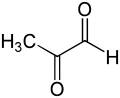
Methylglyoxal
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Oxopropanal | |||
| Other names
Pyruvaldehyde
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 906750 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.001.059 | ||
| KEGG | |||
| MeSH | Methylglyoxal | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H4O2 | |||
| Molar mass | 72.063 g·mol−1 | ||
| Appearance | Yellow liquid | ||
| Density | 1.046 g/cm3 | ||
| Boiling point | 72 °C (162 °F; 345 K) | ||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H290, H302, H315, H317, H318, H319, H335, H341 | |||
| P201, P202, P234, P261, P264, P270, P271, P272, P280, P281, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P390, P403+P233, P404, P405, P501 | |||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Methylglyoxal (MGO) is the organic compound with the formula CH3C(O)CHO. It is a reduced derivative of pyruvic acid. It is a reactive compound that is implicated in the biology of diabetes. Methylglyoxal is produced industrially by degradation of carbohydrates using overexpressed methylglyoxal synthase.[1]
Chemical structure
Gaseous methylglyoxal has two carbonyl groups, an aldehyde and a ketone. In the presence of water, it exists as hydrates and oligomers. The formation of these hydrates is indicative of the high reactivity of MGO, which is relevant to its biological behavior.[2]
Biochemistry
Biosynthesis and biodegradation
In organisms, methylglyoxal is formed as a side-product of several metabolic pathways.[3] Methylglyoxal mainly arises as side products of glycolysis involving glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. It is also thought to arise via the degradation of acetone and threonine.[4] Illustrative of the myriad pathways to MGO, aristolochic acid caused 12-fold increase of methylglyoxal from 18 to 231 μg/mg of kidney protein in poisoned mice.[5] It may form from 3-aminoacetone, which is an intermediate of threonine catabolism, as well as through lipid peroxidation. However, the most important source is glycolysis. Here, methylglyoxal arises from nonenzymatic phosphate elimination from glyceraldehyde phosphate and dihydroxyacetone phosphate (DHAP), two intermediates of glycolysis. This conversion is the basis of a potential biotechnological route to the commodity chemical 1,2-propanediol.[6]
Since methylglyoxal is highly cytotoxic, several detoxification mechanisms have evolved. One of these is the glyoxalase system. Methylglyoxal is detoxified by glutathione. Glutathione reacts with methylglyoxal to give a hemithioacetal, which converted into S-D-lactoyl-glutathione by glyoxalase I.[7] This thioester is hydrolyzed to D-lactate by glyoxalase II.[8]
Biochemical function
Methylglyoxal is involved in the formation of advanced glycation end products (AGEs).[4] In this process, methylglyoxal reacts with free amino groups of lysine and arginine and with thiol groups of cysteine forming AGEs. Histones are also heavily susceptible to modification by methylglyoxal and these modifications are elevated in breast cancer.[9][10]

DNA damages are induced by reactive carbonyls, principally methylglyoxal and glyoxal, at a frequency similar to that of oxidative DNA damages.[12] Such damage, referred to as DNA glycation, can cause mutation, breaks in DNA and cytotoxicity.[12] In humans, a protein DJ-1 (also named PARK7), has a key role in the repair of glycated DNA bases.
Biomedical aspects
Due to increased blood glucose levels, methylglyoxal has higher concentrations in diabetics and has been linked to arterial atherogenesis. Damage by methylglyoxal to low-density lipoprotein through glycation causes a fourfold increase of atherogenesis in diabetics.[13] Methylglyoxal binds directly to the nerve endings and by that increases the chronic extremity soreness in diabetic neuropathy.[14][15]
Occurrence, other
Methylglyoxal is a component of some kinds of honey, including manuka honey; it appears to have activity against E. coli and S. aureus and may help prevent formation of biofilms formed by P. aeruginosa .[16]
Research suggests that methylglyoxal contained in honey does not cause an increased formation of advanced glycation end products (AGEs) in healthy persons.[17][18]
See also
- Dicarbonyl
- 1,2-Dicarbonyl, methylglyoxal can be classified as an 1,2-dicarbonyl
References
- ^ Lichtenthaler, Frieder W. (2010). "Carbohydrates as Organic Raw Materials". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.n05_n07. ISBN 978-3527306732.
- ^ Loeffler, Kirsten W.; Koehler, Charles A.; Paul, Nichole M.; De Haan, David O. (2006). "Oligomer Formation in Evaporating Aqueous Glyoxal and Methyl Glyoxal Solutions". Environmental Science & Technology. 40 (20): 6318–23. Bibcode:2006EnST...40.6318L. doi:10.1021/es060810w. PMID 17120559.
- ^ Inoue Y, Kimura A (1995). "Methylglyoxal and regulation of its metabolism in microorganisms". Adv. Microb. Physiol. Advances in Microbial Physiology. 37: 177–227. doi:10.1016/S0065-2911(08)60146-0. ISBN 978-0-12-027737-7. PMID 8540421.
- ^ a b Bellier, Justine; Nokin, Marie-Julie; Lardé, Eva; Karoyan, Philippe; Peulen, Olivier; Castronovo, Vincent; Bellahcène, Akeila (2019). "Methylglyoxal, a Potent Inducer of AGEs, Connects between Diabetes and Cancer". Diabetes Research and Clinical Practice. 148: 200–211. doi:10.1016/j.diabres.2019.01.002. PMID 30664892. S2CID 58631777.
- ^ Li, YC; Tsai, SH; Chen, SM; Chang, YM; Huang, TC; Huang, YP; Chang, CT; Lee, JA (2012). "Aristolochic acid-induced accumulation of methylglyoxal and Nε-(carboxymethyl)lysine: an important and novel pathway in the pathogenic mechanism for aristolochic acid nephropathy". Biochem Biophys Res Commun. 423 (4): 832–7. doi:10.1016/j.bbrc.2012.06.049. PMID 22713464.
- ^ Sullivan, Carl J.; Kuenz, Anja; Vorlop, Klaus‐Dieter (2018). "Propanediols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_163.pub2. ISBN 978-3527306732.
- ^ Thornalley PJ (2003). "Glyoxalase I—structure, function and a critical role in the enzymatic defence against glycation". Biochem. Soc. Trans. 31 (Pt 6): 1343–8. doi:10.1042/BST0311343. PMID 14641060.
- ^ Vander Jagt DL (1993). "Glyoxalase II: molecular characteristics, kinetics and mechanism". Biochem. Soc. Trans. 21 (2): 522–7. doi:10.1042/bst0210522. PMID 8359524.
- ^ Galligan JJ, Wepy JA, Streeter MD, Kingsley PJ, Mitchener MM, Wauchope OR, Beavers WN, Rose KL, Wang T, Spiegel DA, Marnett LJ (September 2018). "Methylglyoxal-derived posttranslational arginine modifications are abundant histone marks". Proc Natl Acad Sci USA. 115 (37): 9228–33. Bibcode:2018PNAS..115.9228G. doi:10.1073/pnas.1802901115. PMC 6140490. PMID 30150385.
- ^ Zheng Q, Omans ND, Leicher R, Osunsade A, Agustinus AS, Finkin-Groner E, D'Ambrosio H, Liu B, Chandarlapaty S, Liu S, David Y (March 2019). "Reversible histone glycation is associated with disease-related changes in chromatin architecture". Nat Commun. 10 (1): 1289. Bibcode:2019NatCo..10.1289Z. doi:10.1038/s41467-019-09192-z. PMC 6426841. PMID 30894531.
- ^ Oya, Tomoko; Hattori, Nobutaka; Mizuno, Yoshikuni; Miyata, Satoshi; Maeda, Sakan; Osawa, Toshihiko; Uchida, Koji (1999). "Methylglyoxal Modification of Protein". Journal of Biological Chemistry. 274 (26): 18492–502. doi:10.1074/jbc.274.26.18492. PMID 10373458.
- ^ a b Richarme G, Liu C, Mihoub M, Abdallah J, Leger T, Joly N, Liebart JC, Jurkunas UV, Nadal M, Bouloc P, Dairou J, Lamouri A. Guanine glycation repair by DJ-1/Park7 and its bacterial homologs. Science. 2017 Jul 14;357(6347):208-211. doi: 10.1126/science.aag1095. Epub 2017 Jun 8. PMID: 28596309
- ^ Rabbani N; Godfrey, L; Xue, M; Shaheen, F; Geoffrion, M; Milne, R; Thornalley, PJ (May 26, 2011). "Glycation of LDL by methylglyoxal increases arterial atherogenicity. A possible contributor to increased risk of cardiovascular disease in diabetes". Diabetes. 60 (7): 1973–80. doi:10.2337/db11-0085. PMC 3121424. PMID 21617182.
- ^ Spektrum: Diabetische Neuropathie: Methylglyoxal verstärkt den Schmerz: DAZ.online. Deutsche-apotheker-zeitung.de (2012-05-21). Retrieved on 2012-06-11.
- ^ Bierhaus, Angelika; Fleming, Thomas; Stoyanov, Stoyan; Leffler, Andreas; Babes, Alexandru; Neacsu, Cristian; Sauer, Susanne K; Eberhardt, Mirjam; et al. (2012). "Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy". Nature Medicine. 18 (6): 926–33. doi:10.1038/nm.2750. PMID 22581285. S2CID 205389296.
- ^ Israili, ZH (2014). "Antimicrobial properties of honey". American Journal of Therapeutics. 21 (4): 304–23. doi:10.1097/MJT.0b013e318293b09b. PMID 23782759.
- ^ Wallace A, Eady S, Miles M, Martin H, McLachlan A, Rodier M, Willis J, Scott R, Sutherland J (April 2010). "Demonstrating the safety of manuka honey UMF® 20+ in a human clinical trial with healthy individuals". Br J Nutr. 103 (7): 1023–8. doi:10.1017/S0007114509992777. PMID 20064284.
- ^ Degen J, Vogel M, Richter D, Hellwig M, Henle T (October 2013). "Metabolic transit of dietary methylglyoxal". J Agric Food Chem. 61 (43): 10253–60. doi:10.1021/jf304946p. PMID 23451712.