Pilocarpine
This article may require copy editing for grammar, style, cohesion, tone, or spelling. (August 2009) |
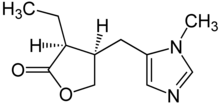 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | gtts, p.o. |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 0.76 hours (5 mg), 1.35 hours (10 mg) |
| Excretion | urine |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.936 |
| Chemical and physical data | |
| Formula | C11H16N2O2 |
| Molar mass | 208.257 g/mol g·mol−1 |
| (verify) | |
Pilocarpine is a parasympathomimetic alkaloid obtained from the leaves of tropical American shrubs from the genus Pilocarpus. It is a non-selective muscarinic receptor agonist[1] in the parasympathetic nervous system, which acts therapeutically at the muscarinic acetylcholine receptor M3 due to its topical application[2], e.g., in glaucoma and xerostomia.
Uses
Clinical
Pilocarpine has been used in the treatment of chronic open-angle glaucoma and acute angle-closure glaucoma for over 100 years.[3] It acts on a subtype of muscarinic receptor (M3) found on the iris sphincter muscle, causing the muscle to contract and engage in miosis. Pilocarpine also acts on the ciliary muscle and causes it to contract. When the ciliary muscle contracts, it opens the trabecular meshwork through increased tension on the scleral spur. This action facilitates the rate that aqueous humor leaves the eye to decrease intraocular pressure.
Pilocarpine is often used as an antidote for scopolamine, atropine, and hyoscyamine poisoning.
In ophthalmology pilocarpine is also used to reduce the possibility of glare at night from lights if the patient underwent implantation of phakic intraocular lenses; the use of pilocarpine would reduce the size of the pupils, relieving these symptoms. The most common concentration for this use is pilocarpine 1%, the weakest concentration.
Pilocarpine is also used to treat dry mouth (xerostomia) which can occur, for example, as a side effect of radiation therapy for head and neck cancers. Pilocarpine stimulates the secretion of large amounts of saliva and sweat.[4]
Pilocarpine is used to stimulate sweat glands in a sweat test to measure the concentration of chloride and sodium that is excreted in sweat. It is used to diagnose cystic fibrosis (CF).
PREPARATION: the drug and powdered leaf of pilocarpin microfelixis are subjected to extracted forotoal alkaloid with ethanol acidified with HCL with solvents removed under reduced pressure and resultant aqueous residue is neutralized with ammonia and kept aside till all the resin is settled down completely. It is filtered and concentrated by sugar solution to a small volume made alkaloid with ammonia and finally extracted with chloroform. The solvent is removed under reduced pressure.
Scientific
Pilocarpine is used to induce chronic epilepsy in rodents, commonly rats, as a means to study the disorder's physiology and to examine different treatments. Smaller doses may be used to induce salivation in order to collect samples of saliva, for instance, to obtain information about IgA antibodies.
Veterinary
Pilocarpine is given in moderate doses (about 2 mg) to induce emesis in cats who have ingested foreign plants, foods, or drugs. One feline trial determined it was effective, even though the choice emetic is xylazine.
Trade names
Pilocarpine is available under several trade names such as: Diocarpine (Dioptic), Isopto Carpine (Alcon), Miocarpine (CIBA Vision), Ocusert Pilo-20 and -40 (Alza), Pilopine HS (Alcon), Salagen (MGI Pharma), Scheinpharm Pilocarpine (Schein Pharmaceutical), and Timpilo (Merck Frosst).
Adverse effects
Use of pilocarpine may result in a range of adverse effects, most of them related to its non-selective action as a muscarinic receptor agonist. Pilocarpine has been known to cause excessive sweating, excessive salivation, bronchospasm, increased bronchial mucus secretion, bradycardia, vasodilation, browache (when used as eye drops) and diarrhea. It can also result in miosis when used chronically as an eye drop. Systemic injection of Pilocarpine can compromise the blood-brain barrier allowing the pilocarpine to gain access to the brain. This can lead to chronic epilepsy.
References
- ^ Spalding et al. 2002
- ^ Pharmacology, (Rang, Dale, Ritter & Moore, ISBN 0443071454, 5:th ed., Churchill Livingstone 2003) Page 144
- ^ Rosin, A. "Pilocarpine. A miotic of choice in the treatment of glaucoma has passed 110 years of use". Oftalmologia. 35 (1). Romania: 53–55. PMID 1811739.
{{cite journal}}:|access-date=requires|url=(help) - ^ "Pilocarpine".
