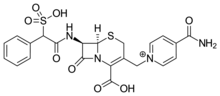
Cefsulodin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H21N4O8S2+ |
| Molar mass | 533.556 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cefsulodin is a third generation cephalosporin antibiotic that has very specific activity against Pseudomonas aeruginosa. It has no significant activity against other Gram-negative bacteria and very limited activity against Gram-positive bacteria and anaerobic bacteria. Cefsulodin was first synthesized and patented by the Takeda Pharmaceutical Company in 1977. In 2002, Takeda stopped production of Cefsulodin. Many years of low-stability cefsulodin production has led to a widespread reduction of laboratory and research usages. Current attempts (i.e. IDEXX Laboratories) of increasing purity and stability of cefsulodin center around recrystallization. Typically the process entails the following: Cefsulodin is dissolved in an organic solvent, sodium, water or any mixture thereof, then subsequently recrystallized through separation of the unwanted fraction. Recently, TOKU-E has found that the main cause of cefsulodin instability stems from one key impurity in 7-ACA (7-aminocephalosporanic acid- a raw material used in the synthesis of cefsulodin). In order to produce high-purity, high-stability cefsulodin, TOKU-E uses industrial HPLC to remove significant quantities of this impurity in 7-ACA and thus produces ultra-pure, ultra-stable, and ultra potent cefsulodin.[1]
General use
Cefuslodin is most commonly used in CIN (cefsulodin-Irgasan-novobiocin) agar to select for Yersinia microorganisms.[2] This agar is most often used in water and beverage testing.
Spectrum of Bacterial Susceptibility and Resistance
Currently almost all the species are resistant to Cefsulodin, except Helicobacter pylori and Pseudomonas aeruginosa have developed in varying degrees.[3]
References
- ^ [1], TOKU-E Technical Application Sheet.
- ^ "BAM Media M35: Cefsulodin-Irgasan Novobiocin Agar or Yersinia Selective Agar". Retrieved 2 September 2012.
- ^ Cefsulodin sodium
