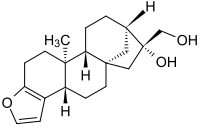
Cafestol
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3bS,5aS,7R,8R,10aR,10bS)-7-(Hydroxymethyl)-10b-methyl-3b,4,5,6,7,8,9,10,10a,10b,11,12-dodecahydro-5a,8-methanocyclohepta[5,6]naphtho[2,1-b]furan-7-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H28O3 | |
| Molar mass | 316.441 g·mol−1 |
| Melting point | 158 to 162 °C (316 to 324 °F; 431 to 435 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cafestol is a diterpenoid molecule present in coffee beans. It is one of the compounds that may be responsible for proposed biological and pharmacological effects of coffee.[1]
Sources
A typical bean of Coffea arabica contains about 0.4-0.7% cafestol by weight.[2] Cafestol is present in highest quantity in unfiltered coffee drinks such as French press coffee or Turkish coffee/Greek coffee. In filtered coffee drinks such as drip brewed coffee, it is present in only negligible amounts, as the paper filter in drip filtered coffee retains the diterpenes.[3]
Research into biological activity
Coffee consumption has been associated with a number of effects on health and cafestol has been proposed to produce these through a number of biological actions.[4] Studies have shown that regular consumption of boiled coffee increases serum cholesterol whereas filtered coffee does not.[5] Cafestol may act as an agonist ligand for the nuclear receptor farnesoid X receptor and pregnane X receptor, blocking cholesterol homeostasis. Thus cafestol can increase cholesterol synthesis.[6]
Cafestol has also shown anticarcinogenic properties in rats.[7]
Cafestol also has neuroprotective effects in a Drosophila fruit fly model of Parkinson's disease.[8]
See also
References
- ^ Ludwig, IA; Clifford, MN; Lean, ME; Ashihara, H; Crozier, A (August 2014). "Coffee: biochemistry and potential impact on health". Food & Function. 5 (8): 1695–717. doi:10.1039/c4fo00042k. PMID 24671262. S2CID 29389074.
- ^ Kitzberger C, Scholz M, Benassi M (2014). "Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions". Food Research International. 61: 61–66. doi:10.1016/j.foodres.2014.04.031.
- ^ Zhang, Chen; Linforth, Robert; Fisk, Ian D. (2012). "Cafestol extraction yield from different coffee brew mechanisms". Food Research International. 49: 27–31. doi:10.1016/j.foodres.2012.06.032.
- ^ Higdon, JV; Frei, B (2006). "Coffee and health: a review of recent human research". Critical Reviews in Food Science and Nutrition. 46 (2): 101–23. doi:10.1080/10408390500400009. PMID 16507475.
- ^ Urgert, R; Katan, MB (1997). "The cholesterol-raising factor from coffee beans". Annual Review of Nutrition. 17: 305–24. doi:10.1146/annurev.nutr.17.1.305. PMC 1295997. PMID 9240930.
- ^ Ricketts ML, Boekschoten MV, Kreeft AJ, Hooiveld GJ, Moen CJ, Müller M, Frants RR, Kasanmoentalib S, Post SM, Princen HM, Porter JG, Katan MB, Hofker MH, Moore DD (2007). "The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors". Molecular Endocrinology. 21 (7): 1603–16. doi:10.1210/me.2007-0133. PMID 17456796.
- ^ National Toxicology Program (NTP): Cafestol (CASRN 469-83-0) and Kahweol (CASRN 6894-43-5) - Review of Toxicological Literature. (PDF) October 1999 Archived November 1, 2004, at the Wayback Machine
- ^ Trinh K, Andrews L, Krause J, Hanak T, Lee D, Gelb M, Pallanck L (April 2010). "Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson's disease through an NRF2-dependent mechanism". J. Neurosci. 30 (16): 5525–32. doi:10.1523/JNEUROSCI.4777-09.2010. PMC 3842467. PMID 20410106.
- Ewen Callaway (23 April 2010). "Parkinson's protection without caffeine or nicotine". New Scientist.
