 2004, p. 4-71, 4-78.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ic0608290, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ic0608290instead. - ^ Noury, Stephane; Silvi, Bernard; Gillespie, Ronald J. (2002). "Chemical bonding in hypervalent molecules: Is the octet rule relevant?" (PDF). Inorganic Chemistry. 41 (8): 2164–2172. doi:10.1021/ic011003v. PMID 11952370. Retrieved 23 May 2012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Greenwood & Earnshaw 1998, pp. 375–376.
- ^ Ellis, Bryan David (2001). Scientific essentialism. Cambridge University Press. p. 69. ISBN 0521800943.
- ^ Aigueperse et al. 2005, p. 28.
- ^ Wiberg, Wiberg & Holleman 2001, p. 897.
- ^ Greenwood & Earnshaw 1998, pp. 198–199.
- ^ a b Raghavan, P. S. (1998). Concepts and problems in inorganic Chemistry. Discovery Publishing House. pp. 164–165. ISBN 9788171414185.
- ^ Aigueperse et al. 2005, p. 37.
- ^ a b Norman, Nicholas C. (1998). Chemistry of arsenic, antimony and bismuth. Springer. p. 97. ISBN 075140389X.
- ^ Lewars 2008, p. 53.
- ^ a b Murthy, C. Parameshwara. University chemistry, Vol. 1. New Age International. pp. 180–182, 206–208. ISBN 8122407420.
- ^ Wiberg, Wiberg & Holleman 2001, p. 435.
- ^ Wiberg, Wiberg & Holleman 2001, p. 436.
- ^ a b Pitzer, Kenneth Sanborn, ed. (1993). Molecular structure and statistical thermodynamics: Selected papers of Kenneth S. Pitzer, Vol. 1. World Scientific. p. 111. ISBN 9810214391.
- ^ Gmelin, Leopold. Gmelin handbook of inorganic chemistry: At—Astatine (8th ed.). Springer-Verlag. p. 224. ISBN 9783540935162.
- ^ Greenwood & Earnshaw 1998, pp. 828–830.
- ^ Patnaik, Pradyot (2007). A comprehensive guide to the hazardous properties of chemical substances. John Wiley & Sons. pp. 478–479. ISBN 9780471714583.
- ^ Wiberg, Wiberg & Holleman 2001, pp. 392–393.
- ^ Wiberg, Wiberg & Holleman 2001, p. 438.
- ^ Wiberg, Wiberg & Holleman 2001, p. 400.
- ^ Lewars 2008, p. 68.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1126/science.139.3559.1047, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1126/science.139.3559.1047instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ic701313h, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ic701313hinstead. - ^ Lewars 2008, p. 67.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/35022551, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/35022551instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1063/1.1506150, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1063/1.1506150instead. - ^ Lewars 2008, p. 71.
- ^ O'Hagan, D. (2008). "Understanding organofluorine chemistry. An introduction to the C–F bond". Chemical Society Reviews. 37 (2): 308–319. doi:10.1039/b711844a. PMID 18197347.
- ^ a b c Siegemund et al. 2005, p. 2.
- ^ a b Siegemund et al. 2005, pp. 7–8.
- ^ F2 Chemicals Limited (2011). "Safety data sheet: Perfluorodecalin" (PDF). Retrieved 14 June 2013.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ Mackay, Donald et al.; Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, Second Edition (2010); p.263; [1]
- ^ Mendicino, L. (1999). Environmental issues in the electronics and semiconductor industries. The Electrochemical Society. p. 116. ISBN 9781566772303.
- ^ a b Knepper, Thomas P.; Lange, Frank T. (2011). Polyfluorinated Chemicals and Transformation Products. Springer. p. 27. ISBN 9783642218712.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Siegemund et al. 2005, p. 29.
- ^ "Acids and bases". askthenerd.com. 2011. Retrieved 18 August 2012.
- ^ a b Siegemund et al. 2005, p. 32.
- ^ Carlson & Scmiegel 2005, p. 3.
- ^ a b Carlson & Scmiegel 2005, pp. 3–4.
- ^ Carlson & Scmiegel 2005, p. 4.
- ^ Rhoades, David Walter (2008). Broadband dielectric spectroscopy studies of Nafion. ProQuest. p. 2. ISBN 9780549785408.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S0013-4686(98)00132-7, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S0013-4686(98)00132-7instead.
Indexed references
- Dean, John A. (1999). Lange's handbook of chemistry (15th ed.). McGraw-Hill, Inc. ISBN 0-07-016190-9.
{{cite book}}: Invalid|ref=harv(help) - Emeléus, H. J.; Sharpe, A. G. (1983). Advances in inorganic chemistry and radiochemistry (27th ed.). Academic Press. ISBN 0-12-023627-3.
{{cite book}}: Invalid|ref=harv(help) - Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the elements (2nd ed.). Butterworth Heinemann. ISBN 0-7506-3365-4.
{{cite book}}: Invalid|ref=harv(help) - Hounshell, David A.; Smith, John Kelly (1988). Science and corporate strategy: DuPont R&D, 1902–1980. Cambridge University Press. ISBN 0-521-32767-9.
{{cite book}}: Invalid|ref=harv(help) - Lewars, Errol G. (2008). Modeling marvels: Computational anticipation of novel molecules. Springer. ISBN 1-4020-6972-3.
{{cite book}}: Invalid|ref=harv(help) - Lide, David R. (2004). Handbook of chemistry and physics (84th ed.). CRC Press. ISBN 0-8493-0566-7.
{{cite book}}: Invalid|ref=harv(help) - Template:Ru icon Lidin, P. A.; Molochko, V. A.; Andreeva, L. L. (2000). Химические свойства неорганических веществ. Khimiya. ISBN 5-7245-1163-0.
{{cite book}}: Invalid|ref=harv(help); Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - Mackay, Kenneth Malcolm; Mackay, Rosemary Ann; Henderson, W. (2002). Introduction to modern inorganic chemistry (6th ed.). CRC Press. ISBN 0-7487-6420-8.
{{cite book}}: Invalid|ref=harv(help) - Ullmann, Franz, ed. (2005). Encyclopedia of Industrial Chemistry. Wiley-VCH. ISBN 978-3-527-30673-2.
- Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, Renée; Cuer, Jean Pierre. "Fluorine Compounds, Inorganic". doi:10.1002/14356007. ISBN 978-3-527-30673-2.
{{cite book}}: Missing or empty|title=(help) - Carlson, D. Peter; Scmiegel, Walter. "Fluoropolymers, Organic". doi:10.1002/14356007.a11_393.
{{cite book}}: Missing or empty|title=(help) - Jaccaud, Michael; Faron, Robert; Devilliers, Didier; Romano, René. "Fluorine". doi:10.1002/14356007.a11_293.
{{cite book}}: Missing or empty|title=(help) - Siegemund, Günter; Schwertweger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blain. "Fluorine Compounds, Organic". doi:10.1002/14356007.a11_349.
{{cite book}}: Missing or empty|title=(help)
- Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, Renée; Cuer, Jean Pierre. "Fluorine Compounds, Inorganic". doi:10.1002/14356007. ISBN 978-3-527-30673-2.
- Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic chemistry. Academic Press. ISBN 978-0-12-352651-9. Retrieved 3 March 2011.
{{cite book}}: Invalid|ref=harv(help) - Yaws, Carl L.; Braker, William (2001). "Fluorine". Matheson gas data book, Book 2001. McGraw-Hill Professional. ISBN 978-0-07-135854-5.
{{cite book}}: Invalid|ref=harv(help)


