Arsenic trioxide

| |

| |
| Names | |
|---|---|
| Other names
Arsenic(III) oxide,
Arsenic sesquioxide, Arsenicum album, Arseneous oxide, Arseneous anhydride, White arsenic[1] | |
| Identifiers | |
| DrugBank | |
| ECHA InfoCard | 100.014.075 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| As2O3 | |
| Molar mass | 197.841 g/mol |
| Appearance | White solid |
| Density | 3.74 g/cm3 |
| Melting point | 312.3°C |
| Boiling point | 465°C |
| 2 g/100 ml (25°C) see text | |
| Solubility | soluble in dilute acids and alkalies, practically insoluble in organic solvents [2] |
| Acidity (pKa) | 9.2 |
| Structure | |
| cubic (α)<180°C monoclinic (β) >180°C | |
| See Text | |
| Zero | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
? J.K–1.mol–1 |
Std enthalpy of
formation (ΔfH⦵298) |
−657.4 kJ/mol |
| Pharmacology | |
| Pharmacokinetics: | |
| 75% bound | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
14.6 mg/kg (rat, oral) |
| Related compounds | |
Other anions
|
Arsenic trisulfide |
Other cations
|
Phosphorus trioxide Antimony trioxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Arsenic trioxide is the inorganic compound with the formula As2O3. This commercially important oxide of arsenic is the main precursor to other arsenic compounds, including organoarsenic compounds. Approximately 50,000 tons are produced annually.[3] Many applications are controversial given the high toxicity of arsenic compounds.
Preparation and properties
Arsenic trioxide can be generated via many routine processing of arsenic compounds including the oxidation (combustion) of arsenic and arsenic-containing minerals in air. Illustrative is the roasting of orpiment, a typical arsenic sulfide ore.
- 2 As2S3 + 9 O2 → 2 As2O3 + 6 SO2
Most arsenic oxide is, however, obtained as a volatile by-product of the processing of other ores. For example arsenopyrite, a common impurity in gold- and copper-containing ores, liberates arsenic trioxide upon heating in air. The processing of such minerals has led to numerous cases of poisonings.[4] Only in China are arsenic ores intentionally mined.[3]
Arsenic trioxide is an amphoteric oxide, and its aqueous solutions are weakly acidic. Thus, it dissolves readily in alkaline solutions to give arsenites. It is less soluble in acids, although it will dissolve in hydrochloric acid, giving chloro compounds, ultimately arsenic trichloride with concentrated acid. Only with strong oxidizing agents such as ozone, hydrogen peroxide, and nitric acid does it give arsenic pentoxide, As2O5. Reduction gives elemental arsenic or arsine (AsH3) depending on conditions. In this regard, arsenic trioxide differs from phosphorus trioxide which readily combusts to phosphorus pentoxide.
Structure
In the liquid and in the gas phase below 800 °C, arsenic trioxide has the formula As4O6 and is isostructural with P4O6). Above 800 °C As4O6 significantly dissociated into molecular As2O3, which adopts the same structure as N2O3. Three forms (polymorphs) are known in the solid state: cubic As4O6, containing molecular As4O6, and two related polymeric forms. The polymers, which both crystallized as monoclinic crystals, feature sheets of pyramidal AsO3 units that share O atoms.[5]
 |
 |
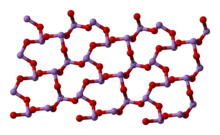
|
(cubic) |
(monoclinic) |
(monoclinic) |
Uses
Large scale applications include its use as a precursor to forestry products, in glass production, and in electronics.[3] Being the main compound of arsenic, the trioxide is the precursor to elemental arsenic, arsenic alloys, and arsenide semiconductors. Organoarsenic compounds, e.g. feed additives (Roxarsone) and pharmaceuticals (Neosalvarsan), are derived from arsenic trioxide. Bulk arsenic-based compounds sodium arsenite and sodium cacodylate are derived from the trioxide.
A variety of applications exploit arsenic's toxicity, including the use of the oxide as a wood preservative. Copper arsenates, which are derived from arsenic trioxide, are used on a large scale as a wood preservative in the US and Malaysia, but such materials are banned in many parts of the world. This practice remains controversial.[3] In combination with copper(II) acetate arsenic trioxide gives the vibrant pigment known as paris green used both in paints and as a rodenticide. This application has been discontinued.
Arsenic trioxide is used for the production of colorless glass.
Medical applications
Despite the well known toxicity of arsenic, arsenic trioxide has long been of biomedical interest, dating to traditional Chinese medicine, where it is known as Pi Shuang and is still used to treat cancer and other conditions.[6] Some discredited patent medicines, e.g. Fowler's solution, contained derivatives of arsenic oxide. Arsenic trioxide under the trade name Trisenox (manufacturer: Cephalon) is a chemotheraputic agent of idiopathic function used to treat leukemia that is unresponsive to "first line" agents. It is suspected that arsenic trioxide induces cancer cells to undergo apoptosis. Due to the toxic nature of arsenic, this drug carries significant risks. Use as a cytostatic in the treatment of refractory promyelocytic (M3) subtype of acute myeloid leukemia.[7][8] The combination therapy of arsenic trioxide and all-trans retinoic acid (ATRA) has been approved by the U.S. FDA for treatment of certain leukemias.[9]
Arsenic trioxide also appears to be a promising therapeutic agent for autoimmune diseases.[10]
The enzyme thioredoxin reductase has recently been identified as a target for arsenic trioxide.[11]
Natural occurrence
Two minerals are known to possess the As2O3 chemical formula: arsenolite(regular) and claudetite (monoclinic). Both are relatively rare secondary minerals found in oxidation zones of As-rich ore deposits (these are often Co-, Ni-, Ag- and U-bearing, too).
Toxicology
- See also: arsenicosis.
Arsenic trioxide is readily absorbed by the digestive system: toxic effects are also well known upon inhalation or upon skin contact. Elimination is rapid at first (half-life of 1–2 days), by methylation to cacodylic acid and excretion in the urine, but a certain amount (30–40% in the case of repeated exposure) is incorporated into the bones, muscles, skin, hair and nails (all tissues rich in keratin) and eliminated over a period of weeks or months.
The first symptoms of acute arsenic poisoning by ingestion are digestive problems: vomiting, abdominal pains, diarrhea often accompanied by bleeding. Sub-lethal doses can lead to convulsions, cardiovascular problems, inflammation of the liver and kidneys and abnormalities in the coagulation of the blood. These are followed by the appearance of characteristic white lines (Mees stripes) on the nails and by hair loss. Lower doses lead to liver and kidney problems and to changes in the pigmentation of the skin. Even dilute solutions of arsenic trioxide are dangerous on contact with the eyes.
The poisonous properties are legendary and the subject of an extensive literature.[12][13][13][14]
Chronic arsenic poisoning is known as arsenicosis. This disorder affects workers in smelters, in populations whose drinking water contains high levels of arsenic (0.3–0.4 ppm), and in patients treated for long periods with arsenic-based pharmaceuticals. Similarly, studies on workers exposed in copper foundries in the U.S., Japan and Sweden indicate a risk of lung cancer 6–10 times higher for the most exposed workers compared with the general population. Long-term ingestion of arsenic trioxide either in drinking water or as a medical treatment can lead to skin cancer. Reproductive problems (high incidence of miscarriage, low birth weight, congenital deformations) have also been indicated in one study of women exposed to arsenic trioxide dust as employees or neighbours of a copper foundry.
In Austria there lived the so called "arsenic eaters", who ingested doses far beyond the lethal dose of arsenic trioxide without any apparent harm. Arsenic is thought to enable strenuous work at high altitudes, e.g. in the Alps.[15][16][17]
The current OSHA 1910.1018 occupational permissible exposure limit for inorganic arsenic compounds in breathing zone air is 0.010 mg/m3.
References
- ^ Shakhashiri BZ, "Chemical of the Week: Arsenic", University of Wisconsin-Madison Chemistry Dept.
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ^ a b c d Sabina C. Grund, Kunibert Hanusch, Hans Uwe Wolf "Arsenic and Arsenic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, VCH-Wiley, 2008, Weinheim.
- ^ "Giant Mine - Northwest Territories Region - Indian and Northern Affairs Canada". Retrieved 2007-08-28.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Marcel Gielen, Edward R. T. Tiekink (2005). Metallotherapeutic Drugs and Metal-Based Diagnostic Agents. Wiley. p. 298.
- ^ Steven L. Soignet; et al. (2001). "United States Multicenter Study of Arsenic Trioxide in Relapsed Acute Promyelocytic Leukemia". Journal of Clinical Oncology. 19 (18): 3852–3860.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Antman, K. H. (2001). "Introduction: The history of arsenic trioxide in cancer therapy". Oncologist. 6(Suppl. 2) (1–2): 2006.
- ^ Jun Zhu, Zhu Chen,Valérie Lallemand-Breitenbach, Hugues de Thé "How Acute Promyelocytic Leukaemia Revived Arsenic," Nature Reviews Cancer 2002, volume 2, 1-9.
- ^ Bobé Pierre, Bonardelle Danielle, Benihoud Karim, Opolon Paule, Chelbi-Alix Mounira (2006). "Arsenic trioxide: A promising novel therapeutic agent for lymphoproliferative and autoimmune syndromes in MRL/lpr mice". Blood. 108 (13): 3967-3975.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lu J, Chew EH, Holmgren A (2007). "Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide". Proc. Natl. Acad. Sci. U.S.A. 104 (30): 12288–93. doi:10.1073/pnas.0701549104. PMID 17640917.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Stanton v Benzler 9716830". U.S. 9th Circuit Court of Appeals. 1998-06-17. Retrieved 2008-06-09.
(...) convicted by a jury of first degree murder for poisoning her ex-husband. Her ex-husband's body was found with traces of arsenic trioxide in it.
- ^ a b Emsley, John (2006). "Arsenic". The Elements of Murder: A History of Poison. Oxford University Press. pp. 93–197. ISBN 9780192806000.
- ^ Madame Bovary by Flaubert
- ^ Arsenic Eaters — New York Times July 26, 1885
- ^ Richard M. Allesch. Arsenik. Seine Geschichte in Österreich. 54. Band. Klagenfurt: Kleinmayr 1959.
- ^ G. Przygoda, J. Feldmann, W. R. Cullen (2001). "The arsenic eaters of Styria: a different picture of people who were chronically exposed to arsenic". Applied Organometallic Chemistry. 15 (6): 457–462. doi:10.1002/aoc.126.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- - Solubility of As2O3 in water as function of temperature
- Case Studies in Environmental Medicine: Arsenic Toxicity
- IARC Monograph – Arsenic and Arsenic Compounds
- International Chemical Safety Card 0378
- NIOSH Pocket Guide to Chemical Hazards
- NTP Report on Carcinogens – Inorganic Arsenic Compounds
- Use of Arsenic Trioxide in Multiple Myeloma Treatment
- The use of Arsenic trioxide in medicine.
- Institut national de recherche et de sécurité (1989). "Trioxyde d'arsenic." Fiche toxicologique n° 89. Paris:INRS. (in French)
- Institute of Chemistry Austria, speciallised on arsenic and various arsenic compounds

