Zinc: Difference between revisions
→Other industrial uses: UV protection for rubber and plastics; refs; ce |
→Occurrence: mention seawater, soil and atmosphere occurance |
||
| Line 25: | Line 25: | ||
{{See also|:Category:Zinc minerals|l1=Zinc minerals}} |
{{See also|:Category:Zinc minerals|l1=Zinc minerals}} |
||
<div style="float:right; margin:5px;">[[Image:Sphalerite4.jpg|thumb|right|Sphalerite (ZnS)]]</div> |
<div style="float:right; margin:5px;">[[Image:Sphalerite4.jpg|thumb|right|Sphalerite (ZnS)]]</div> |
||
Zinc makes up about 75 [[parts-per notation|ppm]] (0.007%) of the [[Earth's crust]], making it the 24th most abundant element there.<ref name="Emsley2001p503">{{harvnb|Emsley|2001|p=503}}</ref> The most heavily-mined zinc-containing [[ore]] is [[sphalerite]], which is a form of [[zinc sulfide]]. Other minerals from which zinc is extracted include [[smithsonite]] (zinc [[carbonate]]), [[hemimorphite]] (zinc [[silicate]]), [[wurtzite]] (another zinc sulfide), and sometimes [[hydrozincite]] (basic zinc carbonate).<ref name="Emsley2001p502"/> |
Zinc makes up about 75 [[parts-per notation|ppm]] (0.007%) of the [[Earth's crust]], making it the 24th most abundant element there.<ref name="Emsley2001p503">{{harvnb|Emsley|2001|p=503}}</ref> Soil contains an average of 64 ppm, but actual soils have 5–770 ppm zinc.<ref name="Emsley2001p503"/> Seawater has only 30 ppb zinc and the atmosphere contains 0.1 to 4 mg/ml.<ref name="Emsley2001p503"/> |
||
The most heavily-mined zinc-containing [[ore]] is [[sphalerite]], which is a form of [[zinc sulfide]]. Other minerals from which zinc is extracted include [[smithsonite]] (zinc [[carbonate]]), [[hemimorphite]] (zinc [[silicate]]), [[wurtzite]] (another zinc sulfide), and sometimes [[hydrozincite]] (basic zinc carbonate).<ref name="Emsley2001p502"/> |
|||
Exploitable reserves of zinc exceed 100 million tonnes<ref name="Emsley2001p502"/> but, at the current rate of consumption, these reserves are estimated to be depleted by 2055.<ref>{{cite journal | title = Earth audit | first = David | last = Cohen | journal = New Scientist | year = 2007 | volume = 194 | issue = 2605 | page =8 | url = http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=27199200&site=ehost-live }}</ref> Based on data gathered by the [[United States Geological Survey]] and the [[University of Augsburg]], the available supply has also been estimated to last for 20 to 30 years.<ref>{{cite web | title = Augsberg University Calculate When Our Materials Run Out | url = http://www.idtechex.com/products/en/articles/00000591.asp | date = 2007-06-04 |publisher = IDTechEx |accessdate = 2008-12-09}}</ref> |
Exploitable reserves of zinc exceed 100 million tonnes<ref name="Emsley2001p502"/> but, at the current rate of consumption, these reserves are estimated to be depleted by 2055.<ref>{{cite journal | title = Earth audit | first = David | last = Cohen | journal = New Scientist | year = 2007 | volume = 194 | issue = 2605 | page =8 | url = http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=27199200&site=ehost-live }}</ref> Based on data gathered by the [[United States Geological Survey]] and the [[University of Augsburg]], the available supply has also been estimated to last for 20 to 30 years.<ref>{{cite web | title = Augsberg University Calculate When Our Materials Run Out | url = http://www.idtechex.com/products/en/articles/00000591.asp | date = 2007-06-04 |publisher = IDTechEx |accessdate = 2008-12-09}}</ref> |
||
Revision as of 03:32, 26 January 2009
 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Zinc | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | silver-gray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Zn) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Zinc in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d10 4s2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 692.68 K (419.53 °C, 787.15 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1180 K (907 °C, 1665 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 7.140 g/cm3 [3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 6.57 g/cm3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 7.32 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 115 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.470 J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −2, 0, +1, +2 (an amphoteric oxide) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.65 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 134 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 122±4 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 139 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) (hP2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constants | a = 266.46 pm c = 494.55 pm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 30.08×10−6/K (at 20 °C)[a] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 116 W/(m⋅K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 59.0 nΩ⋅m (at 20 °C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −11.4×10−6 cm3/mol (298 K)[4] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 108 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 43 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 70 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 3850 m/s (at r.t.) (rolled) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.25 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 327–412 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-66-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Indian metallurgists (before 1000 BCE) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Andreas Sigismund Marggraf (1746) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recognized as a unique metal by | Rasaratna Samuccaya (1300) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of zinc | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zinc (Template:PronEng, from German: Zink) is a metallic chemical element with the symbol Zn and atomic number 30. It is a first-row transition metal of the group 12 of the periodic table. Although zinc was used in the copper-zinc alloy brass since Roman times, and the metal was produced in large scale in India around 1200 AD, the pure metal was unknown to Europe until the end of the 16th century. Industrial-scale production in Europe had not started until the late 18th century. Corrosion-resistant zinc plating of steel is the major application for zinc. Other applications are in batteries and alloys, such as brass. Sphalerite, a zinc sulfide, is the most important zinc ore. Zinc production includes roasting, leaching and, at the end, pyrometallurgic winning or electrowinning.
Zinc is an essential mineral, necessary for sustaining all life. Enzymes with a zinc atom in the reactive center are widespread in biochemistry, such as the alcohol dehydrogenase in humans. Consumption of higher concentrations of zinc can cause ataxia, lethargy and copper deficiency.
A variety of zinc compounds find use industrially, such as zinc chloride (in deodorants), zinc pyrithione (anti-dandruff shampoos), zinc sulfide (in luminescent paints), and zinc methyl or zinc diethyl in the organic laboratory. Roughly one quarter of all zinc output is consumed in the form of zinc compounds.
Characteristics
Physical
Zinc, also referred to in nonscientific contexts as spelter,[6] is a bluish-white and lustrous metal,[7] though most common commercial grades of the metal have a dull finish.[8] Most of the common compounds of zinc are white (colorless when liquid).
The metal is hard and brittle at most temperatures but becomes malleable between 100 and 150 °C.[7][8] Above 210 °C, the metal becomes brittle again and can be pulverized by beating.[9] Zinc is a fair conductor of electricity.[7] For a metal, zinc has relatively low melting and boiling points. Melting at 420 °C, and boiling at 900 °C,[10] it has the lowest melting point of all the transition metals aside from mercury and cadmium.
Many alloys contain zinc, including brass, an alloy of zinc and copper. Other metals long known to form binary alloys with zinc are aluminium, antimony, bismuth, gold, iron, lead, mercury, silver, tin, magnesium, cobalt, nickel, tellurium and sodium.[11] While neither zinc nor zirconium are ferromagnetic, the alloy ZrZn2 exhibits ferromagnetism below 35 K.[7]
Isotopes
Naturally occurring zinc is composed of 5 stable isotopes: 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn. Although it slowly decays, 64Zn is considered stable because its half-life is over 2.3×1018 years.[note 1] The most abundant isotope is 64Zn at 48.6% of all the zinc found in nature, and the least abundant is 70Zn at 0.6%. A number of 25 radioisotopes have been characterized, ranging in atomic mass from 54 to 83. The most stable of these are 65Zn with a half-life of 244.26 days and 72Zn with a half-life of 46.5 hours. The least stable of those whose half-lives have been measured are 55Zn and 56Zn with half-lives of 20 ms and 36 ms, respectively. Zinc also has at least 9 meta states ranging in atomic mass from 61 to 78. 61Zn has three excitation states, the highest of which has a half-life of 130 ms.[12]
Zinc has been proposed as a salting material for nuclear weapons (cobalt is another, better-known salting material). A jacket of isotopically enriched 64Zn, irradiated by the intense high-energy neutron flux from an exploding thermonuclear weapon, would transmute into the radioactive isotope 65Zn with a half-life of 244 days and produce approximately 2.27 MeV of gamma radiation, significantly increasing the radioactivity of the weapon's fallout for several days. Such a weapon is not known to have ever been built, tested, or used.[13]
Occurrence
Zinc makes up about 75 ppm (0.007%) of the Earth's crust, making it the 24th most abundant element there.[14] Soil contains an average of 64 ppm, but actual soils have 5–770 ppm zinc.[14] Seawater has only 30 ppb zinc and the atmosphere contains 0.1 to 4 mg/ml.[14]
The most heavily-mined zinc-containing ore is sphalerite, which is a form of zinc sulfide. Other minerals from which zinc is extracted include smithsonite (zinc carbonate), hemimorphite (zinc silicate), wurtzite (another zinc sulfide), and sometimes hydrozincite (basic zinc carbonate).[15]
Exploitable reserves of zinc exceed 100 million tonnes[15] but, at the current rate of consumption, these reserves are estimated to be depleted by 2055.[16] Based on data gathered by the United States Geological Survey and the University of Augsburg, the available supply has also been estimated to last for 20 to 30 years.[17]
Chemistry and compounds
Zinc is a late, first-row transition metal, and a member of the group 12 elements of the periodic table. It a moderately reactive metal that is covered by a protective thin layer of basic carbonate in air, which tarnishes in moist air. It can also burn in air with a bright bluish-green flame, giving off fumes of zinc oxide. Zinc reacts with acids, alkalis and other non-metals.[18] If not completely pure, zinc reacts with dilute acids to release hydrogen. Zinc metal reacts with hydrochloric and sulfuric acid by respectively forming the soluble salts zinc chloride and zinc sulphate via the following reactions:[19]
- 2 Zn + 2 HCl → 2 ZnCl + H2
- Zn + H2SO4 → ZnSO4 + H2
The one common oxidation state of zinc is +2, forming a typical Zn2+ metallic ion.[8] Rare examples of a +1 oxidation state can also be found.[20]
| -1 anion | -2 anion | -3 anion |
|---|---|---|
| Zn2+ + 2Cl− → ZnCl2 | Zn2+ + O2− → ZnO | 3 Zn2+ + 2N3− → Zn3N2 |
| Zn2+ + 2(OH)− → Zn(OH)2 | Zn2+ + (SO4)2− → ZnSO4 | 3 Zn2+ + 2(PO4)3− → Zn3(PO4)2 |

Binary compounds of zinc with nonmetals are well known. Of the four halides ZnF
2 has the most ionic character, whereas the others, ZnCl
2, ZnBr
2, and ZnI
2, have relatively low melting points and are considered to have considerable covalent character. The chalcogenides ZnO, ZnSe, and ZnTe, the peroxide ZnO
2, the hydride ZnH
2, the pnictogenides Zn
3N
2, Zn
3P
2, Zn
3As
2 and Zn
3Sb
2, and the carbide Zn
2C are also known.
In weak basic solutions containing Zn2+ ions, the hydroxide Zn(OH)
2 forms as a white precipitate. In stronger alkaline solutions, this hydroxide is dissolved to form zincates ([Zn(OH)]2-
4). Other compounds of zinc with arsenic include zinc arsenite (Zn[AsO2]2), a colorless powder, and zinc arsenate octahydrate (Zn[AsO4]2•8H2O), a white powder also referred to as koettigite. The latter two are both used in insecticides and wood preservatives.[21] Other inorganic compounds of zinc include the nitrate Zn(NO
3)
2 (used as oxidizing agent, the chlorate Zn(ClO
3)
2, the sulfate ZnSO
4 (known as "white vitriol"), the phosphate Zn
3(PO
4)
2 (used as primer pigment), the molibdate ZnMoO
4 (used as white pigment), the cyanide [[Zinc cyanide|Zn(CN)
2 (used as a catalyst in a few organic recations[22]) and the chromate ZnCrO
4 (one of the few colored zinc compounds). An example of an organic compound of zinc is the acetate Zn(O
2CCH
3)
2, which has several medicinal applications.

In solution most of the zinc compounds form octahedral or tetrahedral complex, for example [Zn(CN)]2-
4, [Zn(H
2O)]2+
6.[18] While the white insoluble zinc oxide exhibits the cubic wurtzite structure, black zinc sulfide exhibits a hexagonal Zincblende structure.[18] Zinc carbonate, a white powder, forms a rhombohedral crystal structure.[21]

Organozinc compounds are those that contain zinc-carbon covalent bonds. Diethylzinc ((C
2H
5)
2Zn) is an important reagent in synthetic chemistry. It was first reported in 1848 from the reaction of zinc and ethyl iodide, and is the first compound known to contain a metal-carbon sigma bond.[23]
The volatilisation of zinc in combination with zinc chloride at temperatures above 285 °C indicates the formation of Zn2Cl2, a zinc compound with the oxidation state +1.[18] The first such compound containing a zinc-zinc bond observed at room temperature was reported in 2004,[24] followed by several zincocene compounds such as [Zn2(η5-C5Me5)2]- these are organozinc compounds containing metal-carbon pi-bonds.[25]
History

Antiquity
Zinc ores were used to make brass many centuries prior to the discovery of zinc as a separate element. The Book of Genesis, written between the 10th and 5th centuries BC,[26] mentions Tubalcain as an "instructor in every artificer in brass and iron" (Genesis 4:22). Ornaments made of alloys that contain 80 to 90% zinc with lead, iron, antimony, and other metals making up the remainder, have been found that are 2500 years old.[27] A possibly pre-historic statuette containing 87.5% zinc was found in a Dacian archaeological site in Transylvania (modern Romania).[28]
In the West, impure zinc was known since antiquity to exist in the remnants in melting ovens, but it was usually discarded, as it was thought to be worthless. Strabo mentioned an ore from Cyprus that he called pseudo-argyros or "mock silver."[29] The metal was also called Indian tin, tutanego, calamine, and spinter.[27] The Berne zinc tablet is a votive plaque dating to Roman Gaul, probably made from such zinc remnants.

The manufacture of brass was known to the Romans by about 30 BC, using a technique in which powdered calamine (zinc silicate), charcoal and copper were heated together in a crucible.[29] The calamine was reduced and free zinc metal was trapped by the copper, forming an alloy. The resulting calamine brass was then either cast or hammered into shape. Pliny the Elder mentioned an ointment that was probably zinc oxide, which was used to heal wounds and sore eyes.[15]
The zinc mines of Zawar, near Udaipur, India, were active around 400 BC, and there are references to medicinal uses of zinc in the Charaka Samhita, which is believed to have been written as early as 300 BC.[30] An estimated million tonnes of metallic zinc and zinc oxide may have been produced in Zawar from the 12th to 16th centuries.[15]
The Rasaratna Samuccaya, written in approximately the year 800, explains the existence of two types of ores containing zinc metal, one of which is ideal for metal extraction while the other is used for medicinal purposes.[30] Because of the low boiling point and high chemical reactivity of this metal, the true nature of it may not have been fully understood in ancient times.
Smelting and extraction of impure zinc by reducing calamine with wool and other organic substances was accomplished in the 13th century in India.[31][7] Marco Polo documented the production of zinc oxide in Persia also in the 13th century.[15] The Hindu king Madana Pala may have recognized zinc as a metal as early as 1374.[28] The Chinese did not learn of the technique until the 17th century.[31]
The name of the metal was probably first used by Paracelsus, a Swiss-born German chemist, who referred to the metal as "zincum" or "zinken" in his book Liber Mineralium II, in the 16th century.[32][31] The word is probably derived from the German [Zinke] Error: {{Lang}}: text has italic markup (help), and supposedly meant "tooth-like, pointed or jagged part" and, as metallic zinc crystals are needle-like, the derivation appears plausible.[33][note 2]
Pure zinc
In 1597, German metallurgist Andreas Libavius received a quantity of zinc metal in its pure form, which was unknown in the West before then. Libavius referred to it as Indian lead and Malabar lead. It was regularly imported to Europe from the Orient in the 17th and early 18th centuries,[31] but was at times very expensive.[note 3] The sailors may have brought the secret of its smelting,[34] but evidence of this is lacking.
The isolation of metallic zinc in the West may have been achieved independently by several people:
- Flemish metallurgist P.M. de Respour reported that he extracted metallic zinc from zinc oxide in 1668.[15] By the turn of the century, Étienne François Geoffroy described how zinc oxide condenses as yellow crystals on bars of iron placed above zinc ore being smelted.[15]
- Dr. John Lane is said to have carried out experiments, probably at Landore, prior to his bankruptcy in 1726.[35] Postlewayt's Universal Dictionary, a contemporary source giving technological information in Europe, did not mention zinc before 1751.[30]
- In 1738, William Champion patented in Great Britain a process to extract zinc from calamine in a vertical retort style smelter, using a technology somewhat similar to that used at Zawar zinc mines in Rajasthan. However, there is no evidence that he visited the Orient.[36] Champion's process was used through 1851.[31]
- The discovery of pure metallic zinc is often credited to the German Andreas Marggraf in 1746.[31] In his experiment, Marggraf heated a mixture of calamine and charcoal in a closed vessel without copper to obtain a metal that did not match the properties of any known metal.[37] This procedure was made to be commercially practical by 1752.[38]
In 1758, William Champion's brother, John, patented a new process for calcining zinc sulfide into an oxide for use in the retort process.[27] Prior to this only calamine could be used to produce zinc. This process was then used into the 20th century. In 1798, Johann Ruberg built the first horizontal retort smelter in Upper Silesia. This was much more fuel efficient and less labor intensive than the vertical retort process. Jean-Jacques Daniel Dony built a different kind of horizontal zinc smelter in Belgium, which processes more.[31] The gas-fired horizontal retort distillation process did not become successful until 1878 and the electrolytic process was not developed until 1916.[27][clarification needed]
Production
Zinc is the fourth most common metal in use, trailing only iron, aluminium, and copper with an annual production of about 10 million tonnes.[39] The world's largest zinc producer is Nyrstar, a merger of the Australian Zinifex and the Belgian Umicore.[40] About 70% of the world's zinc originates from mining, while the remaining 30% comes from recycling secondary zinc.[41]

There are zinc mines throughout the world, with the main mining areas being China, Australia and Peru.[39] In 2005, China produced almost one-fourth of the global zinc output, reports the British Geological Survey. Zinc mines and refineries in Europe include Tara, Galmoy and Lisheen in Ireland and Zinkgruvan in Sweden.
Zinc metal is produced using extractive metallurgy. Worldwide, 95% of the zinc is mined from sulfidic ore deposits, in which sphalerite ZnS is nearly always mixed with the sulfides of copper, lead and iron. After grinding the ore froth flotation, which selectively separates minerals from gangue by taking advantage of differences in their hydrophobicity, is used to get a ore concentrate. A final concentration of zinc in the range of 40–60% is reached by this process. Roasting converts zinc sulfide to zinc oxide.[43]
- 2 ZnS + 3 O2 → 2 ZnO + 2 SO2
| Rank | Country | tonnes |
|---|---|---|
| 1 | 2,600,000 | |
| 2 | 1,338,000 | |
| 3 | 1,201,794 | |
| 4 | 727,000 | |
| 5 | 710,000 | |
| 6 | 480,000 | |
| 7 | 425,700 | |
| 8 | 420,800 | |
| 9 | 400,000 | |
| 10 | 192,400 |
The sulfur dioxide is used for the production of sulfuric acid, which is necessary for the leaching process. If deposits of zinc carbonate, zinc silicate or zinc spinel, like the Skorpion Deposit in Namibia are used for zinc production the roasting can be omitted.[44] For further processing two basic methods are used pyrometallurgy or electrowinning. The first is the older process in which the zinc oxide is reduced by carbon or carbon monoxide at high temperatures of 950 °C (1,740 °F) into the metal which is distilled of as zinc vapour. The zinc vapour is collected in a condenser.[43]
- 2 ZnO + C → 2 Zn + CO2
- 2 ZnO + 2 CO → 2 Zn + 2 CO2
For the second process the zinc is leached from the ore concentrate by sulfuric acid. The following electrolysis makes it necessary to precipitate the also leached sulfates of other metals, like iron, nickel and copper. After this step electrolysis is used to produce the zinc metal.[43]
- ZnO + H2SO4 → ZnSO4 + H2O
- ZnSO4 + H2O → Zn + H2SO4
Commercially pure zinc is known as Special High Grade, often abbreviated SHG, and is 99.995% pure.[45]
Environmental impact
The production of zinc which is often combined with the production of lead results in environmental problems, because the production for sulfidic ores produces large amounts of sulfur dioxide and cadmium vapor and the smelter slag and other residues contain significant amounts of heavy metals. The Belgian towns of La Calamine and Plombières are good examples for the environmental impacts of the industrial mining and smelting of 1,100,000 tonnes of metallic zinc and 130,000 tonnes of lead between 1806 and 1882.[46] The dumps of the mining operations leach significant amounts of zinc and cadmium and the sediments of the Gaul river contain significant amounts of heavy metals.[46]
Applications
Galvanization

The most important application of zinc and the largest use of zinc metal is galvanization, which is the coating of iron or steel as protection against corrosion.[14] In 2006 in the United States, 56% or 773,000 tonnes of the zinc metal was used for this purpose,[47] while worldwide 47% was used for this purpose.[48]
Although zinc is more reactive than iron or steel it forms a protective oxide and carbonate (Zn5(OH)6(CO3)2) layer which protects the material underneath from oxygen and water. This protection lasts even after the zinc layer is scratched but degrades through time as the zinc corrodes away. Similar corrosion resistance can be achieved by plating with tin or cadmium.
The zinc is applied electrochemically or as molten zinc by hot-dip galvanizing or spraying.[14] The galvanization zinc layer is thinner when applied by hot-dipping, but hot dipping induces a thermal stress in the material, because the molten zinc has a temperature of 460 °C.
Zinc is used on galvanized steel as a sacrificial anode for underground pipelines, chain-link fencing, guard rails, suspension bridges, lightposts, metal roofs, heat exchangers, and car bodies.[14]
Alloys
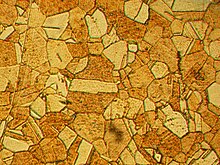
An important alloy of zinc is brass, in which copper is alloyed with anywhere from 9% to 45% zinc, depending upon the type of brass, along with much smaller amounts of lead and tin. Other widely used alloys contain zinc, including nickel silver, typewriter metal, soft and aluminum solder, and commercial bronze.[7] Zinc is also used in contemporary pipe organs as a substitute for the traditional lead/tin alloy in pipes.[49] Alloys of 85–88% zinc, 4–10% copper, and 2–8% aluminium find limited use in certain types of machine bearings. Zinc is the primary metal used in making American one cent coins since 1982.[50] The zinc core is coated with a thin layer of copper to give the impression of a copper coin. In 1994, 33,200 tons of zinc were used to produce 13.6 billion pennies.[51]
Alloys of primarily zinc with small amounts of copper, aluminium, and magnesium are useful in die casting as well as spin casting, especially in the automotive, electrical, and hardware industries.[7] These alloys are marketed under the name Zamak.[52] An example of this is zinc aluminium. The low boiling point together with the low viscosity of the alloy makes the production of small and intricate shapes possible. The low working temperature leads to rapid cooling of the casted products and therefore fast assembly is possible.[48][53][7] Another alloy, marketed under the name Prestal, contains 78% zinc and 22% aluminium and is reported to be nearly as strong as steel but as malleable as plastic.[7] This superplasticity of the alloy allows it to be molded using die casts made of ceramics and cement.[7]
Similar alloys with the addition of a small amount of lead can be cold-rolled into sheets. An alloy of 96% zinc and 4% aluminium is used to make stamping dies for low production run applications for which ferrous metal dies would be too expensive.[54] In building facades, roofs or other applications in which zinc is used as sheet metal and for methods such as deep drawing, roll forming or bending, zinc alloys with titanium and copper are used. Unalloyed zinc is too brittle for these kinds of manufacturing processes.[55]
Cadmium zinc telluride (CZT) is a semiconductive alloy. It can be divided into an array of small sensing devices, similar to an integrated circuit, which can detect the energy levels of incoming gamma rays. When placed behind an absorbing mask, the CZT sensor array can also be used to determine the direction of the rays.[56]
Batteries and fuel cells
With a electrochemical potential of -0.7628 volts, zinc make a good material for anode materials. Zinc is used as part of batteries. The most widespread such use is as the anode in alkaline batteries and the similar zinc-carbon batteries, in which (for both types) the oxidation of the zinc is the reaction at the anode.[57][58]
Zinc is used as the anode or fuel of the zinc-air battery/fuel cell providing the basis of the theorised zinc economy.[59][60][61] Zinc is used as a sacrificial anode on boats and ships that use cathodic protection to prevent corrosion of metals that are exposed to sea water.[62]
Other industrial uses

Roughly one quarter of all zinc output is consumed in the form of zinc compounds; a variety of which are used industrially. Zinc oxide is widely used as a white pigment in paints, and as a catalyst in the manufacture of rubber.[14] It is also used as a heat disperser for the rubber and acts to protect its polymers from ultra violate radiation (the same UV protection is conferred to plastics containing zinc oxide).[14]
Zinc chloride is often added to lumber as a fire retardant[19] and can be used as a wood preservative.[63] It is also used to make other chemicals.[19] Zinc methyl (Zn(CH3)2) is used in a number of organic syntheses since its discovery by Edward Frankland in 1849.[64]
Sulfur-containing compounds are also important. Zinc sulfide is used in luminescent pigments such as on the hands of clocks, x-ray and television screens, and luminous paints.[65] Zinc sulphate is an important chemical in dyes and pigments.[19] Zinc pyrithione is used as in antifouling paints.[66]
The zinc zinc-oxide cycle is a two step thermochemical process based on zinc and zinc oxide for hydrogen production[67] with a typical efficiency around 40%.[68]
Zinc powder is sometimes used as a propellant in model rockets.[69] When a compressed mixture of 70% zinc and 30% sulfur powder is ignited there is a violent chemical reaction. This produces zinc sulfide, together with large amounts of hot gas, heat, and light.[69]
Zinc sheet metal is used in making contemporary zinc bars.[70]
Medicinal

Zinc is included in most single tablet over-the-counter daily vitamin and mineral supplements. It is believed to possess antioxidant properties, which protect against premature aging of the skin and muscles of the body. In larger amounts, taken as zinc alone in other proprietaries, it is believed by some to speed up the healing process after an injury. Preparations include zinc acetate and zinc gluconate.
As an over-the-counter ointment, zinc oxide is applied as a thin coating on the exposed skin of the face or nose to prevent dehydration of the area of skin. It can protect against sunburn in the summer and windburn in the winter.[29] Applied thinly to a baby's diaper area (perineum) with each diaper change, it can protect against rash.[29]
As determined in the Age-Related Eye Disease Study, it can be part of an effective treatment for age-related macular degeneration.[71] Zinc supplementation is also an effective treatment for acrodermatitis enteropathica, a genetic disorder affecting zinc absorption that was previously fatal to babies born with it.[29]
Lotions made of calamine, a mix of Zn-(hydroxy-)carbonates and silicates, are used to treat skin rash. Zinc gluconate glycine and zinc acetate are used in throat lozenges or tablets to reduce the duration and the severity of cold symptoms.[72] Zinc chloride is used as a deodorant.
Zinc lactate is used in toothpaste to prevent halitosis.[73] Zinc pyrithione is widely applied in shampoos because of its anti-dandruff function.[74] Zineb a polymeric zinc dithiocarbamate complex is used as a fungicide.[75]
Zinc salts are effective against pathogens in direct application. Gastroenteritis is strongly attenuated by ingestion of zinc, and this effect could be due to direct antimicrobial action of the zinc ions in the GI tract, or to the absorption of the zinc and re-release from immune cells (all granulocytes secrete zinc), or both.[76][77][note 4]
Biological role
Uptake and use
Zinc is an essential trace element, necessary for sustaining all animal life and is thought to protect plants from drought and disease.[78] Concentrations of zinc in humans are highest in the eye, kidney, liver, muscle, and prostrate.[78] It is particularly rich in semen and is a key factor in prostate gland function and reproductive organ growth.[78]
It is absorbed 15 to 40 percent in the intestines, with higher absorption when zinc status is low. Once absorbed, it may be held in metallothionein reserves within the intestines or the liver. Excess zinc is also stored in bones and the spleen but it can not be easily released from these tissues to make up for a dietary deficiency.[78] About 1% of total zinc is lost from the body per day; 90% of that is excreted through the intestines and the rest via urine (5%) and sweat (5%).[78]
Zinc is transported through the blood by albumin and transferrin. Since transferrin also transports iron, excessive iron reduces zinc absorption, and vice-versa. A similar situation exists with zinc and copper.[79] Plasma zinc concentrations are insensitive indicators of zinc status since a narrow homeostatic range is maintained in the body.[80]: 447

An estimated 3,000 proteins in the human body contain zinc prosthetic groups. The most-important types of protein that contain zinc are enzymes and transcription factors; two examples are metalloenzyme and zinc finger, respectively. There are over 200 enzymes that contain zinc as a cofactor and about the same number of transcription factors.[78] Zinc-containing enzymes are used by the body to regulate growth and development, promote fertility, and aid digestion and nucleic acid synthesis.[81]
Carbonic anhydrase uses zinc to quickly catalyze the waste gas carbon dioxide into bicarbonate and protons in vertebrate blood.[81] The non-related β-carbonic anhydrase is required in plants for leaf formation, the synthesis of indole acetic acid (auxin) and anaerobic respiration (alcoholic fermentation).[82] Alcohol dehydrogenase uses zinc in the liver to metabolize alcohol.[29]
Zinc is a good lewis acid, making it a useful catalytic agent in hydroxylation and other enzymatic reactions. Also zinc has a flexible coordination geometry, allowing enzymes using zinc to rapidly shift conformations and perform biological reactions.[83]
In addition, there are over a dozen types of cells in the human body that secrete zinc ions, and the roles of these secreted zinc signals in medicine and health are now being actively studied. Zinc is also recycled through the pancreas, which secretes zinc-containing enzymes into the intestines at mealtimes in a process called enteropancreatic circulation.
Zinc ions are now considered to be neurotransmitters[citation needed]. Cells in the salivary gland, prostate, immune system and intestine use zinc signaling.[84] Zinc is especially needed by tissues that have a high rate of new cell development, such as bone marrow, immune system cells and the lining of the gut.[81] Zinc is involved in olfaction: the olfactory receptors contain zinc binding sites and a deficiency in zinc causes anosmia.[citation needed]
As of 2005 an effective measure of zinc status in humans "remained elusive", although a method involving reverse transcription polymerase chain reaction showed promise.[85][clarification needed]
Food sources

| Food | mg Zn/100 g |
|---|---|
| oysters | 7 |
| liver | 6 |
| beef | 4 |
| wheat | 4 |
| cheese | 3 |
| shrimp | 2 |
| eggs | 1 |
| milk | 0.4 |
| fruit | ~0.15 |
Red meats, especially beef, lamb and liver have some of the highest concentrations of zinc in food.[81] A turkey's neck and beef's chuck or shank also contain significant amounts of zinc. Tubers, such as potatoes or cassava, have very low levels of zinc.[81]
The concentration of zinc in plants varies based on levels of the element in soil. When there is adequate zinc in the soil, the food plants that contain the most zinc are wheat (20–60 ppm), sweet corn (about 20 ppm) and lettuce (12 ppm) while fruits have the lowest zinc content; apples and oranges only contain 1 ppm zinc or less (all fresh weights).[78] Zinc is also found in beans, nuts, almonds, whole grains, pumpkin seeds, sunflower seeds and blackcurrant.[86] Soil conservation is needed to make sure that crop rotation will not deplete the zinc in soil.
Other sources include fortified food and dietary supplements, which come in various forms. A 1998 review concluded that zinc oxide, one of the most common supplements in the United States, and zinc carbonate are nearly insoluble and poorly absorbed, and cited studies which found low plasma zinc concentrations after zinc oxide and zinc carbonate consumption relative to the plasma concentrations seen after consumption of zinc acetate and sulfate salts.[87] For fortification, however, a 2003 review recommended zinc oxide in cereals as cheap, stable, and as easily absorbed as more expensive forms.[88] A 2005 study found that various compounds of zinc, including oxide and sulfate, did not show statistically significant differences in absorption when added as fortificants to maize tortillas.[89]
Deficiency
Zinc deficiency occurs where insufficient zinc is available for metabolic needs. It is usually nutritional, but can also be associated with malabsorption, acrodermatitis enteropathica, chronic liver disease, chronic renal disease, sickle cell disease, diabetes, malignancy, and other chronic illnesses. Phytic acid decreases zinc absorption by forming insoluble zinc phytate.[81] So diets rich in foods that contain a lot of phytic acid, such as cereals and legumes, decreases zinc absorption into the body.
The minimum absorption of zinc needed to avoid deficiency in humans is 2–3 mg per day but not all zinc ingested can be absorbed by the body. Assuming 30% of zinc ingested is absorbed for a particular diet, then men require 7.5 mg of zinc in their food per day and women need 5.5 mg.[81] An average diet contains about 10 mg zinc per day for men and around 8 mg per day for women.[81] Absorption of zinc during pregnancy and lactation increases, resulting in sufficient zinc assuming an average diet is maintained.[81][note 5]
Animals with a diet deficient in zinc require twice as much food to attain the same weight gain as animals given sufficient zinc.[65] Symptoms of chronic zinc deficiency are stunted growth and retarded sexual development.[29] The condition may also be on factor responsible for low sperm counts.[78]
Zinc has been identified as one of ten major factors contributing to disease in developing nations. About half of the world's population is at risk for zinc deficiency.[81] In Southeast Asia and sub-Saharan Africa, zinc intake is inadequate for one-third of the population and stunted growth affects 40% of children. Although the case for food fortification or dietary supplementation seems strong, it has been relatively neglected; the World Health Organization only advocates zinc supplementation for severe malnutrition and diarrhea. Evidence suggests that zinc supplements prevent disease and reduce mortality, especially among children with low-birth weight or stunted growth. However, zinc supplements should not be administered alone, since many in the developing world have several deficiencies, and zinc interacts with other micronutrients.[90]
Precautions
Toxicity
Even though zinc is a very essential requirement for a healthy body, excess zinc can be harmful. Excessive absorption of zinc can also suppress copper and iron absorption. The free zinc ion in solution is highly toxic to plants, invertebrates, and even vertebrate fish. The Free Ion Activity Model (FIAM) is well-established in the literature, and shows that just micromolar amounts of the free ion kills some organisms. A recent example showed 6 micromolar killing 93% of all Daphnia in water.[91]
The free zinc ion is also a powerful Lewis acid up to the point of being corrosive. Stomach acid contains hydrochloric acid, in which metallic zinc dissolves readily to give corrosive zinc chloride. Swallowing a post-1982 American one cent piece (97.5% zinc) can cause damage to the stomach lining due to the high solubility of the zinc ion in the acidic stomach.[92]
There is evidence of induced copper deficiency at low intakes of 100–300 mg Zn/d. The USDA RDA is 15 mg Zn/d. Even lower levels, closer to the RDA, may interfere with the utilization of copper and iron or to adversely affect cholesterol.[93]
There is also a condition called the zinc shakes or "zinc chills" that can be induced by the inhalation of freshly formed zinc oxide formed during the welding of galvanized materials.[65]
Poisoning
In 1983, the United States Mint began minting pennies coated in copper but made primarily of zinc. With the new zinc pennies, there is the potential for zinc toxicosis, which can be fatal. One reported case of chronic ingestion of 425 pennies (over 1 kg of zinc) resulted in death due to gastrointestinal bacterial and fungal sepsis, while another patient, who ingested 12 grams of zinc, only showed lethargy and ataxia (gross lack of coordination of muscle movements).[94] Several other cases are reported of humans suffering zinc intoxication by the ingestion of zinc coins.[95][96]
Pennies and other small coins are sometimes ingested by dogs, resulting in the need for medical treatment to remove the foreign body. The zinc content of some coins can also cause zinc toxicity, which is commonly fatal in dogs, where it causes a severe hemolytic anemia, also liver or kidney damage; vomiting and diarrhoea are possible symptoms.[97][98][99] Zinc is highly toxic in parrots and poisoning can often be fatal.[100] Storing fruit juices in galvanized cans has resulted in mass poisonings of zinc.[29]
Notes
- ^ For comparison, the age of the Universe is believed to be less than 1.4×1010 years, or less than a millionth the time required for a significant amount of 64Zn to decompose.
- ^ Some sources say that a "Löhneyes" first used the term "Zinc" in 1697.(Lehto 1968, p. 822)
- ^ An East India Company ship carrying a cargo of nearly pure zinc metal from the Orient sank off the coast Sweden in 1745.(Emsley 2001, p. 502)
- ^ In clinical trials, both zinc gluconate and zinc gluconate glycine (the formulation used in lozenges) have been shown to shorten the duration of symptoms of the common cold.
- Godfrey, J. C. (1996). "Zinc for treating the common cold: Review of all clinical trials since 1984". Alternative Therapies in Health and Medicine. PMID 8942045.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
- Hulisz, Darrell T. "Zinc and the Common Cold: What Pharmacists Need to Know". uspharmacist.com. Retrieved 2008-11-28.
- Godfrey, J. C. (1996). "Zinc for treating the common cold: Review of all clinical trials since 1984". Alternative Therapies in Health and Medicine. PMID 8942045.
- ^ Human breast milk contains the 2 mg zinc needed by the baby each day but milk from cows needs to be fortified in zinc to provide adequate nutrition for the baby.(Emsley 2001, p. 500)
References
- ^ "Standard Atomic Weights: Zinc". CIAAW. 2007.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "Spelter". American Heritage Dictionary of the English Language. Houghton Mifflin. 2000. Retrieved 2008-11-29.
- ^ a b c d e f g h i j CRC 2006, p. 4-41
- ^ a b c d Heiserman 1992, p. 123
- ^ Scoffern, John (1861). The Useful Metals and Their Alloys (in German). Houlston and Wright.
- ^ http://www.galvanizeit.org/aga/designing-fabricating/design-considerations/zinc-metal-properties
- ^ Walter Renton Ingalls (1902). Production and Properties of Zinc: A Treatise on the Occurrence and Distribution of Zinc Ore, the Commercial and Technical Conditions Affecting the Production of the Spelter, Its Chemical and Physical Properties and Uses in the Arts, Together with a Historical and Statistical Review of the Industry. The Engineering and mining journal. pp. 142–146.
- ^ Audi, Georges (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A. 729. Atomic Mass Data Center: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ Win, David Tin (2003). "Weapons of Mass Destruction" (PDF). AU Journal of Technology. 6 (4). Assumption University: 199–219.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h Emsley 2001, p. 503
- ^ a b c d e f g Emsley 2001, p. 502
- ^ Cohen, David (2007). "Earth audit". New Scientist. 194 (2605): 8.
- ^ "Augsberg University Calculate When Our Materials Run Out". IDTechEx. 2007-06-04. Retrieved 2008-12-09.
- ^ a b c d Holleman, Arnold F. (1985). "Zink". Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 1034–1041. ISBN 3-11-007511-3.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: extra punctuation (link) Cite error: The named reference "Holl" was defined multiple times with different content (see the help page). - ^ a b c d Heiserman 1992, p. 124
- ^ Gmelin, Leopold (1853). "Zink". Handbuch der anorganischen Chemie (in German). Vol. 3. Karl Winter. pp. 1–49.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Perry, D. L. (1995). Handbook of Inorganic Compounds. CRC Press. pp. 448–458. ISBN 0-8492-8671-3.
{{cite book}}: Check|isbn=value: checksum (help) - ^ Rasmussen J. K., Heilmann S. M. (1990). "In situ Cyanosilylation of Carbonyl Compounds: O-Trimethylsilyl-4-Methoxymandelonitrile". Organic Syntheses, Collected Volume]. 7: 521.
- ^ E. Frankland (1850). "On the isolation of the organic radicals". Quarterly Journal of the Chemical Society. 2: 263. doi:10.1039/QJ8500200263.
- ^ Parkin, G (2004). "Chemistry. Zinc-zinc bonds: a new frontier". Science (New York, N.Y.). 305 (5687): 1117–8. doi:10.1126/science.1102500. PMID 15326345.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ del Río, Diego; Galindo, Agustín; Resa, Irene; Carmona, Ernesto (2005). "Theoretical and synthetic studies on Zn2(eta5-C5Me5)2: analysis of the Zn-Zn bonding interaction". Angewandte Chemie (International ed. In English). 44 (8): 1244–7. doi:10.1002/anie.200462175. PMID 15662655.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lerner, Gerda (2003). "Religion and the Creation of Feminist Consciousness". Harvard Divinity Bulletin. 23 (1).
- ^ a b c d Lehto 1968, p. 822
- ^ a b Weeks 1933, p. 20
- ^ a b c d e f g h Emsley 2001, p. 501
- ^ a b c Craddock, P. T. (1983). "Zinc production in medieval India". World Archaeology. 15 (2): 211–21.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h Habashi, Fathi. "Discovering the 8th Metal" (PDF). International Zinc Association (IZA). Retrieved 2008-12-13.
- ^ . p. 409. ISBN 0766131971.
{{cite book}}: Missing or empty|title=(help) - ^ http://www.etymonline.com/index.php?search=zinc&searchmode=term
- ^ Metals Reference and Encyclopedia (Atlas Publishing Co, 1968).
- ^ R. O. Roberts, 'Dr John Lane and the foundation of the non-ferrous metal industry in the Swansea valley' Gower 4 (1951), 19–24; F. V. Emery, 'Further light on Dr John Lane' Gower 20 (1969), 8–13; R. O. Roberts, 'Further note on Dr John Lane' Gower 22 (1972), 23-5.
- ^ Rhys Jenkins, 'The Zinc Industry in England: the early years up to 1850' Transactions of the Newcomen Society 25 (1945–7), 41–52.
- ^ Weeks 1933, p. 21
- ^ Heiserman 1992, p. 122
- ^ a b c "Zinc: World Mine Production (zinc content of concentrate) by Country" (PDF). 2006 Minerals Yearbook: Zinc. Washington, D.C.: United States Geological Survey. February 2008. p. Table 15. Retrieved 2009-01-19.
- ^ "Zinifex and Umicore to create largest zinc producer". Retrieved 2008-11-24.
{{cite news}}: Unknown parameter|published=ignored (help) - ^ "Zinc Recycling". International Zinc Association. Retrieved 2008-11-28.
- ^ Jasinski, Stephen M. "Mineral Commodity Summaries 2007: Zinc" (PDF). United States Geological Survey. Retrieved 2008-11-25.
- ^ a b c Porter, Frank C. (1991). Zinc Handbook. CRC Press. ISBN 9780824783402.
- ^ Borg, Gregor (2003). "Geology of the Skorpion Supergene Zinc Deposit, Southern Namibia". Economic Geology. 98 (4): 749–771. doi:10.2113/98.4.749.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Special High Grade Zinc (SHG) 99.995% (PDF), Nyrstar, 2008, retrieved 2008-12-01
- ^ a b Kucha, H. (1996). "Primary minerals of Zn-Pb mining and metallurgical dumps and their environmental behavior at Plombières, Belgium". Environmental Geology. 27 (1): 1. doi:10.1007/BF00770598.
{{cite journal}}: More than one of|pages=and|page=specified (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Tolcin, Amy C. "Mineral Yearbook 2006: Zinc" (PDF). United States Geological Survey.
- ^ a b Panagapko, Doug (2006). "Zinc". Natural Resources Canada. Retrieved 2008-12-12.
- ^ Bush, Douglas Earl (2006). The Organ: An Encyclopedia. Routledge. p. 679. ISBN 9780415941747.
{{cite book}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ "Coin Specifications". United States Mint. Retrieved 2008-10-08.
- ^ Jasinski, Stephen M. "Mineral Yearbook 1994: Zinc" (PDF). United States Geological Survey. Retrieved 2008-11-13.
- ^ Eastern Alloys contributors. "Diecasting Alloys". Maybrook, NY: Eastern Alloys. Retrieved 2009-01-19.
{{cite web}}:|author=has generic name (help) - ^ Apelian, D. (1981). "Casting with Zinc Alloys". Journal of Metals. 33: 12–19.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Samans, Carl Hubert (1949). Engineering Metals and Their Alloys. Macmillan Co.
- ^ Porter, Frank (1994). "Wrought Zinc". Corrosion Resistance of Zinc and Zinc Alloys. CRC Press. pp. 6–7. ISBN 9780824792138.
- ^ Katz, Johnathan I. (2002). The Biggest Bangs. pp. 18 publisher = Oxford University Press. ISBN 0-19-514570-4.
{{cite book}}: Missing pipe in:|pages=(help) - ^ Besenhard, Jürgen O. "Handbook of Battery Materials" (PDF). Retrieved 2008-10-08.
- ^ Wiaux, J. -P. (1995). "Recycling zinc batteries: an economical challenge in consumer waste management". Journal of Power Sources. 57 (1–2): 61–65. doi:10.1016/0378-7753(95)02242-2.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Culter, T. (1996). "A design guide for rechargeable zinc-air battery technology". Southcon/96. Conference Record: 616. doi:10.1109/SOUTHC.1996.535134.
{{cite journal}}: More than one of|pages=and|page=specified (help) - ^ Whartman, Jonathan. "Zinc Air Battery-Battery Hybrid for Powering Electric Scooters and Electric Buses" (PDF). The 15th International Electric Vehicle Symposium. Retrieved 2008-10-08.
{{cite web}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Cooper, J. F. "A refuelable zinc/air battery for fleet electric vehicle propulsion". Society of Automotive Engineers future transportation technology conference and exposition. Retrieved 2008-10-08.
{{cite web}}: More than one of|author=and|last=specified (help) - ^ Bounoughaz, M. (2003). "A comparative study of the electrochemical behaviour of Algerian zinc and a zinc from a commercial sacrificial anode". Journal of Materials Science. 38 (6): 1139–1145. doi:10.1023/A:1022824813564.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Blew, Joseph Oscar (1953). Wood preservatives. Madison, Wis.
- ^ Frankland, Edward (1849). "Notiz über eine neue Reihe organischer Körper, welche Metalle, Phosphor u. s. w. enthalten". Liebig's Annalen der Chemie und Pharmacie (in German). 71 (2): 213–216. doi:10.1002/jlac.18490710206.
- ^ a b c CRC 2006, p. 4-42
- ^ Konstantinou, I. K. (2004). "Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: a review". Environment International. 30 (2): 235–248. doi:10.1016/S0160-4120(03)00176-4.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Weimer, Al (2006-05-17). "Development of Solar-powered Thermochemical Production of Hydrogen from Water" (PDF). U.S. Department of Energy. Retrieved 2009-01-10.
- ^ "Novel Method for Solar Hydrogen Generation" (PDF). ETH Zurich. Retrieved 2009-01-19.
- ^ a b Boudreaux, Kevin A. "Zinc + Sulfur". Angelo State University. Retrieved 2008-10-08.
- ^ Technical Information, Zinc Counters, 2008, retrieved 2008-11-29
- ^ "Age-Related Eye Disease Study Research Group". www.pubmed.gov. Retrieved 2007-11-13.
- ^ Ananda S., Prasad (2000). "Duration of Symptoms and Plasma Cytokine Levels in Patients with the Common Cold Treated with Zinc Acetate: A Randomized, Double-Blind, Placebo-Controlled Trial" (PDF). Annals of Internal Medicine. 133 (4): 245–252.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Roldán, S. (2003). "The effects of a new mouthrinse containing chlorhexidine, cetylpyridinium chloride and zinc lactate on the microflora of oral halitosis patients: a dual-centre, double-blind placebo-controlled study". Journal of Clinical Periodontology. 30 (5): 427–434. doi:10.1034/j.1600-051X.2003.20004.x.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Marks, R. (1985). "The effects of a shampoo containing zinc pyrithione on the control of dandruff". British Journal of Dermatology. 112 (4): 415–422. doi:10.1111/j.1365-2133.1985.tb02314.x.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ullmann's Agrochemicals. Wiley-Vch (COR). 2007. p. 592. ISBN 3527316043.
- ^ Aydemir, T. B. (2006). "Zinc Supplementation of Young Men Alters Metallothionein, Zinc Transporter, and Cytokine Gene Expression in Leucocyte Populations". PNS. 103 (3): 1699–1704. doi:10.1073/pnas.0510407103.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Valko,, M. (2005). "Metals, Toxicity and Oxidative stress". Current Medicinal Chemistry. 12 (12): 1161–1208. doi:10.2174/0929867053764635.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: extra punctuation (link) - ^ a b c d e f g h Emsley 2001, p. 499
- ^ Whitney E, Rolfes SR. (2005). Understanding Nutrition, pp. 447–449. Tenth Edition. Thomson Learning.
- ^ United States National Research Council, Institute of Medicine. (2000). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press. Free full-text
- ^ a b c d e f g h i j k Emsley 2001, p. 500
- ^ Gadallah, M.A.A. (2000). "Effects of indole-3-acetic acid and zinc on the growth, osmotic potential and soluble carbon and nitrogen components of soybean plants growing under water deficit". Journal of Arid Environments. 44 (4): 451–467. doi:10.1006/jare.1999.0610.
- ^ Stipanuk, Martha H. (2006). Biochemical, Physiological & Molecular Aspects of Human Nutrition. W.B. Saunders Company. pp. 1043–1067. ISBN 9780721644523.
- ^ Hershfinkel, Michal (2007). "The Zinc Sensing Receptor, a Link Between Zinc and Cell Signaling". Molecular Medicine. 13 (7–8): 331–336. doi:10.2119/2006-00038.Hershfinkel.. PMID 17728842.
{{cite journal}}: Check|doi=value (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help) - ^ Aydemir, T. B.; Blanchard, R. K.; Cousins, R. J. (2006). "Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations". The Proceedings of the National Academy of Sciences U.S.A. 103 (6): 1699–704. doi:10.1073/pnas.0510407103. PMC 1413653. PMID 16434472.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Zinc content of selected foods per common measure" (PDF). USDA National Nutrient Database for Standard Reference, Release 20. United States Department of Agriculture. Retrieved 2007-12-06.
- ^ Allen, Lindsay H. (1998). "Zinc and micronutrient supplements for children". American Journal of Clinical Nutrition. 68 (2 Suppl): 495S–498S. PMID 9701167.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Rosado, J. L. (2003). "Zinc and copper: proposed fortification levels and recommended zinc compounds". Journal of Nutrition. 133 (9): 2985S–2989S. PMID 12949397.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hotz, C.; DeHaene, J.; Woodhouse, L. R.; Villalpando, S.; Rivera, J. A.; King, J. C. (2005). "Zinc absorption from zinc oxide, zinc sulfate, zinc oxide + EDTA, or sodium-zinc EDTA does not differ when added as fortificants to maize tortillas". Journal of Nutrition. 135 (5): 1102–1105. PMID 15867288.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Shrimpton, R. (2005). "Zinc deficiency: what are the most appropriate interventions?". British Medical Journal. 330 (7487): 347–259. doi:10.1136/bmj.330.7487.347. PMC 548733. PMID 15705693.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Muyssen, Brita, T. A. (2006). "Mechanisms of chronic waterborne Zn toxicity in Daphnia magna". Aquatic Toxicology. 77 (4): 393–401. doi:10.1016/j.aquatox.2006.01.006.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Bothwell, Dawn N. (2003). "Chronic Ingestion of a Zinc-Based Penny". Pediatrics. 111 (3): 689–691. doi:10.1542/peds.111.3.689. PMID 12612262.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fosmire, G. J. (1990). "Zinc toxicity". American Journal of Clinical Nutrition. 51 (2): 225–227. PMID 2407097.
{{cite journal}}: Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help) - ^ Barceloux, Donald G. (1999). "Zinc". Clinical Toxicology. 37 (2): 279–292. doi:10.1081/CLT-100102426.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Bennett, Daniel R. M.D. (1997). "Zinc Toxicity Following Massive Coin Ingestion". American Journal of Forensic Medicine & Pathology. 18 (2): 148–153. doi:10.1097/00000433-199706000-00008.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fernbach, S. K. (1986). "Coin ingestion: unusual appearance of the penny in a child". Radiology. 158 (2): 512–512. PMID 3941880.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help) - ^ Stowe, C. M. (1978). "Zinc phosphide poisoning in dogs". Journal of the American Veterinary Medical Association. 173 (3): 270. PMID 689968.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Stowe C. M., Nelson R., Werdin R., et al: Zinc phosphide poisoning in dogs. JAVMA 173:270, 1978
- ^ Wendy C. Brooks (2008-03-11). "Zinc Poisoning". Pet Health Library. VeterinaryPartner.com. Retrieved 2008-12-09.
- ^ "Helath Risks - For Your Parrot". Pet Parrots 101. 2006-10-09. Retrieved 2009-01-20.
Bibliography
- Craddock, P.T. et al. (1983). Zinc production in medieval India, World Archaeology, vol. 15, no. 2, Industrial Archaeology.
- CRC contributors (2006). David R. Lide (ed.). Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida: CRC Press, Taylor & Francis Group. ISBN 0-8493-0487-3.
{{cite book}}:|author=has generic name (help) - Emsley, John (2001). "Zinc". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 499–505. ISBN 0198503407.
{{cite book}}: CS1 maint: ref duplicates default (link) - Greenwood, N. N. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: ref duplicates default (link) - Heiserman, David L. (1992). "Element 30: Zinc". Exploring Chemical Elements and their Compounds. New York: TAB Books. ISBN 0-8306-3018-X.
{{cite book}}: CS1 maint: ref duplicates default (link) - Lehto, R. S. (1968). "Zinc". In Clifford A. Hampel (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 822–830. LCCN 68-29938.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help)CS1 maint: ref duplicates default (link) - Stwertka, Albert (1998). "Zinc". Guide to the Elements (Revised ed.). Oxford University Press. ISBN 0-19-508083-1.
{{cite book}}: CS1 maint: ref duplicates default (link) - Weeks, Mary Elvira (1933). "III. Some Eighteenth-Century Metals". The Discovery of the Elements. Easton, PA: Journal of Chemical Education. ISBN 0766138720.
{{cite book}}: CS1 maint: ref duplicates default (link)
External links
- Los Alamos National Laboratory - Zinc
- WebElements.com – Zinc
- History & Etymology of Zinc
- Statistics and Information from the U.S. Geological Survey
- Reducing Agents > Zinc
- American Zinc Association Information about the uses and properties of zinc.
- Outline safety data for zinc
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).


