DADLE
| |
| Names | |
|---|---|
| IUPAC name
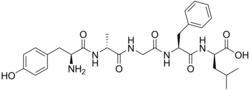
(2R)-2-[[(2S)-2-[[2-[[(2R)-2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]propanoyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoic acid
| |
| Other names
• [D-Ala2, D-Leu5]-Enkephalin
• L-Tyrosyl-D-alanylglycyl-L-phenylalanyl-D-Leucine • N-(N-(N-(N-L-tyrosyl-D-alanyl)glycyl)-L-phenylalanyl)-D-Leucine • Tyr-D-Ala-Gly-Phe-D-Leu-OH | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.059.337 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C29H39N5O7 | |
| Molar mass | 569.659 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
DADLE ([D-Ala2, D-Leu5]-Enkephalin) is a synthetic opioid peptide with analgesic properties. Although it is often considered a selective delta opioid receptor agonist, it also binds to the μ1 subtype of mu opioid receptors.
Treatment with DADLE results in transient depression of mean arterial blood pressure and heart rate.[1][2]
Its peptide sequence is Tyr-D-Ala-Gly-Phe-D-Leu.
See also
References
