Formoterol
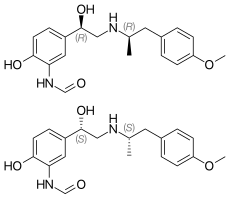 | |
 Formoterol (top), (R,R)-(−)-formoterol (center) and (S,S)-(+)-formoterol (bottom) | |
| Clinical data | |
|---|---|
| Trade names | See below |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Inhalation (capsules for oral inhalation, DPI, MDI) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 61–64% |
| Metabolism | Hepatic demethylation and glucuronidation (CYP2D6, CYP2C19, CYP2C9 and CYP2A6 involved) |
| Elimination half-life | 10 hours |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.131.654 |
| Chemical and physical data | |
| Formula | C19H24N2O4 |
| Molar mass | 344.405 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Formoterol (INN) or eformoterol (former BAN) is a long-acting β2 agonist (LABA) used in the management of asthma and COPD. It is marketed in three forms: a dry-powder inhaler, a metered-dose inhaler and an inhalation solution, under various trade names including Foradil/Foradile, Oxeze/Oxis and many others. It is also marketed in the combination formulations budesonide/formoterol and mometasone/formoterol.
Formoterol is a long-acting β2 agonist that has an extended duration of action (up to 12 hours) compared to short-acting β2 agonists such as salbutamol (albuterol), which are effective for 4–6 hours. LABAs such as formoterol are used as "symptom controllers" to supplement prophylactic corticosteroid therapy (e.g., fluticasone). A "reliever" short-acting β2 agonist (e.g., salbutamol) is still required, since LABAs are not recommended for the treatment of acute asthma.
Mechanism of action
Inhaled formoterol works like other β2 agonists, causing bronchodilation by relaxing the smooth muscle in the airway so as to treat the exacerbation of asthma. The long duration of formoterol action occurs because the formoterol molecules initially diffuse into the plasma membrane of the lung cells, and then are slowly released back outside, where they can come into contact with β2 adrenergic receptors. Formoterol has been demonstrated to have a faster onset of action than salmeterol as a result of lower lipophilicity, and has also been demonstrated to be more potent – a 12 µg dose of formoterol has been demonstrated to be equivalent to a 50 µg dose of salmeterol.
Safety
In November 2005, the US FDA released a health advisory alerting the public to findings that show the use of long-acting β2 agonists could lead to a worsening of wheezing symptoms in some patients.[1]
At the current time, available long-acting β2 agonists include salmeterol, formoterol, bambuterol, and sustained-release oral salbutamol. Combinations of inhaled steroids and long-acting bronchodilators are becoming more widespread – combination preparations include fluticasone/salmeterol and budesonide/formoterol.
Additional potential uses
β2 agonists in treatment of obesity
β2 agonists increase energy utilization and fat metabolism, however, therapeutic use for obesity has been limited by the concomitant activation of β1 receptors resulting in excessive increases in heart rate. A recent 2012 dose-finding study (in healthy men) demonstrated significantly increased resting energy expenditure, and fat oxidation at a dose of 160 μg of formoterol per day without significantly increased heart rate. Formoterol's specificity for the β2 receptor (relative to β1 receptors) may facilitate its use for this purpose. A combination of a highly selective β2 agonist like formeterol with a low dose highly selective β1 blocker may further improve the differential therapeutic ability to selectively agonize β2 receptors for metabolic benefits in treating obesity without adverse β1 agonist effects.[2]
Stimulation of mitochondrial biogenesis
Formoterol may also help stimulate mitochondrial biogenesis. Mitochondrial dysfunction is related to many degenerative diseases — particularly neurodegenerative disorders.[3]
Treatment for Down syndrome
Preliminary research offers hope that formoterol may be a useful treatment in Down syndrome. In a mouse model of the disease, the drug strengthened nerve connections in the hippocampus, a brain center used for spatial navigation, paying attention and forming new memories.[4]
Trade names
- Foradil/Foradile capsules for oral inhalation (Schering-Plough in the U.S., Novartis rest of world)
- Oxeze/Oxis Turbuhaler DPI (AstraZeneca)
- Atock (Astellas)
- Atimos/Atimos Modulite MDI (Chiesi)
- Perforomist inhalation solution (Mylan N.V.)
See also
- Arformoterol ((R,R)-(−)-formoterol) — an enantiopure compound used in the management of COPD
- Combination drugs:
References
- ^ Center for Drug Evaluation and Research. "Advair Diskus, Advair HFA, Brovana, Foradil, Perforomist, Serevent Diskus, and Symbicort Information (Long Acting Beta Agonists)". Postmarket Drug Safety Information for Patients and Providers.
- ^ Lee, P; Day, RO; Greenfield, JR; Ho, KKY (May 29, 2012). "Formoterol, a highly β2-selective agonist, increases energy expenditure and fat utilisation in men". International Journal of Obesity. 37 (4): 593–597. doi:10.1038/ijo.2012.90. ISSN 1476-5497. PMID 22641064.
- ^ Wills, Lauren P; Trager, Richard E; Beeson, Gyda C; Lindsey, Christopher C; Peterson, Yuri K; Beeson, Craig C; Schnellmann, Rick G (April 6, 2012). "The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis". The Journal of Pharmacology and Experimental Therapeutics. 342 (1): 106–118. doi:10.1124/jpet.112.191528. ISSN 1521-0103. PMC 3383035. PMID 22490378.
- ^ Dang, Van; Medina, Brian; Das, Devsmita; Moghadam, Sarah; Martin, Kara J.; Lin, Bill; Naik, Priyanka; Patel, Devan; Nosheny, Rachel; Wesson Ashford, John; Salehi, Ahmad (February 2014). "Formoterol, a Long-Acting β2 Adrenergic Agonist, Improves Cognitive Function and Promotes Dendritic Complexity in a Mouse Model of Down Syndrome". Biological Psychiatry. 75 (3): 179–188. doi:10.1016/j.biopsych.2013.05.024. ISSN 1873-2402. PMID 23827853.
{{cite journal}}:|access-date=requires|url=(help)
